Abstract
Objective
Antidepressant side-effects are a significant public health issue, associated with poor adherence, premature treatment discontinuation and in rare cases significant harm. This is especially relevant for older adults, who assume the largest and most serious burden of medication side-effects. We investigated the association between antidepressant side-effects and genetic variation in the serotonin system in anxious, older adults participating in a randomized, placebo-controlled trial of the SSRI escitalopram.
Method
Adults (n=177) aged ≥ 60 years were randomized to active treatment or placebo for 12-weeks. Side-effects were assessed using the UKU side effect rating scale. Genetic polymorphisms were putative functional variants in the promoters of the serotonin transporter and 1A and 2A receptors (5-HTTLPR (L/S + rs25531), HTR1A rs6295, HTR2A rs6311, respectively).
Results
Four significant drug-placebo side-effect differences were found, including increased duration of sleep, dry mouth, diarrhea and diminished sexual desire. Analyses using putative high- vs low-transcription genotype groupings revealed 6 pharmacogenetic effects: greater dry mouth and decreased sexual desire for the low- and high-expressing genotypes of the serotonin transporter, respectively, and greater diarrhea with the low-transcription genotype of the 1A receptor. Diminished sexual desire was experienced significantly more in those with high-expressing genotype and either the serotonin transporter, 1A or 2A receptors. There was not a significant relationship between drug concentration and side-effects nor a mean difference in drug concentration between low- and high-expressing genotypes.
Conclusion
Genetic variation in the 5HT system may predict who develops common SSRI side-effects and why. More work is needed to further characterize this genetic modulation and to translate research findings into strategies useful for more personalized patient care.
Keywords: SSRI, side-effects, serotonin, pharmacogenetic, older adults
Objective
In the United States, 14.5% of people aged 60 and older take an antidepressant medication. (1) Adherence to antidepressant pharmacotherapy is suboptimal, with one of the reasons being patient intolerance of side effects, even minor side-effects. (2) Side effect burden has improved with the advent of the selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs), however, discontinuation due to adverse reactions is still problematic; almost 40% of patients taking an SSRI experience at least one side-effect. (3, 4) Antidepressants are increasingly prescribed for older adults with depression and anxiety, (5, 6) a population especially vulnerable to side effects. (7, 8) Side-effects constitute a large public health burden which disproportionately falls on elderly patients. Older adults have less physiologic reserve than younger patients (7) and SSRI treatment has specifically been associated with weight gain, (9) concentration and attention impairment, (10, 11) falls, (12–14) syndrome of inappropriate antidiuretic hormone secretion (SIADH),(3) and serotonin syndrome (15) in this population. In addition to more severe side-effects that happen rarely, common, less severe side-effects also play an important role in patient adherence to SRIs.
One way of addressing side-effects of antidepressant treatment is through prevention. Patients with high genetic susceptibility to side-effects of a specific antidepressant could be offered a different treatment regimen. This level of treatment personalization is not currently available, but is within the sights of pharmacogenomics. Understanding the genetic basis of treatment-emergent adverse events and using this information to improve treatment quality is the goal of this line of research.
Pharmacogenetic effects are variations in drug effect by genotype, in this case the effect being a side-effect. Currently, there is no consensus on the pharmacogenetics of SSRI side-effects. Available studies lack consistency in samples selected and method of their assessment. (4) The assessment of SSRI side-effects is difficult due to individual differences in onset, duration, remission and recurrence. We addressed these issues through systematic, longitudinal assessment of individual side-effects both before and throughout the 12-week treatment trial. Studying side-effects within a placebo-controlled trial allows us to distinguish between true drug side-effects and placebo symptoms that occur naturalistically. Previous work has shown that some individuals are more likely to experience side effects, regardless of treatment. (16)
Our study examined genetic variation in the serotonin transporter and 1A and 2A receptors in relation to SSRI side-effects. The primary aim of this investigation was to determine if SSRIs were associated with genetic variation in the serotonin system. Additionally, our secondary aim was to determine if drug concentration was a moderator of side-effects. The serotonin transporter is the main target for all SSRIs, which increase serotonin in the synapse through inhibition of the serotonin transporter. Downstream, this affects serotonergic transmission at proximal targets including the 1A and 2A receptors. (17) The receptors are located both centrally and peripherally and thus have the potential to influence SSRI side-effects through both central nervous system (CNS) and peripheral changes. The serotonin receptors are known to have functional genetic variation, with high- and low- expressing (gene transcription) genotypes. These functional genotypic differences in expression may influence the occurrence of SSRI side-effects phenotypically. We hypothesized that there would be pharmacogenetic differences in SSRI side-effects, related to genetic variation in these 3 genes.
Methods
Data for this study come from a 12-week, double-blind, randomized controlled trial comparing escitalopram and placebo. (18) Participants were age 60 and older, with a principal diagnosis of generalized anxiety disorder (GAD) (according to the Structured Diagnostic Interview for DSM-IV axis I diagnoses (SCID))(19) and a score of ≥17 on the Hamilton Anxiety Rating Scale. (20) Written informed consent was obtained from all participants and the study was approved by the University of Pittsburgh Institutional Review Board. Participants were recruited from primary care, specialty mental health practices and advertisements. Co-morbid diagnoses including unipolar depression and other anxiety disorders were allowed while the following were excluded: lifetime psychosis or bipolar disorder, dementia, medical instability, exogenous steroid use (including inhaled steroids), and antidepressant or anxiolytic coprescription (with the exception of continuing low-dose benzodiazepines if already in use for at least two months). Participants were randomized to 10 mg of escitalopram (increased to 20 mg after four weeks if tolerated and as needed) or placebo.
Side-effects were assessed using the Udvalg fur Kliniske Undersøgelser (UKU) side effect rating scale (21) at baseline and weeks 1, 2, 3, 4, 6, 8, 10 and 12 of the clinical trial. The “side-effects” may be more accurately characterized as symptoms in patients receiving placebo and at baseline, therefore we report symptoms when describing analyses that include untreated patients and/or baseline data and report side-effects in analyses that include only escitalopram treated patients. The UKU assesses 46 side-effects experienced during treatment with psychotropics in the categories of psychic, neurologic, autonomic and other. Items are scored 0–3 for extent of side-effect experienced. The scale, including all 46 side-effects, was administered by trained raters at the clinic visits listed previously. For this project, we only evaluated the 17 side-effects listed in Table 3 because in preliminary analyses escitalopram treated patients reported higher mean scores, averaged across administrations, on these 17 side-effects compared to placebo patients. Those symptoms eliminated because escitalopram-treated patients did not have significantly higher mean scores include: failing memory; reduced duration of sleep; hyperkinesia/akathisia; parasthesias; accommodation disturbances; increased salivation; nausea/vomiting; constipation; palpitations/tachycardia; weight loss. Additinally, we eliminated from consideration a priori those symptoms that were target symptoms of the medication (depression; tension/inner unrest; emotional indifference), and UKU items specific to pre-menopausal women (menoragia; amenorrhea; galactorrhea; gynaecomastia) and that were too rare to analyze due occurrence in less than 1% of participants (rigidity; hypokinesia/akinesia; epileptic seizures; increased sexual desire) or had extremely low response rate (erectile dysfunction; ejaculatory dysfunction; orgasmic dysfunction; dry vagina). We excluded one UKU item (sleepiness/sedation) due to inconsistency with the other items; this item was based upon observation of the participant during the clinic visit while the other items were based on reported symptoms over the past week. Our statistical analyses focused on the remaining UKU items (Table 3).
Table 3.
UKU side-effects arranged by significance. Beta, standard error and p-values were calculated using GEE models adjusting for baseline UKU scores and represent the difference in reported SSRI side-effects between escitalopram-treated and placebo participants.
| UKU Item | Side-effect | β (SE) for escitalopram>placebo difference | p |
|---|---|---|---|
| Q7 | Increased duration sleep | 1.13 (.36) | 0.002 |
| Q22 | Dry mouth | .55 (.23) | 0.02 |
| Q42 | Diminished sexual desire | 1.10 (.47) | 0.02 |
| Q24 | Diarrhea | .46 (.23) | 0.04 |
| Q32 | Pruritus | 1.30 (.22) | 0.05 |
| Q26 | Micturition disturbance | .75 (40) | 0.06 |
| Q30 | Increased tendency to sweat | .44 (27) | 0.10 |
| Q15 | Tremor | .52 (.36) | 0.15 |
| Q2 | Asthenia/Lassitude/Fatigue | .27 (.20) | 0.17 |
| Q11 | Dystonia | .72 (.55) | 0.19 |
| Q33 | Photosensitivity | .72 (.56) | 0.20 |
| Q27 | Polyuria/Polydipsia | .31 (.25) | 0.21 |
| Q9 | Increased dreaming | .18 (.25) | 0.47 |
| Q31 | Rash | .28 (.39) | 0.48 |
| Q34 | Increased pigmentation | .29 (.49) | 0.56 |
| Q19 | Headache | .08 (.19) | 0.67 |
| Q1 | Concentration difficulties | .08 (.21) | 0.71 |
Proc Genmod in SAS was used to calculate GEE models, with a Z test used to test for the significance of the GEE parameter.
DNA was extracted from blood using standard procedures. For genotyping all serotonin receptor polymorphisms other than the serotonin transporter, we used Sequenom™ technology. For the serotonin transporter polymorphisms we followed a protocol which allows for genotyping of the SLC6A4 promoter haplotype. (22) The genotypes examined in this sample were found to be in Hardy Weinberg Equilibrium. Caucasians were examined alone and then both Caucasian and African American samples were examined together. There were no significant differences in findings so results from the combined analyses are presented.
At weeks 2, 8 and 12 plasma samples for escitalopram levels were obtained. We assessed escitalopram concentrations using high performance liquid chromatography with ultraviolet detection. (23) Using nonlinear mixed effects modeling with the NONMEM computer program (Version 5, level 1.1; University of California at San Francisco, CA, USA)(24) average concentration was calculated for each subject at a given dose. Average escitalopram concentration at the modal dose during the 12-week trial was utilized as the variable for analysis. (25)
Statistical Analysis
Characteristics of participants in the active and placebo groups at baseline were compared using t-tests and chi-square tests. When data were not normally distributed, the corresponding nonparametric equivalent was computed. Generalized Estimating Equations analyses (GEE) were used to examine the between-treatment group differences for the various symptoms. GEE analysis is a type of regression analysis that is similar to repeated measures ANOVA. GEE models the inherent correlations in time-course data. GEE, introduced by Liang and Zeger (1986)(26), is a method of analyzing correlated data that otherwise could be modeled as a generalized linear model. GEEs have become an important strategy in the analysis of correlated data. Such data sets arise from longitudinal studies, in which subjects are measured at different points in time, as we have in the current study. SAS/STAT® software’s GENMOD procedure enables one to perform GEE analysis by specifying a REPEATED statement in which one provides clustering information and a working correlation matrix. The generalized linear model estimates are used as the starting values. The focus of GEE is on estimating the average response over the population (“population-averaged” effects). Since our outcome measures are ordinal, the ordinal multinomial model was selected in PROC GENMOD. The multinomial option was used in the model statement. The multinomial probability distribution l specifies an ordinal model, as our side effect data have a natural order. Lipsitz, Kim, and Zhao (1994)(27) and Miller, Davis, and Landis (1993)(28) describe how to extend GEEs to multinomial data.
The GEE models (17 comparisons) were computed to determine which reported symptoms were more common in escitalopram-treated participants compared to placebo participants, controlling for baseline occurrence of symptoms. In pharmacogenetic analyses (15 comparisons), data from only the escitalopram-treated patients was used – eighty-five originally randomized to treatment excluding those without viable gene expression data, list-wise, for each pharmacogenetic analysis. We dichotomized subjects within each genotype into “high-expressing” or “low-expressing” groups based on the presence or absence of at least one high-transcription allele to maximize power, with groupings shown in Table 1. For example, the serotonin transporter promoter has a triallelic polymorphism composed of S, LG and LA alleles. These are commonly grouped by level of expression, with the low-expressing S and LG alleles showing little difference in expression from each other, but great difference from the high-expressing LA allele. (29) The presence of an LA allele leads to higher expression for the serotonin transporter, the G allele for the 1A receptor (rs6295) and the A allele for 2A receptor (rs6311). We tested the hypothesis that there would be a difference in the reporting of side-effects between high- and low-transcription genotypes, using GEE models for ordinal data, controlling for baseline UKU scores. In order to determine whether there was a significant pharmacogenetic effect, meaning that individuals with high-expressing vs low-expressing genotypes reported significantly different levels of side-effects, we tested for equality of GEE regression coefficients using the equation specified by Paternoster et al. (1998). (30) A probable pharmacogenetic effect was defined as a statistically significant difference in magnitude of GEE regression coefficients at the p<.05 significance level.
Table 1.
Serotonin transporter and receptor polymorphisms and genetic groupings.
| Chromosome | Polymorphism | rs number | Genotypes (n) | High-exp genotype | Allele Freq |
|---|---|---|---|---|---|
|
| |||||
| 5 | HTR1A (C-1019G) | rs6295 | CC (33) | CC | C .48 |
| CG (79) | G .52 | ||||
| GG (38) | |||||
|
| |||||
| 13 | HTR2A (G-1438A) | rs6311 | GG (52) | G .58 | |
| GA (70) | A .42 | ||||
| AA (27) | AA | ||||
|
| |||||
| 17 | 5-HTT triallelic haplotype | 5HTTLPR+rs25531 | LA0 (44) | L .57 | |
| LA1 (54) | L+A | S .43 | |||
| LA2 (35) | A .89 | ||||
| G .11 | |||||
We also examined pharmacokinetic effects by computing the Spearman’s rank correlation between modal escitalopram concentration (derived from the NONMEM model) and the side-effects of interest (4 comparisons). Additionally, we used t-tests to compare the mean dose of escitalopram for each high- and low-expressing genotype (8 comparisons). Analyses were performed using SAS 9.1.3 (SAS Institute, Cary, NC) and alpha levels were set at 0.05 with 2-tailed tests.
Seven participants dropped out of the trial due to side-effects. For these participants, we imputed a score of ‘3’ on the UKU item or items corresponding to the side-effect leading to discontinuation, at the week of discontinuation.
Results
Characteristics of the sample are shown in Table 2. There were no statistically significant differences between participants randomized to escitalopram treatment or placebo for demographic or clinical characteristics. The following number of patients were lost from each arm: dropped-out due to or side-effects or symptoms (3 escitalopram; 4 placebo), dropped-out due to unrelated medical complications (1 escitalopram), withdrew consent or lost to follow-up (12 escitalopram; 12 placebo), no viable gene-expression data (5HTT (13 escitalopram; 14 placebo); HTR1A (15 escitalopram; 12 placebo); HTR2A (15 escitalopram; 13 placebo); no escitalopram concentration data (18 escitalopram). There were not any statistically significant differences in the number of patients lost at any stage between treatment groups. In Table 3 the UKU symptoms assessed are presented in descending order of significance for difference between escitalopram-treated and placebo groups, with p-values derived from GEE models for ordinal data, controlling for baseline reporting of symptoms. We found a significantly greater severity of symptom report in the escitalopram-treated patients compared to placebo for the following UKU items, shown in Table 3: increased duration of sleep, dry mouth, diarrhea and diminished sexual desire. Therefore, these items became candidates for genetic analysis. For ease of interpretability, we calculated the percent of patients reporting at least one of these symptoms across the time-course of the trial for both escitalopram-treated and placebo participants. We found the following: 31% of treated participants reported increased duration of sleep at least once compared to 16% of placebo participants; 46% of treated participants reported dry mouth at least once compared to 34% of placebo participants; 14% of treated participants reported diminished sexual desire at least once compared to 10% of placebo participants and 51% of treated participants reported diarrhea at least once compared to 35% or placebo participants.
Table 2.
Characteristics of escitalopram-treated and placebo participants at baseline.
| Characteristic | Escitalopram (n=85) | Placebo (n=92) | p-value |
|---|---|---|---|
| Age, mean (SD) | 71.1 (7.4) | 72.2 (8.2) | .56 |
| White, No. % | 68 (80.0) | 78 (84.8) | .49 |
| Men, No. % | 24 (28.3) | 34 (37.0) | .22 |
| Education, mean (SD) | 14.0 (2.5) | 13.8 (3.3) | .47 |
| Hamilton Anxiety Rating Scale, Mean (SD) | 22.9 (4.3) | 23.1 (4.9) | .92 |
| Hamilton Depression Rating Scale, Mean (SD) | 11.8 (3.4) | 12.3 (4.3) | .98 |
| Age of onset GAD, Mean (SD) | 43.3 (25.9) | 37.8 (27.8) | .18 |
For continuous variables, such as age, a two sample t test was used, degrees of freedom=175.
For dichotomous variables, such as gender, a chi square test was used, degrees of freedom=1
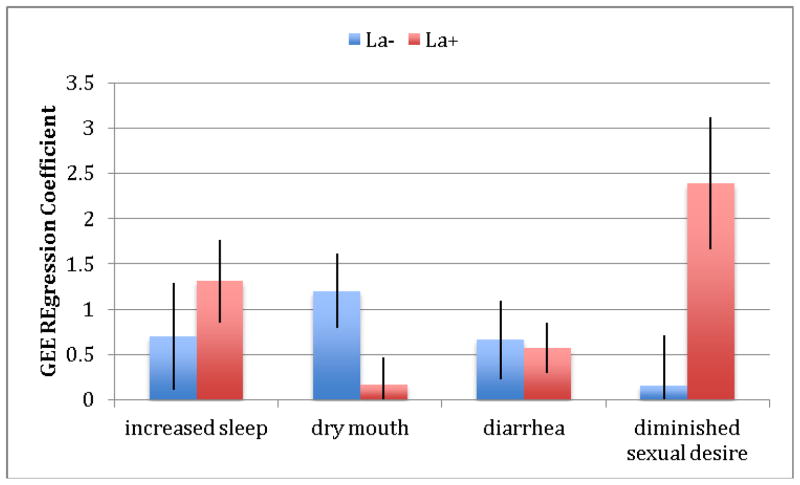
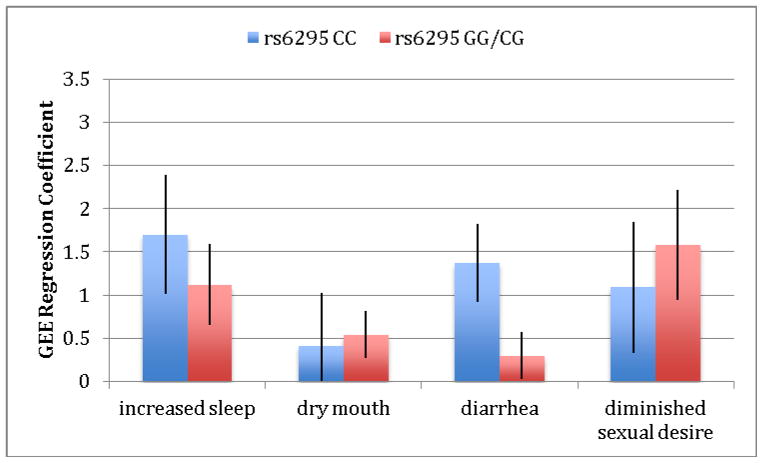
We next explored the relationship of the four significant patient-reported UKU side-effects -- increased duration of sleep, dry mouth, diminished sexual desire and diarrhea -- with genetic variation in the serotonin system. Analysis of these four UKU items revealed three pharmacogenetic effects, as shown in Table 4. For the serotonin transporter, we found greater dry mouth in participants with the LA− (low-expressing) genotype compared to those with the LA+ (high-expressing) genotype. We also found greater reported diminished sexual desire in the LA+ genotype. Additionally, we found greater reported diarrhea in the CC (low-expressing) genotype at the 1A receptor compared to the GG/GC (high-expressing) genotype and greater reported diminished sexual desire in the GG/CG (high-expressing) compared to the low-expressing genotype. Finally, we found greater reported diminished sexual desire in the AA/AG (high-expressing) genotype at the 2A receptor. Figures 1–3 show differences in GEE regression coefficients for low- and high-expressing genotypes for the pharmacogenetic effects in Table 4.
Table 4.
Serotonin transporter, 1A and 2A receptors by low- and high-expressing genotype groupings for significant drug effects. Highlighted pairs represent ‘pharmacogenetic effects’ where there is a significant difference in regression coefficients between the expression-based groupings for a side-effect.
| UKU | 5-HTTLPR triallelic haplotype | HTR1A rs6295 | HTR2A rs6311 | |||
|---|---|---|---|---|---|---|
| genotype (expression- level) | LA− (low) | LA+ (high) | CC (low) | GG/CG (high) | GG (low) | AA/AG (high) |
| n | 49 | 102 | 35 | 116 | 53 | 97 |
| Increased sleep β (SE) | .70 (.59) | 1.32 (.47) | 1.70 (.69) | 1.12 (.47) | 1.39 (.51) | 1.10 (.53) |
| Dry mouth β (SE) | 1.20 (.41)* | .17 (.30)* | .41 (.61) | .54 (.27) | .89 (.38) | .30 (.32) |
| Diarrhea β (SE) | .66 (.43) | .57 (.28) | 1.37 (.45)* | .30 (.27)* | 1.15 (.40) | .42 (.30) |
| Diminished Sexual Desire β (SE) | .16 (.55)* | 2.39 (.73)* | 1.09 (.76)* | 1.58 (.64)* | .89 (.66)* | 2.25 (.85)* |
p<.05 between GEE regression coefficients for low- and high-expressing genotypes
A Z test was used to test for the equality of regression coefficients (30).
Figure 1.
Difference in severity of side-effects, expressed as GEE regression coefficients, between low-expressing (LA−) and high-expressing (LA+) genotype groups for the triallelic haplotype of the serotonin transporter promoter polymorphism (5-HTTLPR). The comparisons between groups for dry mouth and diminished sexual desire are statistically significant at p<.05 between GEE regression coefficients for low- and high-expressing genotypes. A Z test was used to test for the equality of regression coefficients (30).
Figure 3.
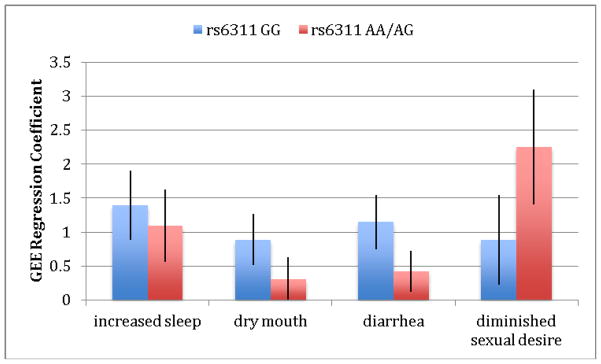
Difference in severity of side-effects, expressed as GEE regression coefficients, between low-expressing (GG) and high-expressing (AA/AG)) genotype groups for the HTR2A receptor. The comparison between groups for diminished sexual desire is statistically significant at p<.05 between GEE regression coefficients for low- and high-expressing genotypes. A Z test was used to test for the equality of regression coefficients (30).
In the pharmacokinetic analysis, there were no statistically significant relationships between drug concentration and side-effects (data not shown). There were also not any significant differences in concentration between low- and high-expressing genotypes. (Table 5; Supplemental Digital Content).
Conclusions
With their increased vulnerability to antidepressant side-effects, identifying older adults who may be at increased risk for adverse consequences is particularly important. Our goal was to identify pharmacogenetic effects in the serotonin system associated with SSRI side-effects. We found four symptoms occurring more frequently with escitalopram treatment than placebo: increased duration of sleep, dry mouth, diarrhea and diminished sexual desire. Importantly, data for this study were collected during a randomized, placebo-controlled trial. Additionally, we systematically assessed individual medication symptoms in both the treatment and placebo groups beginning prior to treatment and at 8 time-points during the 12-week trial. We selected only those symptoms that were significantly greater in treated participants, after controlling for any pre-treatment differences not addressed by random assignment to groups.
We found six pharmacogenetic effects for SSRI side-effects: dry mouth in the serotonin triallelic haplotype low-expressing genotype (LA−), diminished sexual desire in the high-expressing genotype of the triallelic haplotype (LA+) and diarrhea in the HTR1A low-expressing genotype (CC), diminished sexual desire in the HTR1A high-expressing genotype (GG/CG) and diminished sexual desire in the HTR2A receptor high-expressing genotype (AA/AG). These are all common, problematic side-effects with SSRI treatment, so finding genetic moderation is a positive development in the efforts to bring genetically informed prescribing to the clinic.
Our finding of two pharmacogenetic effects of opposite direction of function for the serotonin transporter promoter polymorphism is not surprising given existing evidence and the physiology of the serotonin system. The serotonin transporter is located in the CNS, but also is found in platelets, bone and most commonly in the gut. (31) Because serotonin does not cross the blood-brain barrier, the central (brain-derived) and peripheral serotonin systems (gut-derived) operate separately. Sexual side-effects may be centrally mediated while dry mouth and diarrhea may be peripherally mediated. Consistent with our finding that the serotonin transporter is involved with risk for SSRI side-effects, a recent, extensive review of antidepressant pharmacogenetic findings by Kato and Serretti (2010) concluded that there was evidence from 9 studies with 2,642 subjects for significant risk modulation associated with the serotonin transporter gene promoter polymorphism (5-HTTLPR).(32) Our study extends these previous findings by demonstrating specific symptoms tied to genetic variation.
The HRT1A is widely distributed in the enteric nervous system making it plausible that the rs6295 polymorphism low expressing genotype (CC) is linked to diarrhea from SSRIs. Considering this receptor as a target for a 1A receptor agonist antidote medication in people genetically susceptible to diarrhea may be warranted.
Overall, little is known about the physiological pathways by which SSRIs induce many of their side effects. Diarrhea is likely a peripheral effect – and one that unexpectedly appeared later in treatment in the low-expressing HTR1A group. Because it takes a few weeks to manifest, it is likely that downstream effects on receptor function are involved. Dry mouth is typically an anticholinergic effect, although salivation can be moderated by 5-HT. It actually appears that the placebo group and the La group (with placebo or drug) both slightly improve over time in having dry mouths. Rather than having worse dry mouth, the La− group simply doesn’t improve much. Thus, one could argue that La− (having low SERT) results in being insensitive to this beneficial effect. Effects on libido can plausibly be CNS effects in the reward system and in the hypothalamus. There is also a known peripheral SSRI effect in the sexual organs (mediated by HTR2A) that results in difficulty having an orgasm, which may indirectly influence libido.
Limitations
The findings in this study should be considered as preliminary and need to be confirmed. Limitations include the potential for both type 1 and type 2 error. We explicitly state the number of comparisons we performed for each analysis in out methods; we did not control for the number of comparisons. In a post hoc analysis, we ran our pharmacogenetic findings through a False Discovery Rate (FDR) filter. Three main findings survived the FDR at the p<.10 level (data not shown) including dry-mouth and diminished sexual desire at 5-HTTLPR and diarrhea at HTR1A. In power analyses, we found that a 20% difference between genotype expression-level groups would be necessary to achieve 80% power in the current sample size. Our differences are on the order of 3–18%.
Several pharmacogenetic effects (particularly with the 5HTR2A polymorphism) were of sufficient magnitude to be clinically significant, but were not statistically significant. Future work in this area can benefit from larger controlled studies that include serial administration of a side-effect rating scale that systematically rates presence and severity of individual symptoms. Those side-effects that we did not find significantly associated with SSRI exposure may still be good targets for future assessment in larger samples. Additionally, genetic samples were obtained at week 2 vs at randomization, meaning that we did not obtain genetic samples from patients who dropped-out before week 2. This could decrease our chance of finding pharmacogenetic effects if these patients were experiencing side-effects but did not contribute to the genetic samples.
The most important overall finding of this study is that there may be a pharmacogenetic basis for some common SSRI side-effects. Future work should confirm additional genetic variation associated with side-effects. It was not our intention to examine genetic predictors throughout the genes of interest but to examine whether the known (or putative) sources of functional variation predict side effects. Since it appears that they do, it would be important for larger, replication studies to also examine the entire genes with tag SNPs because additional functional variation might heighten the ability to discriminate between patients who will or will not develop side effects. Identifying these associations moves us closer to the goal of genetically informed treatment designed to maximize benefits while minimizing risks of antidepressant treatment.
Supplementary Material
Table 5. Mean concentration escitalopram by low- and high-expressing serotonin genotypes for treated patients
Figure 2.
Difference in severity of side-effects, expressed as GEE regression coefficients, between low-expressing (CC) and high-expressing (GG/CG)) genotype groups for the HTR1A receptor. The comparisons between groups for diarrhea and diminished sexual desire are statistically significant at p<.05 between GEE regression coefficients for low- and high-expressing genotypes. A Z test was used to test for the equality of regression coefficients (30).
Acknowledgments
Sources of financial and material support
Lundbeck, Roche, Johnson & Johnson (EJL)
2T32HL007456 (LDG)
MH070547 (EJL, DD, PD)
MH090250 (FEL)
The authors would like to acknowledge Dr. Eric Westhus for his assistance in preparing figures.
Footnotes
Previous presentations
This project was presented at the 11th Annual Pharmacogenetics in Psychiatry Meeting, March 30–31, 2012, New York, NY.
Disclaimer statements
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pratt LJ, Brody DJ, Gu Q. Antidepressant use in persons aged 12 and older: United States, 2005–2008. Hyattsville, MD: National Center for Health Statistics; 2011. [Google Scholar]
- 2.Schatzberg AF. Safety and tolerability of antidepressants: Weighing the impact in treatment decisions. J Clin Psychiatry. 2007;68:26–34. [PubMed] [Google Scholar]
- 3.Gartlehner G, Hansen RA, Carey TS, et al. Discontinuation rates for selective serotonin reuptake inhibitors and other second-generation antidepressants in outpatients with major depressive disorder: A systematic review and meta-analysis. International Clinical Psychopharmacology. 2005;20:59–69. doi: 10.1097/00004850-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cascade E, Kalali AH, Kennedy SH. Real-world data on SSRI antidepressant side effects. Psychiatry (Edgemont) 2009;6:16–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Mamdani M, Rapoport M, Shulman KI, et al. Mental health-related drug utilization among older adults: prevalence, trends, and costs. Am J Geriatr Psychiatry. 2005;13:892–900. doi: 10.1176/appi.ajgp.13.10.892. [DOI] [PubMed] [Google Scholar]
- 6.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 7.Pollock BG. Adverse reactions of antidepressants in elderly patients. The Journal of clinical psychiatry. 1999;60 (Suppl 20):4–8. [PubMed] [Google Scholar]
- 8.Mark TL, Joish VN, Hay JW, et al. Antidepressant use in geriatric populations: the burden of side effects and interactions and their impact on adherence and costs. Am J Geriatr Psychiatry. 2011;19:211–221. doi: 10.1097/jgp.0b013e3181f1803d. [DOI] [PubMed] [Google Scholar]
- 9.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. The Journal of clinical psychiatry. 2010;71:1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 10.Lenze EJ, Dixon D, NOWOTNY P, et al. Escitalopram reduces attentional performance in anxious older adults with high-expressing genetic variants at serotonin 2A and 1B receptors. International Journal of Neuropsychopharmacology. 2012 doi: 10.1017/S1461145712000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drueke B, Baetz J, Boecker M, et al. Differential effects of escitalopram on attention: a placebo-controlled, double-blind cross-over study. Psychopharmacology (Berl) 2009;207:213–223. doi: 10.1007/s00213-009-1649-6. [DOI] [PubMed] [Google Scholar]
- 12.Gagne JJ, Patrick AR, Mogun H, et al. Antidepressants and fracture risk in older adults: a comparative safety analysis. Clinical pharmacology and therapeutics. 2011;89:880–887. doi: 10.1038/clpt.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo JH, Lenze EJ, Mulsant BH, et al. Risk factors for falls during treatment of late-life depression. J Clin Psychiatry. 2002;63:936–941. doi: 10.4088/jcp.v63n1012. [DOI] [PubMed] [Google Scholar]
- 14.Laghrissi-Thode F, Pollock BG, Miller MC, et al. Double-blind comparison of paroxetine and nortriptyline on the postural stability of late-life depressed patients. Psychopharmacol Bull. 1995;31:659–663. [PubMed] [Google Scholar]
- 15.Anderson HD, Pace WD, Libby AM, et al. Rates of 5 common antidepressant sode effects among new adult and adolescent cases of depression: A retrospective US claims study. Clinical Theraputics. 2012;34:113–123. doi: 10.1016/j.clinthera.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Lotrich F, Bies R, Muldoon M, et al. Neuroendocrine response to intravenous ncitalopram in healthy control subjects: Pharmacokinetic influences. Psychopharmacology. 2005;178:268–275. doi: 10.1007/s00213-004-2006-4. [DOI] [PubMed] [Google Scholar]
- 17.Lotrich FE, Pollock BG. Candidate genes for antidepressant response to selective serotonin reuptake inhibitors. Neuropsychiatr Dis Treat. 2005;1:17–35. doi: 10.2147/nedt.1.1.17.52301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenze EJ, Rollman BL, Shear MK, et al. Escitalopram for older adults with Generalized Anxiety Disorder: a placebo-controlled trial. JAMA. 2009;301:296–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version: Administration Booklet. Washington, DC: 1996. [Google Scholar]
- 20.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 21.Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 22.Wendland JR, Martin BJ, Kruse MR, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 23.Foglia JP, Sorisio D, Kirshner M, et al. Quantitative determination of paroxetine in plasma by high-performance liquid chromatography and ultraviolet detection. J Chromatogr B Biomed Sci Appl. 1997;693:147–151. doi: 10.1016/s0378-4347(97)00010-8. [DOI] [PubMed] [Google Scholar]
- 24.Beal SL, Sheiner LB. NONMEM Users Guides. San Francisco, CA: 1992. [Google Scholar]
- 25.Jin Y, Pollock BG, Frank E, et al. The effect of reporting methods for dosing times on the estimation of pharmacokinetic parameters of escitalopram. J Clin Pharmacol. 2009;49:176–184. doi: 10.1177/0091270008327538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.Lipsitz SH, Kim K, Zhao L. Analysis of repeated categorical data using generalized estimating equations. Statistics in Medicine. 1994;13:1149–1163. doi: 10.1002/sim.4780131106. [DOI] [PubMed] [Google Scholar]
- 28.Miller ME, Davis CS, Landis JR. The analysis of longitudinal polytomous data: Generalized estimating equations and connections with weighted least squares. Biometrics. 1993;49:1033–1044. [PubMed] [Google Scholar]
- 29.Smits KM, Smits LJ, Peeters FP, et al. Serotonin transporter polymorphisms and the occurrence of advers events during treatment with sekectuve serotonin reuptake inhibotors. Int Clin Psychopharmacol. 2007;22:137–143. doi: 10.1097/YIC.0b013e328014822a. [DOI] [PubMed] [Google Scholar]
- 30.Paternoster R, Brame R, Mazerolle P, et al. Using the correct statistical test for the equality of regression coefficients. Criminology. 1998;36:859–866. [Google Scholar]
- 31.Berger M, Gray JA, Roth BR. The expanded biology of serotonin. Annual Review of Medicine. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive diorder. Molecular Psychiatry. 2010;15:473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 5. Mean concentration escitalopram by low- and high-expressing serotonin genotypes for treated patients