Abstract
The objective of the study was to assess the effects of diabetic polyneuropathy (DPN) on muscle contractile properties in humans, and how these changes are related to alterations in muscle morphology and denervation. Patients with DPN (n = 12) were compared with age- and sex-matched controls (n = 12). Evoked and voluntary contractile properties, including stimulated twitch responses and maximal voluntary contractions, of the dorsiflexor muscles were assessed using an isometric ankle dynamometer. Motor unit number estimates (MUNE) of the tibialis anterior (TA) were performed via quantitative electromyography and decomposition-enhanced spike-triggered averaging. Peak tibialis anterior (TA) cross-sectional area (CSA; cm2), and relative proportion of contractile to noncontractile tissue (%) was determined from magnetic resonance images. Patients with DPN demonstrated decreased strength (−35%) and slower (−45%) dorsiflexion contractile properties for both evoked and voluntary contractions (P < 0.05). These findings were not accounted for by differences in voluntary activation (P > 0.05) or antagonist coactivation (P > 0.05). Additionally, patients with DPN were weaker when strength was normalized to TA total CSA (−30%; P < 0.05) or contractile tissue CSA (−26%; P < 0.05). In the DPN patient group, TA MUNEs were negatively related to both % noncontractile tissue (P < 0.05; r = 0.72) and twitch half-relaxation time (P < 0.05; r = 0.60), whereas no relationships were found between these variables in controls (P > 0.05). We conclude that patients with DPN demonstrated reduced strength and muscle quality as well as contractile slowing. This process may contribute to muscle power loss and functional impairments reported in patients with DPN, beyond the loss of strength commonly observed.
Keywords: diabetes mellitus, postactivation potentiation, coactivation, contractile slowing, weakness
diabetes mellitus (DM) and its common complication, diabetic polyneuropathy (DPN), are associated with changes in the neuromuscular system and motor dysfunction (4). Motor dysfunction as a result of DPN can manifest broadly as muscle atrophy, weakness, and increased susceptibility to fatigue (1, 5, 26, 48, 49). These changes may be due to a combination of DM-induced alterations to the α-motor neuron, neuromuscular junction, and skeletal muscle fibers (14, 21, 47). Despite many studies conducted using diabetic animal models, relatively few have attempted to directly quantify neuromuscular and motor unit properties in humans with DPN. Thus the current study was aimed at investigating alterations in contractile properties and morphology of skeletal muscle in patients with DPN in association with changes in motor unit properties and denervation.
In rodent models of DM, weakness, as measured through induced tetanic stimulation, has been attributed partially to muscle atrophy, particularly in fast-twitch fibers (16). However, reduction in strength persists when muscle is compared relative to weight (16) or cross-sectional area (57). Studies investigating DPN in humans have reported length-dependent atrophy of skeletal muscle using magnetic resonance imaging (MRI) and dual energy x-ray absorptiometry (DXA) imaging techniques, in association with weakness (5, 26, 60). Strength loss in DPN has also been linked to excess fat infiltration of skeletal muscle (31) and preferential loss of type II muscle fibers related perhaps to their selective denervation (27). Other studies using experimental rodent models of DPN report distal motor axon or neuromuscular junction deficits, as well as excitation-contraction uncoupling and disruption of Ca2+ handling as contributing factors to strength loss (14, 22, 34). Thus there is no consensus on which factors, or combination of key factors, explain DM or DPN-associated weakness in humans or rodents. In humans with DPN, a potential unexplored source and complication of muscle weakness may be due to increased antagonist coactivation. In other neurological disorders (i.e., stroke, muscular dystrophy, Parkinson's disease), increased levels of coactivation have been reported to contribute to muscle weakness (13, 33) which may occur as a compensatory strategy to maintain joint stability. In addition to weakness, reductions in ankle plantar and dorsiflexion isokinetic muscle power (31) and rate of torque development (27) have been reported in DPN patients. These changes have been associated with decrements to balance and physical function. To date, the relationships between muscle morphological changes and reduction in rate of torque development with a quantification of denervation, or motor unit loss, have not been explored in DPN patients.
Whereas a loss of power production in DPN patients may be accounted for partly by a loss in strength, a reduction in contractile velocity could also play an important role. Studies investigating neuromuscular function in healthy adult aging have explored this link (40, 46). In diabetic rodent skeletal muscle, reduced contractile speed of muscle fibers has been shown (16, 34, 45, 57); however, these studies have utilized rodent models of diabetes, and mechanically skinned single-muscle fiber preparations, and thus the translation of these results to humans is unclear. A study in the tibialis anterior (TA) of humans reported no differences in evoked contractile properties between patients with DM and controls (54), but it is important to note the patient group studied featured DM, and not DPN. The presence of DPN could reflect a longer duration or more severe history of DM, and indeed DPN can result directly in greater muscle atrophy, weakness, and neuromuscular remodeling than DM alone (10, 11). Indeed, DPN has been associated with reduced motor unit number estimates (MUNEs; 2, 30), although how this chronic process of muscular denervation may affect muscle morphology has not been comprehensively explored.
Additionally, the potential slowing of muscle contractile properties has been purported to be associated with reduced motor unit (MU) firing rates (62). Previous investigations in rodent and human muscle have found muscle fiber contractile velocities match the firing rate properties of the motor neurons supplying their innervation (24, 61). This has been reported for some muscles in humans in relation to adult aging, including the TA (15). It is conceivable that the lower maximal motor unit firing rates reported in DPN patients may be linked with slowed contractile properties relative to controls. However, although contractile slowing has been reported numerous times in rodent models of DM and DPN, this slowing has not been shown in humans with DPN.
The purpose of this study was to assess the neuromuscular contractile properties of the TA in patients with DPN compared with age- and sex-matched controls. In addition to being affected by DPN earlier and to a greater extent than more proximal muscles, the TA has important functional roles in balance and gait, and has been extensively studied in health, aging, and disease (2, 40, 52, 60). Our specific objectives were threefold: 1) to investigate evoked twitch and voluntary contractile properties of dorsiflexors, including antagonist (plantar flexor) coactivation; 2) to determine peak TA muscle cross-sectional area (CSA), normalized strength (strength per unit of muscle tissue), and relative contractile and noncontractile tissue; 3) and to determine the relationship of the morphological measures stated above as they relate to TA denervation. We hypothesized patients with DPN would have slowed contractile properties compared with controls. Conjunctly, we hypothesized slowed contractile properties in DPN patients would be positively associated with lower mean firing rates (relative to controls) in TA motor units during a sustained, low-intensity dorsiflexion contraction. We hypothesized patients with DPN would be weaker and demonstrate lower maximal rates of torque development during maximal isometric voluntary contractions (MVCs), and that these decrements would be positively related to increased antagonist coactivation. Additionally, we hypothesized DPN patients would possess smaller TA CSAs with greater relative amounts of noncontractile tissue which would be negatively related to the number of functioning motor units estimated in the TA.
METHODS
Participants.
With ethical approval from the local university's Research Ethics Board, informed oral and written consent was obtained from 12 patients (7 men, 5 women; ages 32–78 yr) with DPN. These patients met the criteria for diagnosis of type 2 non-insulin-dependent DM with clinical and electrophysiological features of confirmed DPN (20). Medications used by individual patients enrolled in this study included metformin (n = 5), insulin intensive therapy (n = 2), glicazide (n = 2), metformin plus insulin (n = 1), and metformin and glicazide (n = 2). DPN patients were not taking any confounding medications that could exacerbate their neuropathy or contribute to differences in their neuromuscular status (e.g., statins). Additionally, they had a thorough history, and clinical and electrophysiological examination by an experienced neurologist with specialized training in neuromuscular disease to exclude other causes of nerve injury and or neuropathy (i.e., compressive mononeuropathies or radiculopathies), and other neurological, metabolic, or vascular conditions unrelated to DM or DPN. To provide electrophysiological evidence of neuropathy all DPN patients had motor nerve conduction studies done of the fibular nerve, as well as sensory nerve conduction studies of the sural nerve. Data regarding blood lipid profiles and glycemic control were obtained through clinical chart review. The DPN patient group was compared with 12 healthy, age- and sex-matched controls (7 men, 5 women; ages 29–77 yr), who were recruited from the community and provided written informed consent. Control participants were recreationally active, living independently in the community, free from medications, and were screened by physicians (neurologists) to ensure they met inclusion criteria.
Evoked contractile properties and strength of tibialis anterior.
To assess voluntary and electrically stimulated properties of the TA, participants were seated in a custom isometric dorsiflexion dynamometer (38). The right ankle was positioned at 30° of plantar flexion, while both knee and hip angles were maintained at 90°. Movement at the hip was minimized by securing a padded, C-shaped brace to the distal aspect of the right thigh. Inelastic straps were wrapped over the dorsum of the foot to secure the foot to the dynamometer (1, 2, 39).
All testing was performed on the right (dominant) leg. Maximal evoked twitch and compound muscle action potential (CMAP) responses of the TA were obtained by supramaximal, percutaneous electrical stimulation of the fibular nerve just distal to the fibular head. Stimulation was performed through a bar stimulating electrode using single, 100-μs square-wave pulses via a constant-voltage electrical stimulator (Digitimer stimulator, model DS7AH; Digitimer, Welwyn Garden City, UK). Participants performed three dorsiflexion maximal voluntary contractions (MVCs), with at least 3 min of rest between attempts. Participants were instructed to contract as hard and as fast as possible to ensure maximal torque, and rate of torque development, were achieved. Each MVC was held for approximately 3–4 s. Participants were provided with real-time visual feedback of their torque on a computer monitor and were verbally encouraged throughout the contraction. Voluntary activation during the 2nd and 3rd MVC attempts was assessed using the interpolated twitch technique (58). This technique involves supramaximal electrical stimulation of the fibular nerve before, during, and after a voluntary MVC. The amplitude of the interpolated torque electrically evoked from a single 100-μs stimulus during the plateau of the MVC was compared with a single (100 μs) resting twitch evoked ∼1 s following the MVC. Voluntary activation was calculated as a percent using the following equation: [1 − (interpolated twitch/resting twitch)] × 100. The twitch evoked following the MVC was used to evaluate postactivation potentiation (PAP) torque. The peak torque of the three MVC attempts was taken as the maximal torque for the participant. All torque signals were collected and sampled online at 500 Hz using Spike2 software (Version 7.11; Cambridge Electronic Design, Cambridge, UK) and analyzed off-line to determine voluntary isometric torques (strength) and voluntary rate of torque development (RTD) (N·m/s).
Surface electromyography (EMG) was collected from the TA and soleus (SOL) using self-adhering Ag-AgCl electrodes (1 cm × 3 cm; GE Healthcare, Helsinki, Finland). For the TA, the active electrode was placed over the motor point, ∼7 cm distal to the tibial tuberosity and 2 cm lateral to the anterior border of the TA, whereas the reference electrode was placed over the distal tendon of the TA. For the SOL, the active electrode was placed 2 cm below the gastrocnemii border, along the longitudinal axis over the SOL, whereas the reference electrode was placed over the distal tendon of the triceps surae. Electrode placement was adjusted to maximize the TA and SOL compound muscle action potential (CMAP) negative peak amplitudes as necessary. Two ground electrodes were placed over the patella. Electrically evoked CMAPs were elicited from the SOL using a bar electrode held in the distal portion of the popliteal fossa between the origins of the heads of the gastrocnemius to stimulate the tibial nerve. Surface EMG signals were preamplified (×100), amplified (×2), band pass filtered (10 Hz to 1 kHz), converted by a 12-bit analog-to-digital converter (Power 1401, Cambridge Electronic Design), and sampled online at 2,000 Hz. To calculate the level of coactivation, a 0.5-s period of EMG was analyzed from the SOL corresponding to the point of peak torque during a dorsiflexion MVC. The mean root mean square (RMS) of this EMG signal was calculated and normalized to the maximal SOL CMAP [%antagonist coactivation = (mean MVC SOL RMS EMG/peak RMS SOL CMAP) × 100].
Decomposition-based quantitative electromyography of TA.
To obtain MUNE and mean MU firing rates in the TA, decomposition-based quantitative electromyography (DQEMG) data were acquired using decomposition enhanced spike triggered averaging (DE-STA) software, described in detail elsewhere (56). Intramuscular EMG signals were recorded via a disposable concentric needle electrode (model N53153; Teca, Hawthorne, NY) inserted into the TA, 5–10 mm distal to the active surface electrode. The surface and intramuscular EMG signals were band pass filtered at 5 Hz to 5 kHz and 10 Hz to 10 kHz, respectively. Surface EMG was sampled at 3 kHz; intramuscular EMG was sampled at 30 kHz. Participants matched a target line of 25% MVC, visible on a computer monitor, for all isometric dorsiflexion contractions while the intramuscular needle electrode was inserted and manipulated in the muscle. This contraction intensity has been shown to be the most effective intensity for obtaining a representative MUNE in the TA based on the limitations of the technique (40). Surface and intramuscular EMG were collected while participants sustained a steady target torque for each contraction held for ∼30 s. Between contractions, the concentric needle electrode was repositioned to obtain a sample from different motor units. These procedures were repeated until at least 20 suitable trains of motor unit potentials (MUPs) and their respective surface-motor unit potentials (S-MUPs) were collected.
Decomposed EMG signals were reviewed off-line to determine the acceptability of the needle-detected MUP trains and their corresponding S-MUPs. A computer algorithm aligned the negative onset markers for all accepted S-MUPs and created a mean S-MUP template based upon their data-point by data-point average. All MUP and S-MUP markers were subsequently reviewed visually by the operator. A MUNE was derived by dividing the negative-peak amplitude of the CMAP by the negative peak amplitude of the mean S-MUP (2) (MUNE = CMAP/mean S-MUP). In addition to MUNEs, the DQEMG software measures firing rates (Hz) of individual motor unit action potential trains (MUAPTs) and provides overall mean firing rates per participant at a relative target level of contraction. The investigator was blinded to the status of the participant during off-line analysis.
Magnetic resonance imaging of TA.
Magnetic resonance images were acquired via serial axial plane in a 3.0-T magnet (Verio MRI, Siemens; Erlangen, Germany). Proton density images were acquired using the following parameters: 1500-ms repetition time (TR), 14-ms echo time (TE), 256 × 192 matrix, 243 × 325 mm field of view, and 5-mm slice thickness with slice separation of 2 mm. Participants were inserted into the magnet bore feet first, in the supine position, with the motor point on their right leg isocentered to the bore of the magnet. To ensure minimal movement the feet and knees were strapped together using inelastic, Velcro straps. The entire musculature of the leg from the tibial plateau to the malleoli was imaged.
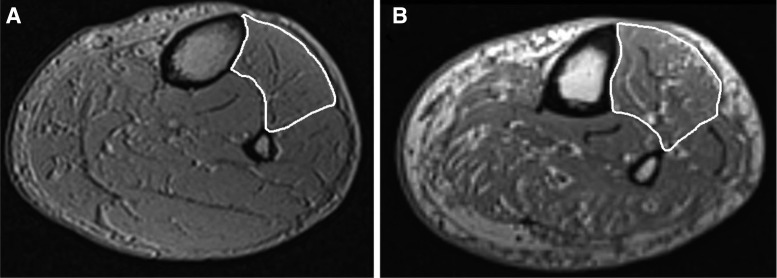
From the images, total TA CSAs were calculated pixelwise using a combination of manual and semiautomated techniques with open-source OsiriX image processing software (version 4.1, Geneva, Switzerland). Muscle CSAs were measured at the slice with the largest CSA. Analysis began proximally from the first slice, in which the TA appeared to the most distal slice containing the TA. A region of interest (ROI) was manually outlined on the TA muscle with the brush tool and repeated every five slices; missing ROIs on the skipped slices were automatically interpolated. With the TA outlined, all pixels outside the ROIs were set to zero. To quantify the contractile-only tissue, a three-dimensional threshold-growing tool was used to ensure only muscular tissue was included in the ROIs (excluding noncontractile tissue and septal spaces). Any errors produced by the automatic generation were corrected manually. The software calculated muscle CSA and volume for the ROIs. Sample images of a control leg and patient with DPN leg are depicted in Fig. 1. The investigator was blinded to the status of the participant during off-line analysis. Previous research has shown a high degree of intra- (ICC = 0.997) and interrater reliability (ICC = 0.997) with this analysis technique (9). Normalized strength was calculated by relating MVC torque values to TA CSA (N·m/cm2).
Fig. 1.
Sample MRI images of age-matched (∼65 yr old) male control (A) and diabetic polyneuropathy (DPN) patient (B) leg. The tibialis anterior (TA) is outlined in white. Note the greater amounts of intramuscular fatty infiltration and noncontractile tissue found in the DPN patient leg compared with the control leg (also see Table 3).
Statistics.
Mean values ± SDs are presented in the text. Normally distributed data were analyzed using a one-way ANOVA (group); the level of significance was set at P ≤ 0.05. Normality was assessed using the Shapiro-Wilk normality test; the only variables found to have a nonnormal distribution were mean SMUPs. Nonnormally distributed data were analyzed using a Kruskal-Wallis one-way ANOVA on ranks. Relationships among variables of interest were tested using Pearson's product moment correlation. Data analyses were performed using SigmaPlot software (version 12.2; Systat Software, Chicago, IL).
RESULTS
Participant characteristics.
Participant characteristics are presented in Table 1. No significant differences were detected for age (P = 0.42) or height (P = 0.29) between groups. DPN patients had significantly greater body mass (P = 0.008) and BMI (P = 0.02) than controls. Data characterizing the clinical history and status of the DPN patient group are also presented in Table 1.
Table 1.
Participant characteristics
| Controls (n = 12) | DPN Patients (n = 12) | |
|---|---|---|
| Anthropometric parameters | ||
| Male/Female | 7/5 | 7/5 |
| Age, yr | 64.5 ± 14.7 | 65.6 ± 14.6 |
| Height, m | 1.8 ± 0.1 | 1.7 ± 0.1 |
| Weight, kg | 73.8 ± 6.1 | 83.1 ± 7.4* |
| BMI, kg/m2 | 24.4 ± 3.1 | 28.9 ± 3.7* |
| Diabetic characteristics | Range or limit of normal | |
| Duration of diabetes, yr | 14.1 ± 11.2 | |
| Duration of DPN, yr | 9.2 ± 8.1 | |
| HbA1c, % | <6.0 | 7.4 ± 1.4 |
| Triglycerides, mmol/l | 0.77–1.7 | 1.9 ± 1.3 |
| Cholesterol, mmol/l | 3.0–5.0 | 4.1 ± 0.3 |
| HDL cholesterol, mmol/l | 0.9–2.0 | 1.3 ± 0.5 |
| LDL cholesterol, mmol/l | 2.0–3.0 | 2.1 ± 0.8 |
| Total cholesterol:HDL ratio | <5.0 | 3.9 ± 0.9 |
| Nerve conduction studies | Range or limit of normal | |
| Sural nerve SNAP amplitude, μV | >5 | 1.3 ± 2.5# |
| TA CMAP amplitude, mV | >4.5 | 5.0 ± 1.7 |
| Fibular nerve CV, m/s | >40.0 | 41.4 ± 10.4 |
| FDI CMAP amplitude, mV | >10.0 | 10.3 ± 2.7 |
| Ulnar nerve CV, m/s | >53.0 | 49.2 ± 3.4 |
Values are means ± SD, unless otherwise indicated. HDL, high-density lipoprotein; LDL, low-density lipoprotein; DPN, diabetic polyneuropathy; BMI, body mass index; SNAP, sensory nerve action potential; TA, tibialis anterior; CMAP, compound muscle action potential; CV, conduction velocity.
Significant difference between groups (P < 0.05).
SNAP responses were absent in 10 of 12 patients.
Dorsiflexor contractile properties.
Dorsiflexion contractile properties are presented in Table 2. DPN patient MVCs were 35% lower with 48% slower rates of maximal voluntary RTD compared with controls (P = 0.0001), although these differences did not appear to be due to any differences in voluntary activation (P = 0.57). DPN patients featured weaker and slower evoked twitch responses as evidenced by lesser peak twitch torque (∼Δ35%; P = 0.02), lesser average rate of twitch rise (∼Δ40%; P = 0.01), greater half-relaxation time (∼Δ35%; P = 0.001), and greater twitch contraction duration (∼Δ22%; P = 0.003). Additionally, the twitch response of DPN patients potentiated significantly less (∼Δ40%; P = 0.01) following a 3 s MVC compared with controls.
Table 2.
Dorsiflexion (TA) contractile properties in controls and patients with DPN
| Muscle Property | Control (n = 12) | DPN (n = 12) | %Difference |
|---|---|---|---|
| MVC, N·m | 34.1 ± 9.5 | 22.3 ± 7.2* | −34.6% |
| Maximal MVC RTD, N·m/s | 180.5 ± 49.9 | 93.9 ± 33.1* | −47.9% |
| Voluntary activation, % | 98.3 ± 1.9 | 97.6 ± 2.1 | |
| Peak twitch tension, N·m | 5.4 ± 1.9 | 3.5 ± 1.3* | −35.2% |
| Twitch average rate of rise, N·m/ms | 0.05 ± 0.01 | 0.03 ± 0.01* | −40.0% |
| Time to peak tension, ms | 105.3 ± 17.1 | 113.6 ± 25.6 | |
| Half-relaxation time, ms | 121.8 ± 16.6 | 164.3 ± 32.1* | +34.8% |
| Twitch rate of relaxation, N·m/ms | 0.04 ± 0.01 | 0.02 ± 0.01* | −50.0% |
| Twitch contraction duration TPT + HRT, ms | 227.1 ± 24.6 | 277.9 ± 43.9* | +22.0% |
| Potentiated twitch tension, N·m | 8.8 ± 2.7 | 4.8 ± 1.5* | −45.5% |
| %Potentiation | 65.4 ± 22.1 | 39.7 ± 17.3* | −39.3% |
Values are means ± SD. MVC, maximal voluntary contraction; RTD, rate of torque development; TPT, time to peak tension; HRT, half-relaxation time.
Significant difference between groups (P < 0.05).
TA CSA and normalized strength.
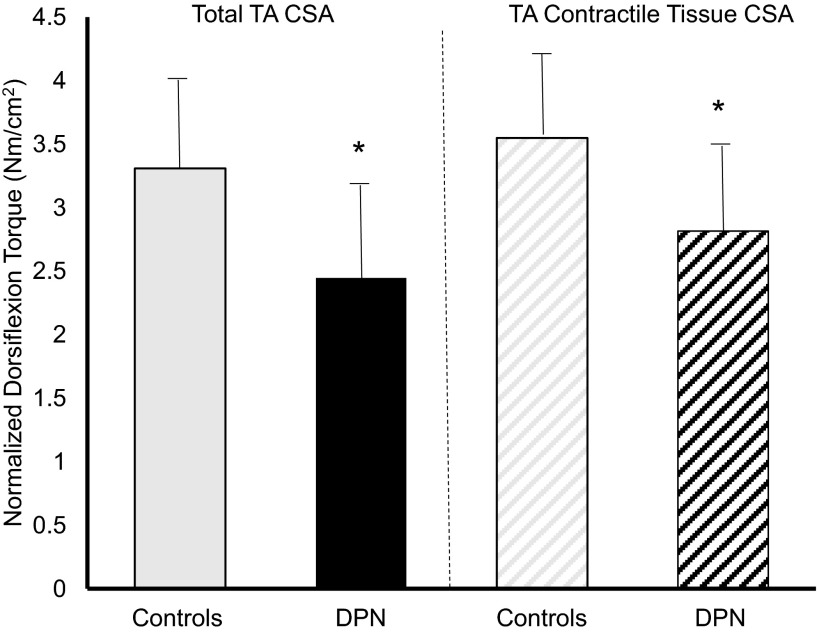
Both groups featured similar peak absolute TA total size (P = 0.21), contractile tissue (P = 0.18), and noncontractile tissue (P = 0.14; Table 3). However, as a relative percentage of total size, DPN patients featured 5% less contractile tissue and 5% more noncontractile tissue (P = 0.0003). When normalized to total TA CSA DPN patients were 30% weaker than controls, although total TA CSA contains both contractile and noncontractile tissue (P = 0.008; Fig. 2). However, DPN patients were also found to be weaker when strength was normalized to maximal TA contractile tissue CSA alone (P = 0.03; Fig. 2).
Table 3.
TA cross-sectional area and normalized dorsiflexion strength
| Tibialis Anterior Parameter | Control (n = 12) | DPN (n = 12) | %Difference |
|---|---|---|---|
| Total CSA, cm2 | 10.3 ± 2.3 | 9.5 ± 2.4 | |
| Contractile tissue CSA, cm2 | 9.6 ± 2.0 | 8.3 ± 2.2 | |
| Contractile tissue CSA, % | 93.2 ± 2.8 | 87.8 ± 4.3* | −4.8% |
| Noncontractile CSA, cm2 | 0.8 ± 0.4 | 1.1 ± 0.4 | |
| Noncontractile CSA, % | 7.7 ± 2.8 | 12.2 ± 4.5* | +74.3% |
| Strength normalized to total CSA, N·m/cm2 | 3.5 ± 0.8 | 2.5 ± 0.9* | −28.6% |
| Strength normalized to contractile CSA, N·m/cm2 | 3.8 ± 0.6 | 2.8 ± 0.9* | −26.3% |
Values are means ± SD. CSA, cross-sectional area.
Significant difference between groups (P < 0.05).
Fig. 2.
Dorsiflexion strength normalized to total TA cross-sectional area (CSA) in controls (solid gray) and DPN patients (solid black) and TA contractile tissue CSA in controls (hatched gray) and DPN patients (hatched black). *Significant difference between groups (P < 0.05).
Relationships between muscular denervation, function, and morphology.
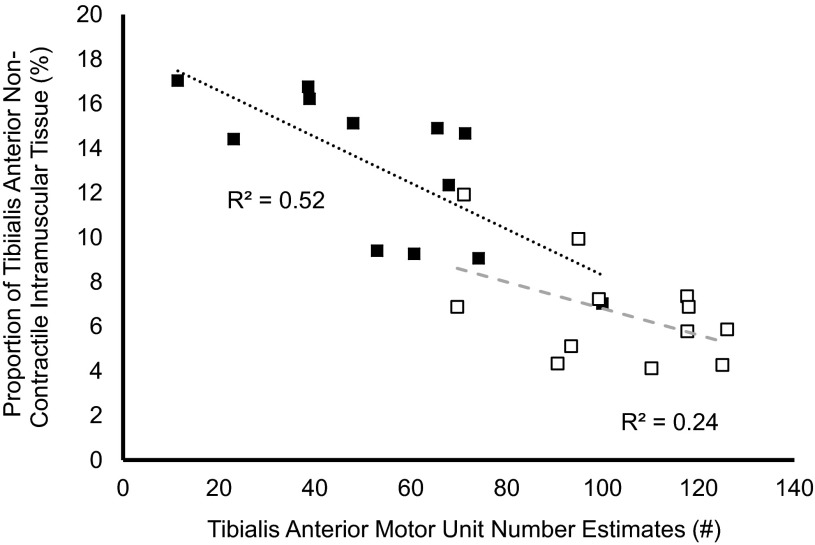
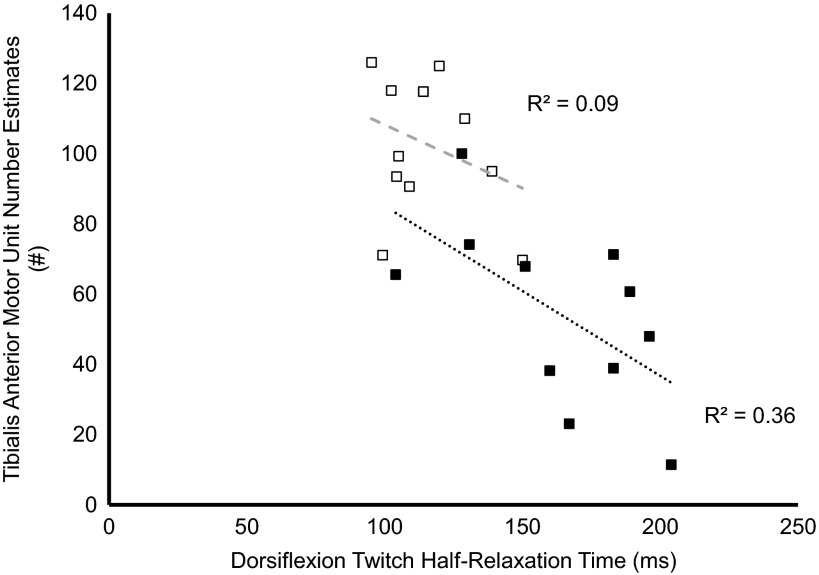
DPN patients featured 25% smaller TA CMAPs (P = 0.002), 50% larger mSMUPs (P = 0.03), and 45% reduced MUNEs (P = 0.0001) compared with controls (Table 4). Between groups, no differences were found in antagonist coactivation during MVCs (P = 0.07). In DPN patients, TA MUNEs were negatively related to the proportion of TA noncontractile tissue (P = 0.007, r = 0.72; Fig. 3), whereas no significant relationships were found between TA MUNEs and contractile/noncontractile tissue in the control group (P = 0.10, r = 0.49; Fig. 3). A significant negative relationship was found between dorsiflexion twitch half-relaxation time and TA MUNEs in the DPN patient group (P = 0.04; r = −0.61), but no significant relationship was found in the control group (P = 0.32, r = −0.31) (Fig. 4). In the DPN patient group, a negative relationship was found between antagonist coactivation and dorsiflexion MVC strength (P = 0.03, r = −0.64) but not RTD (P = 0.24, r = −0.36). During 25% MVC dorsiflexion contractions, mean TA firing rates were significantly lower in patients with DPN (10 vs. 12 Hz; P = 0.009). Finally, within the patient group, mean firing rates were negatively correlated with twitch half-relaxation time (P = 0.009, r = −0.77), whereas no significant relationship was found within the control group (P = 0.29, r = −0.36).
Table 4.
Needle and surface electromyography of TA and plantar flexors
| Electrophysiological Parameter | Control (n = 12) | DPN (n = 12) | %Difference |
|---|---|---|---|
| TA CMAP, mV | 6.8 ± 0.9 | 5.0 ± 1.7* | −26.5% |
| TA mSMUP, μV | 77.0 ± 20.0 | 116.0 ± 61.0* | +50.6% |
| TA MUNE, # | 99.0 ± 19.0 | 54.0 ± 25.0* | −45.5% |
| Mean TA firing rate at 25% MVC, Hz | 12.2 ± 1.7 | 10.1 ± 1.2* | −17.2% |
| MVC % antagonist (plantar flexor) coactivation | 2.7 ± 0.9 | 4.4 ± 2.4 |
Values are means ± SD. mSMUP, mean surface detected motor unit potential; MUNE, motor unit number estimation.
Significant difference between groups (P < 0.05).
Fig. 3.
Relationships between relative TA noncontractile tissue with TA motor unit number estimations (MUNE) in controls (open symbols) and DPN patients (closed symbols).
Fig. 4.
Relationships between dorsiflexion twitch half-relaxation time and TA MUNE in controls (open symbols) and DPN patients (closed symbols).
DISCUSSION
The main findings of this study were 1) patients with DPN had significantly lower and slowed evoked and voluntary contractile properties of the dorsiflexors; 2) no differences between groups were found in antagonist coactivation during voluntary contractions; 3) DPN patients featured less dorsiflexion postactivation potentiation; 4) DPN patients had less relative contractile tissue and greater relative noncontractile tissue in the TA than controls; 5) compared with controls, DPN patients featured less specific strength when torque was related to total TA CSA or contractile TA tissue CSA; and 6) MUNEs were positively related to relative TA contractile tissue CSA (and negatively related to TA noncontractile tissue CSA) in DPN patients, but not controls.
In the present study, our patient group featured clear evidence of DM and DPN through elevated glycosylated hemoglobin (HbA1c) levels and reduced or absent sural nerve sensory nerve action potential amplitudes (SNAPs) (Table 1). The slowed dorsiflexor contractile properties in patients with DPN are consistent with previous findings reported in rodent models of experimental diabetes (16, 34, 45, 57). This contractile slowing is evidenced by a decreased twitch average rate of rise (−40%), prolonged evoked half-relaxation times (+35%) and contraction durations (+22%), as well as decreased maximal RTD during voluntary MVCs (−50%) (Table 3). In addition to slowing, DPN patients featured ∼40% less postactivation potentiation (PAP) compared with controls (Table 3). Our results may be due to a variety of nonmutually exclusive factors, both myogenic and neurogenic in nature, and these factors may be directly related to mechanisms underlying decreases in strength and muscle quality.
Muscle strength, quality, and denervation.
In humans, it has been well established that DM and DPN are associated with a loss of muscle mass and strength (1, 3, 6, 44, 60). Indeed, our DPN group featured ∼35% weaker dorsiflexion strength than the control group (Table 2). In DPN, this weakness is related to the severity of the neuropathy and is likely caused by motor axonal degeneration and subsequent muscle fiber loss and atrophy (1, 30). In addition to the present study, previous reports have shown patients with DM have reduced muscle quality (torque per unit of muscle) compared with healthy controls (Fig. 2; 44, 60). Thus the present study importantly extends and focuses these previous findings by investigating patients with DPN, and relating neurogenic changes (i.e., motor axon or motor unit loss) with changes to muscle morphology. From our results a greater loss of motor units is associated with greater proportions of noncontractile intramuscular tissue (r = −0.72) and proportional loss of contractile tissue (r = 0.72) in patients with DPN (Fig. 3). This process may be caused by denervation of muscle fibers and insufficiency of surviving motor neurons in providing collateral reinnervation, thus failing to rescue these orphaned muscle fibers (1, 30, 47). If denervated muscle fibers fail to acquire a new source of innervation, they may atrophy, die, and subsequently be replaced by intramuscular fat deposits or other noncontractile tissue (19, 32, 35).
Based on our results, strength and muscle quality differences cannot be attributed to differences in voluntary activation of the TA, as both groups were able to activate their muscle similarly, and near maximally (>95%). Additionally, no differences were found between groups for antagonist coactivation during voluntary MVCs (Table 4). However, within the DPN group, there was a significant negative relationship (r = −0.64) between antagonist coactivation % and strength; thus perhaps with greater disease severity antagonist coactivation may become more pronounced to help stabilize the ankle joint during contraction. Alternatively, this pattern of weakness and increased coactivation may be related to a loss of proprioception associated with DPN (59). In either case, changes in agonist-antagonist coactivation in DPN patients seem comparable to contraction strategies used in other patient populations with neurological disorders such as stroke, muscular dystrophy, and Parkinson's disease (13, 33). Finally, myocellular gene expression of key contractile and regulatory proteins are markedly reduced in diabetes, particularly in the absence of insulin (and presumably established insulin resistance as occurs in type 2 DM) (55). Some of these downregulated genes control the synthesis of myosin heavy chain polypeptide, myosin light chain, actin, troponin, tropomyosin, and calmodulin proteins (55). In a single myocyte, reduced transcription of these genes could result in less contractile (e.g., actin, myosin) and contractile-supporting proteins (e.g., troponin, tropomyosin), potentially resulting in less tension produced per muscle fiber.
Muscle contractile slowing and DPN.
Conflicting results have been reported from studies that have explored the impact of diabetes on muscle fiber type composition, which can affect contractile speed and postactivation potentiation (PAP). Using an experimental animal model of diabetes, one investigation showed slow oxidative muscle fibers were least affected by DM, whereas fast oxidative and fast glycolytic muscle fibers were reduced in proportion and size (7). In contrast, using human cross-sectional studies, patients with non-insulin-dependent DM (type 2 DM) had lower proportions of type I muscle fibers and greater proportions of type II fibers (especially type IIb) in skeletal muscle (vastus lateralis) compared with healthy controls (37, 53). It is important to note that the animal model of experimental diabetes (7) represented type 1 DM in humans, whereas the muscle fiber composition studies in humans investigated patients with type 2 DM. Thus the latter may better reflect the physiological status of the patients included in the present study, who had type 2 DM. Given this information, faster contractile properties might be expected to accompany DM, contrary to the results presented in this study. However, it is possible changes in excitation-contraction (EC) coupling and Ca2+ handling supersede the effects of muscle fiber type and MHC expression on contractile speed, or they are competing factors which are differently expressed depending on the muscle or disease severity. Indeed, it is important to note the durations of diabetes in the studies that tested human vastus lateralis muscles (37, 53) are unknown, and this relatively proximal muscle may be less affected (vs. TA) by the length-dependent neurogenic changes associated with DPN. The muscle fiber compositions in the patients included in those prior studies with more recent disease onset may be more a reflection of the risk of development of DM as opposed to the effects of DM on potential muscle fiber type changes (43). Additionally, studies investigating muscle fiber composition and DM have not explored the impacts of DPN on this relationship.
Although motor unit or axon loss is a feature of DPN (1, 2, 30, 47) it is not known whether there is a preferential loss of larger, faster (type 2) motor units. If type 2 motor units are lost preferentially, this could drive the observed slowing of muscle contractile properties via the collateral reinnervation of orphaned muscle fibers by slow, type 1 motor neurons. Subsequently, these reinnervated muscle fibers acquire the characteristics of their new parent motor neuron (12). Indeed, there is some evidence to suggest this may be the case in DPN. For example, our results show a negative relationship between MUNEs and dorsiflexion twitch half-relaxation time (r = −0.61). This relationship could reflect a slowing of muscle properties due to a preferential loss of faster motor units associated with muscular denervation (Fig. 4). The significantly lesser degree of PAP in muscles from DPN patients reported in this study may also reflect a greater proportional loss of fast, type 2 motor units because muscle potentiation is known to be heavily influenced by fiber type composition (28). Also, motor unit firing rates are reduced in DM and DPN patients at a given contractile intensity in both the TA (Table 5; 2) and vastus lateralis (62), which may indicate a preferential loss of faster motor units, or slowing of the firing rates of surviving motor units. This process matches the contractile slowing reported in the present study and corroborates findings in healthy development and aging (15, 61). Additionally, within the DPN group, prolonged twitch half-relaxation times were negatively associated with decreases in mean firing rates (r = −0.77); however, it is not known if one alteration is necessarily dependent on the other.
Changes in EC coupling and Ca2+ handling may partially be responsible for slowing of contractile properties of patients with DPN. It has been reported that baseline myocellular Ca2+ levels are elevated in diabetic rats (42), which can cause attenuation in the rate of Ca2+ release from the sarcoplasmic reticulum (SR) during contraction (8, 18). This would lead to a decrease in RTD in evoked or voluntary contractions. Additional considerations regarding the reported reductions in strength and slowed contractile properties in patients with DPN include alterations in muscle architecture and tendon mechanical properties. Whereas aspects of muscle architecture, such as pennation angle, fascicle length, and sarcomere number and orientation, were not directly measured in this study, the increased proportion of noncontractile tissue in the TA of DPN patients may reflect broader architectural changes. Heterogeneous development of intramuscular noncontractile tissue and fatty infiltration (31) may not only reduce muscle CSA, but it may also disrupt normal fascicular organization. Structurally, an important determinant of whole muscle contractile speed is the number of sarcomeres in series (36), and fascicular disruption via noncontractile tissue could result in contractile slowing. With regard to tendon mechanical properties, previous studies have found the tendons of DM and DPN patients appear to show signs similar to those undergoing accelerated aging (25, 29). Specifically, these changes include altered tendon fibril shape, increases in fibril packing density, and increased tendon stiffness (6, 25). However, these DM-related changes in tendon properties are unlikely to lead to a slowed or weaker contractile response as a stiffer tendon would be conducive to a faster RTD. Slowed contractile properties as a result of DPN may be related to important functional decrements in conjunction with strength loss, specifically impaired muscle power generation. As power is a function of both muscle torque-generating capacity and contractile velocity, a decrease in either or both aspects will lead to reductions in power. Indeed, previous reports show patients with DM feature a loss of isokinetic power generation relative to controls, and the degree of power loss is greater than reductions in torque-generating capacity (3, 31). Additionally, this loss of power in DPN patients may be more important than strength loss alone in terms of functional consequences in dynamic muscle activities and the onset of physical disability (50). Finally, one possible confound related to our findings regarding contractile slowing is the potential difference between groups in physical activity levels. A previous study has shown that with adult aging, more sedentary individuals demonstrate greater contractile slowing than those who are more physically active (17). Our control subjects and nearly all of our DPN patients were living independently in the community with no mobility issues (2 DPN patients had impaired mobility and utilized a wheelchair or a cane). We do not believe there were substantive differences in everyday physical activity levels between the two groups that are as extreme as those reported elsewhere for example (17). Thus, whereas a lesser engagement in physical activity may be responsible for some of the slowing observed in our patient group, DPN per se seems to be the main contributor.
Summary.
The present study reports contractile slowing, loss of muscle CSA, loss of strength, and reduced contractile quality of lower limb muscles in association with DPN in humans. We propose these neuromuscular alterations may be caused by a combination of factors related to both myogenic and neurogenic changes that are known to occur with DPN. However, the relationships between muscle morphology and contractile slowing with denervation (MUNE) suggests changes in muscle properties may be secondary to neurogenic processes. With regard to functional consequences, these findings may represent key underlying mechanisms leading to DPN-related impairments in gait (41, 60), impaired balance, and increased fall risk (51, 52), and reduced walking speed (51).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.A., K.K., T.J.D., and C.L.R. conception and design of research; M.D.A. performed experiments; M.D.A. and B.M. analyzed data; M.D.A., B.M., K.K., T.J.D., and C.L.R. interpreted results of experiments; M.D.A. prepared figures; M.D.A. drafted manuscript; M.D.A., B.M., K.K., T.J.D., and C.L.R. edited and revised manuscript; M.D.A., B.M., K.K., T.J.D., and C.L.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Butler for assistance regarding magnetic resonance imaging and all those who participated in the study. This work was supported by the Natural Sciences and Engineering Research Council of Canada. M. D. Allen is supported by the Ontario Graduate Scholarship program.
REFERENCES
- 1.Allen MD, Choi I, Kimpinski K, Doherty TJ, Rice CL. Motor unit loss and weakness in association with diabetic neuropathy in humans. Muscle Nerve 48: 298–300, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Allen MD, Kimpinski K, Doherty TJ, Rice CL. Length dependent loss of motor axons and altered motor unit properties in human diabetic polyneuropathy. Clin Neurophysiol 10.1016/j.clinph201309037 [DOI] [PubMed] [Google Scholar]
- 3.Andersen H, Poulsen PL, Mogensen CE, Jakobsen J. Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes 45: 440–445, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Andersen H. Motor dysfunction in diabetes. Diabetes Metab Res Rev 28: 89–92, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Andersen H, Gadeberg PC, Brock B, Jakobsen J. Muscular atrophy in diabetic neuropathy: a stereological magnetic resonance imaging study. Diabetologia 40: 1062–1069, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Andreassen TT, Seyer-Hansen K, Bailey AJ. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim Biophys Acta 12: 313–317, 1981 [DOI] [PubMed] [Google Scholar]
- 7.Armstrong RB, Gollnick PD, Ianuzzo CD. Histochemical properties of skeletal muscle fibers in streptozotocin-diabetic rats. Cell Tissue Res 13: 387–394, 1975 [DOI] [PubMed] [Google Scholar]
- 8.Benders AA, Oosterhof A, Wevers RA, Veerkamp JH. Excitation-contraction coupling of cultured human skeletal muscle cells and the relation between basal cytosolic Ca2+ and excitability. Cell Calcium 21: 81–91, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Berger MJ, McKenzie CA, Ches DG, Goela A, Doherty TJ. Quadriceps neuromuscular function and self-reported functional ability in knee osteoarthritis. J Appl Physiol 113: 255–262, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Boulton AJ, Ward JD. Diabetic neuropathies and pain. Clin Endocrinol Metab 15: 917–931, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Bril V, Werb MR, Greene DA, Sima AF. Single-fiber electromyography in diabetic peripheral polyneuropathy. Muscle Nerve 19: 2–9, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol 150: 417–439, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busse ME, Wiles CM, van Deursen RW. Co-activation: its association with weakness and specific neurological pathology. J Neuroeng Rehabil 20: 26, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron NE, Cotter MA, Robertson S. Changes in skeletal muscle contractile properties in streptozotocin-induced diabetic rats and role of polyol pathway and hypoinsulinemia. Diabetes 39: 460–465, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Connelly DM, Rice CL, Ross MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87: 843–852, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Cotter M, Cameron NE, Lean LR, Robertson S. Effects of long-term streptozotocin diabetes on the contractile and histochemical properties of rat muscles. Q J Exp Physiol 74: 65–74, 1989 [DOI] [PubMed] [Google Scholar]
- 17.D'Antona G, Pellegrino MA, Carlizzi CN, Bottinelli R. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol 100: 603–611, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Delbono O. Ca2+ modulation of sarcoplasmic reticulum Ca2+ release in rat skeletal muscle fibers. J Membr Biol 146: 91–99, 1995 [PubMed] [Google Scholar]
- 19.Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol 74: 868–874, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Dyck PJ, Albers JW, Andersen H, Arezzo JC, Biessels G, J, Bril V, el al. Diabetic polyneuropathies: Update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 27: 620–628, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Fahim MA, Hasan MY, Alshuaib WB. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J Appl Physiol 89: 2235–2240, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Fahim MA, el-Sabban F, Davidson N. Muscle contractility decrement and correlated morphology during the pathogenesis of streptozotocin-diabetic mice. Anat Rec 251: 240–244, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Ganguly PK, Mathur S, Gupta MP, Beamish RE, Dhalla NS. Calcium pump activity of sarcoplasmic reticulum in diabetic rat skeletal muscle. Am J Physiol Endocrinol Metab 251: E515–E523, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Gardiner PF, Kernell D. The “fastness” of rat motoneurones: time-course of afterhyperpolarization in relation to axonal conduction velocity and muscle unit contractile speed. Pflügers Arch 415: 762–766, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Grant WP, Sullivan R, Sonenshine DE, Adam M, Slusser JH, Carson KA, Vinik AI. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg 36: 272–278, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Greenman RL, Khaodhiar L, Lima C, Dinh T, Giurini JM, Veves A. Foot small muscle atrophy is present before the detection of clinical neuropathy. Diabetes Care 28: 1425–1430, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez EM, Helber MD, Dealva D, Ashton-Miller JA, Richardson JK. Mild diabetic neuropathy affects ankle motor function. Clin Biomech 16: 522–528, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Hamada T, Sale DG, MacDougall JD, Tarnopolski MA. Interaction of fibre type, potentiation and fatigue in human knee extensor muscles. Acta Physiol Scand 178: 165–173, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hamlin CR, Kohn RR, Luschin JH. Apparent accelerated aging of human collagen in diabetes mellitus. Diabetes 24: 902–904, 1975 [DOI] [PubMed] [Google Scholar]
- 30.Hansen S, Ballantyne JP. Axonal dysfunction in the neuropathy of diabetes mellitus: a quantitative electrophysiological study. J Neurol Neurosurg Psychiatry 40: 555–564, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther 88: 1336–1344, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol 88: 662–668, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Lamontagne A, Malouin F, Richards CL, Dumas F. Mechanisms of disturbed motor control in ankle weakness during gait after stroke. Gait Posture 15: 244–255, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Lesniewski LA, Miller TA, Armstrong RB. Mechanisms of force loss in diabetic mouse skeletal muscle. Muscle Nerve 28: 493–500, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 23: 1647–1666, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Marin P, Andersson B, Krotkiewski M, Bjorntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17: 382–386, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Marsh E, Sale D, McComas AJ, Quinlan J. Influence of joint position on ankle dorsiflexion in humans. J Appl Physiol 51: 160–167, 1981 [DOI] [PubMed] [Google Scholar]
- 39.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. The effect of contraction intensity on motor unit number estimates of the tibialis anterior. Clin Neurophysiol 116: 1342–1347, 2005 [DOI] [PubMed] [Google Scholar]
- 40.McNeil CJ, Vandervoort AA, Rice CL. Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol 102: 1962–1968, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Meier MR, Desrosiers J, Bourassa P, Blaszczk J. Effect of type II diabetic peripheral neuropathy on gait termination in the elderly. Diabetologia 44: 585–592, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa M, Kobayashi S, Kimura I, Kimura M. Diabetic state-induced modification of Ca, Mg, Fe and Zn content of skeletal, cardiac and smooth muscles. Endocrinol Jpn 36: 795–807, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Pagel-Lagenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev 31: 25–51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging and body composition study. Diabetes 55: 1813–1816, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Paulus SF, Grossie J. Skeletal muscle in alloxan diabetes. A comparison of isometric contractions in fast and slow muscle. Diabetes 32: 1035–1039, 1983 [DOI] [PubMed] [Google Scholar]
- 46.Power GA, Dalton BH, Rice CL. Human neuromuscular structure and function in old age: a brief review. J Sport and Health Sci 2: 215–226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramji N, Toth C, Kennedy J, Zochodne DW. Does diabetes mellitus target motor neurons? Neurobiol Dis 26: 301–311, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc 27: 875–881, 1995 [PubMed] [Google Scholar]
- 49.Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelson AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol 85: 310–317, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Resnick HE, Stansbery KB, Harris TB, Tirivedi M, Smith K, Morgan P, Vinik AI. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve 25: 43–50, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Simmons RW, Richardson C. The effects of muscle activation on postural stability in diabetes mellitus patients with cutaneous sensory deficit in the foot. Diabetes Res Clin Pract 53: 25–32, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83: 166–171, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Singh-Peters LA, Jones GR, Kenno KA, Jakobi JM. Strength and contractile properties are similar between persons with type 2 diabetes and age-, weight-, gender- and physical activity matched controls. Can J Diabetes 31: 357–364, 2007 [Google Scholar]
- 55.Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 51: 1913–1920, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Stashuk DW. Decomposition and quantitative analysis of clinical electromyographic signals. Med Eng Phys 21: 389–404, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Stephenson GM, OCallaghan A, Stephenson DG. Single-fiber study of contractile and biochemical properties of skeletal muscles in streptozotocin-induced diabetic rats. Diabetes 43: 622–628, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Todd G, Gorman RB, Gandevia SC. Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve 29: 834–842, 2004 [DOI] [PubMed] [Google Scholar]
- 59.van Deursen RW, Simoneau GG. Foot and ankle sensory neuropathy, proprioception and postural stability. J Orthop Sports Phys Ther 29: 718–726, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, Zuliani G, Ferrucci L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care 35: 1672–1679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vrbova G, Navattete R, Lowrie M. Matching of muscle properties and motoneurones firing patterns during early stages of development. J Exp Biol 155: 113–123, 1985 [DOI] [PubMed] [Google Scholar]
- 62.Watanabe K, Gazzoni M, Holobar A, Miyamoto T, Fukuda K, Merletti R, et al. Motor unit firing pattern of vastus lateralis muscle in type 2 diabetes mellitus patients. Muscle Nerve 48: 806–813, 2013 [DOI] [PubMed] [Google Scholar]