Abstract
Lung cancers are highly heterogeneous and resistant to available therapeutic agents, with a five year survival rate of less than 15%. Despite significant advances in our knowledge of the genetic alterations and aberrations in signaling pathways, it has been difficult to determine the basis of lung cancer heterogeneity and drug ressitance. Cancer stem cell model has attracted a significant amount of attention in recent years as a viable explanation for the heterogeneity, drug resistance, dormancy and recurrence and metastasis of various tumors. At the same time, cancer stem cells have been relatively less characetrized in lung cancers. This review summarizes the current understanding of lung cancer stem cells, including their molecular features and signaling pathways that drive their stemness. This review also discusses the potential startegies to inhibit the signaling pathways driving stemness, in an effort to eradicate these cells to combat lung cancer.
1. Introduction: Stem cell model of cancer
Lung cancers cause maximum number of cancer-related deaths worldwide and is highly correlated with smoking (Proctor, 2001) (Parkin et al., 2005) (Siegel et al., 2012). The risk of lung cancer remains significantly high for long-term heavy smokers even after smoking cessation. Fifty percent of new lung cancer patients are former smokers and many of them stopped smoking five years or more prior to diagnosis (Halpern and Warner, 1993; Tong et al., 1996). According to an estimate made by the World Health Organization (WHO), lung cancer will cause about 2.5 million deaths per year by the year 2030 (Proctor, 2001). In the United States, approximately 85% of the patients diagnosed with lung cancer die of this disease within five years and this rate has not changed significantly since 1970s (Jemal et al., 2008a; Jemal et al., 2008b). Despite significant advances in our knowledge about cancer, our ability to develop effective therapies to combat lung cancer has been limiting (Hanahan and Weinberg, 2011). Treatment of the primary lesions rarely prevents the development of the distant metastases, which is the major cause for fatality (Jemal et al., 2008b). These facts highlight a need for better understanding the cellular and molecular events underlying the genesis and metastasis of this disease for designing novel therapeutic strategies. In this context, a school of thought has emerged in the recent years that suggest that tumors arise from a subset of cancer cells, called cancer stem cells, which may remain dormant, have the capacity to evade therapeutic drugs and metastasize. This concept is different from the prevailing theory where all the cancer cells have equal and similar proliferative capacity and opportunity for initiating tumor growth and spread (Nowell, 1976; Visvader and Lindeman, 2008). Further, the cancer stem cell model suggests that cancers are organized into aberrant cell hierarchies which are driven by a subset of cells that have the ability to self-renew themselves and generate heterogeneous lineages of other cell types that comprise the tumor (Figure 1)(Bonnet and Dick, 1997; Clarke et al., 2006). Thus, in principle, agents that can eliminate such cancer stem cells or tumorinitiating cells might be highly effective as anti-cancer agents.
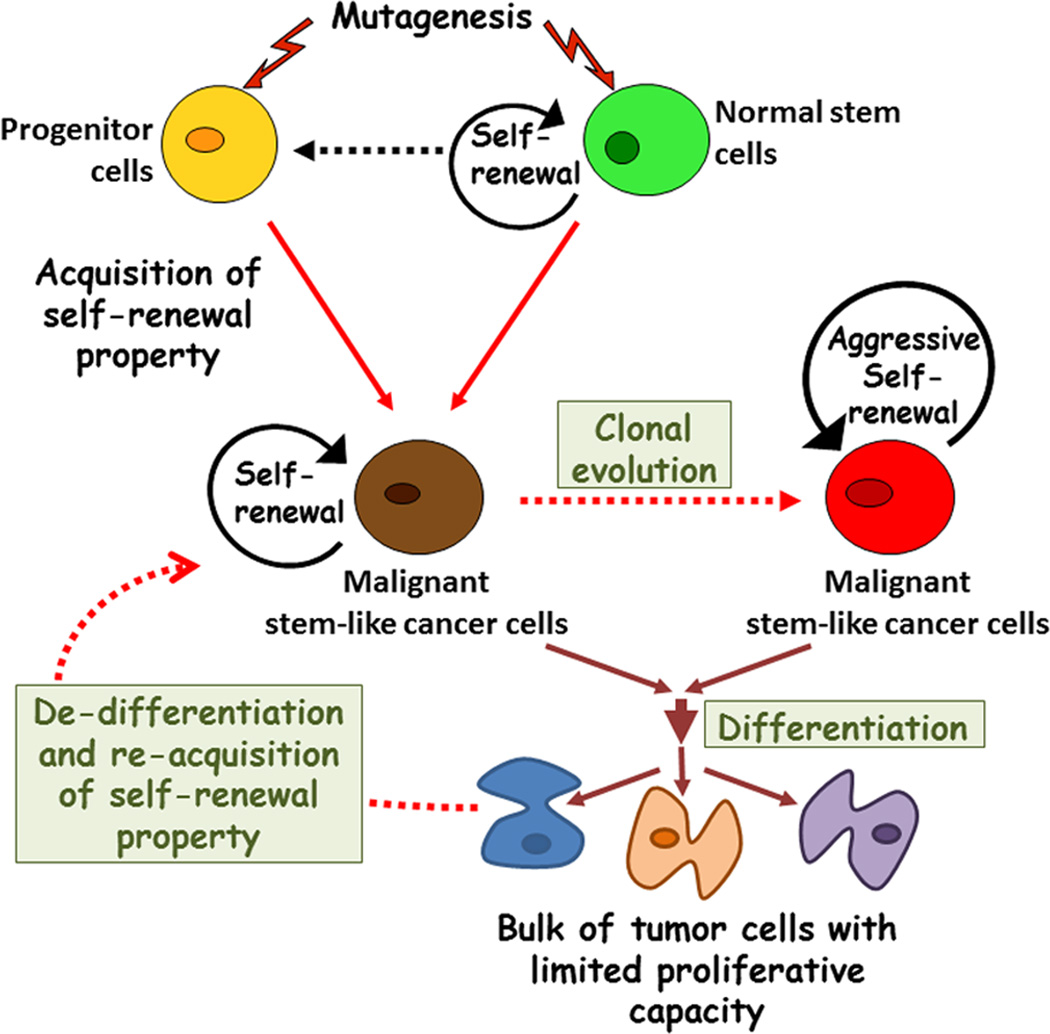
Figure 1. Origin of heterogeneity among stem-like cancer cells.
This diagram depicts our current understanding of stem cell model of cancer. Normal tissue stem cells with intrinsic properties of selfrenew and multi-lineage differentiation acquire oncogenic mutations which results in their deregulated self-renewal and give rise to stem-like cancer cells. Additionally, mutations might also cause restricted progenitor cells to acquire self-renewal property and become malignant stem-like cancer cells. These cells self-renew themselves as well as differentiate to generate phenotypically diverse cancer cells, which constitute the bulk of the heterogeneous tumor. During cancer progression stem-like cancer cells may evolve and change in genotype and phenotype to produce subclonal heterogeneity. Recent evidence also suggests the potential for reversal of mature cancer to re-acquire the stem-like properties through de-differentiation.
First experimental evidences for the existence of cancer stem cells came in the year 1997, with the identification of leukemia stem cells (Bonnet and Dick, 1997; Lapidot et al., 1994). Later, in the year 2003, the first evidence for hierarchical stem cell origin of solid tumor was experimentally demonstrated in breast cancer (Al-Hajj et al., 2003). However the de novo existence of cancer stem cells within solid tumors had remained controversial until very recently (For review, see (Medema, 2013)). In these recent studies using mouse models of brain (Chen et al., 2012), skin (Driessens et al., 2012) and intestinal (Schepers et al., 2012) tumors, three independent groups have provided convincing evidence that cancer stem cells do exist and are responsible for maintaining tumor growth in intact organs.
Self-renewal is a characteristic property of stem cells that allows them to maintain their numbers through symmetric or asymmetric mitotic cell division (Morrison and Kimble, 2006). During asymmetric division, each stem cell generates one daughter cell with stem cell fate (self-renewal) and one daughter cell (progenitor cell) that is destined to differentiate (Clevers, 2005). However, upon injuries or when stem cell pool has to be developed during development, stem cells undergo symmetric cell division where all the divided cells have stem cell fate (Morrison and Kimble, 2006). The fine balance between symmetric and asymmetric modes of division maintains the number of stem cells and its differentiated progeny depending on the developmental signals (Morrison and Kimble, 2006). It is believed that oncogenic transformation of the normal stem cells or the progenitor cells that were derived from normal stem cells results in deregulated self-renewal and give rise to cancer stem cells (Fisher et al., 2001; Hochedlinger et al., 2005; Lo Celso et al., 2004; Smalley and Ashworth, 2003). Aberrant symmetric or asymmetric cell division maintains the number of cancer stem cells within the tumor, whereas its descendent progeny constitute the bulk of the heterogeneous tumor (Clevers, 2005; Morrison and Kimble, 2006; Pardal et al., 2003; Visvader and Lindeman, 2008). These cells eventually undergo different stages of differentiation and are unable to initiate the formation of tumors. Being the only cell type with cancer initiating and driving ability and endowed with extensive replicative potential, the stem-like cancer cells must act as unit of selection during the progression of cancer (Greaves and Maley, 2012). Therefore, it is important to note that the clonal evolution model may co-exist with the cancer stem cell model. Cancer stem cells may themselves undergo “clonal evolution” by acquiring further mutations to undergo more aggressive self-renewal and growth as demonstrated for leukemia stem cells (Passegue et al., 2003; Visvader and Lindeman, 2008). Adding to the complexity, small number of recent studies now suggests that the differentiation of cancer stem cells may be reversible in certain cancers, and non-tumorigenic cells may also acquire cancer stem cell-like tumorigenic ability under specific circumstances (Chaffer et al., 2011; Chaffer et al., 2013; Gupta et al., 2011; Roesch et al., 2010); however the efficiency of this process under in vivo conditions is not clear.
2. Stem Cells in Lung cancer
Comparatively less is known about the biology of lung cancer stem cells compared to stem cell progenitors of other types of tumors. This is in part due to the complexity of the disease in terms of its phenotypic diversity and anatomically distinct sites of origin in pulmonary airways. Lung cancer can be subdivided into small cell lung cancer (SCLC), and three types of non-small cell lung cancer (NSCLC), which are squamous cells carcinoma (SCC), adenocarcinoma and large cell carcinoma. SCLC and SCC occur in the proximal region of the respiratory tract whereas adenocarcinoma originates in the distal airway (Giangreco et al., 2007). The presence of a diverse pool of self-renewing lung epithelial stem cells present in different regions of the respiratory tract has been thought to be responsible for this regional diversity. In support of this hypothesis, originating sites of SCLC, SCC, and adeno-/broncheoalviolar carcinomas appear to coincide with airway stem cell niches in murine models (Giangreco et al., 2009; Rawlins et al., 2009).
Induction of lung cancer in animal models using genetic modification suggests that lung cancer originates from resident stem cells. Classically, genetic modifications utilized comprise of transgenic expression of oncogenes or knockout of tumor suppressor genes, alone or in combination, under the control of lung epithelial cell-specific promoters (Dutt and Wong, 2006). These genetic modifications generate identical mutations throughout the large proportion of the lung and therefore should be capable of generating cancers throughout the entire lung. However, the most originating sites for various lung cancers appear to coincide with identified airway stem cell niches, and therefore suggest their stem cell origin (Rawlins and Hogan, 2006).
SCLC has very poor prognosis and high rate of metastatic dissemination (Jemal et al., 2008b). Human SCLCs predominately localize to mid-level bronchioles and typically express a range of neuroendocrine cell markers, including CGRP and other markers generally expressed within pulmonary Neuroendocrine Cells (PNECs), while markers associated with other cell types are not expressed (Gazdar et al., 1985). The loss of Retinoblastoma (Rb) and p53 functions are closely associated with human SCLC (Meuwissen and Berns, 2005). Despite Rb gene deletion throughout the airways, hyperplasia of PNECs was exclusive in a lung-specific conditional Rb inactivation model (Meuwissen et al., 2003). Similarly, deletion of both Rb and p53 in adult mice resulted in progressive epithelial hyperplasia that was restricted to the neuroepithelial bodies (NEB) microenvironment (Meuwissen et al., 2003; Minna et al., 2003). Importantly, these lesions progressed to form metastatic tumors resembling human SCLC (Meuwissen et al., 2003). On the basis of these observations, SCLCs are proposed to originate from PNECs. In a recent report, a direct approach has been taken to identify the cell of origin for SCLCs using a cell-type specific Cre-loxP expression model in adult mouse lung (Sutherland et al.). Depletion of Rb and p53 specifically in tracheal and bronchial Clara cells, BASCs, AT-2 cells and PNECs suggested that PNECs are indeed the predominant cancer-initiating population in SCLC. In addition, data also supported the presence of a SPC-positive progenitor cell population that could give rise to SCLC following loss of p53 and Rb, although with a lower efficiency (Sutherland et al.).
Since genetically modified mouse models of human lung-SCC do not exist, SCC is generally induced in mice using chemical carcinogenesis (Henry et al., 1981; Wang et al., 2004; Yoshimoto et al., 1977; Yoshimoto et al., 1980). Carcinogen induced murine SCCs generally occur in the proximal airways down to the second or third bifurcation and are rarely observed distally, which coincide with the suggested niche for basal stem/progenitor cells (Borthwick et al., 2001; Evans et al., 2001). Histologically, carcinogen induced SCCs are found to be initiated with generalized basal cell hyperplasia (Jeremy George et al., 2007). Additionally, hyperproliferative and preneoplastic lesions in SCC were also found to be basal stem cells specific Keratin-14-positive (Barth et al., 2000). Although direct approaches are needed to confirm the cell of origin for SCCs, current data support a direct relationship between proximal airway basal progenitors and SCC in murine models.
Bronchiolar adenocarcinomas and bronchioalveolar cell carcinomas are the most common lung cancer types among smokers as well as non-smokers (Jemal et al., 2008b). In murine models, adenocarcinomas arise from the junction between the terminal bronchiole and the alveolus termed the “bronchioalveolar duct junction” (BADJ) (Kim et al., 2005). Murine as well as human adenocarcinoma cells express Clara Cell Secretory Protein (CCSP) and/or Surfactant Protein-C (SP-C) markers suggesting that Clara or type II alveolar cells (AT-2) cells function as the originating cells for adenocarcinomas (Fisher et al., 2001; Kim et al., 2005). Additionally, in mouse models where CCSP or alveolar-SP-C promoters were utilized to express oncogenic proteins like mutated epidermal growth factor (EGF) receptor (Ji et al., 2006; Politi et al., 2006), active K-Ras (G12D) (Kim et al., 2005; Xu et al., 2012), dominant negative transforming growth factor-β (Bottinger et al., 1997), or large T antigen (DeMayo et al., 1991; Wikenheiser et al., 1992), all resulted in tumors closely resembling human bronchioalveolar carcinoma. As described above, napthalene resistant, NEB-independent, CCSP and SP-C expressing, Sca1-positive, BASCs were discovered in BADJ region of napthalene injured mouse lung (Kim et al., 2005). In the same study, using the cell-type specific Cre-loxP expression model, Kim and colleagues have demonstrated selective, dose-dependent BASC expansion and bronchioalveolar carcinoma induction after introducing K-Ras (G12D) mutation (Kim et al., 2005). In contrary, recent studies have shown that depletion of K-Ras and p53 or sustained EGFR signaling in SP-C+ cells led to the development of alveolar tumors without the involvement of BASCs (Lin et al., 2012). Whereas, another study suggested that expression of K-Ras in Clara cells and BASCs results in lung hyperplasia; whereas, its expression in AT-2 cells resulted to lung-adenocarcinoma (Xu et al., 2012).
The above findings provide evidence that normal airway stem cells can act as originating cells for lung cancers. However, in an interesting study, specific expression of H-Ras to pulmonary PNECs using a calcitonin gene-related peptide (CGRP) promoter-driven transgenic construct resulted in the formation of bronchial adenocarcinomas but not SCLCs (Sunday et al., 1999). Similarly, in recent studies, introduction of an activated H-Ras (V12) could convert neuroendocrine type SCLC cells into a completely changed phenotype more closely resembling NSCLC adenocarcinomas (Calbo et al.). These results suggest that the physiological effects of different genetic lesions can also drive the same target cells into divergent differentiation paths. Moreover, it should also be mentioned that the cell of origin is described for those self-renewing stem cells that acquire first oncogenic mutation to destabilize its growth. However, CSC may also arise from restricted progenitors, which acquires the self-renewing properties through genetic or epigenetic mechanisms. For lung tumorigenesis, this hypothesis proposal is supported by a recent report where Sca-1-positive-BASCs were originally proposed as cell of origin for K-Ras (G12D) driven bronchioalveolar carcinoma. However, within the tumors, both Sca-1-positive as well as negative cells acquired cancer stem cells properties, as demonstrated by their ability to initiate secondary tumors when implanted in recipient mice (Curtis et al.). Therefore, cancer stem cells may show more distinct markers than their proposed cell of origin, which represents the major challenge in identification and isolation of these Cancer stem cells from tumors.
3. Detection and association of human lung cancer stem cells with disease progression
The first experimental evidence for the existence of a stem-like clonogenic subpopulation in lung cancer was demonstrated in 1980s (Carney et al., 1982; Carney et al., 1980). In these pioneering studies, only a small proportion of SCLC and lung adenocarcinoma cells from patient samples demonstrated the ability to generate colonies in soft agar (Carney et al., 1982; Carney et al., 1980). Transplantation of these colony forming cells displayed tumorigenic potential in athymic nude mice (Carney et al., 1982; Carney et al., 1980). Since then, researchers have attempted to define lung cancer stem cells by various experimental approaches. Presently, these cells are isolated by flow cytometry using the presence of stem cell specific cell surface markers or by their functional properties, as described below.
The side population phenotype is a specific functional property described to isolate normal human hematopoietic stem cells from bone marrow population (Goodell et al., 1996). It is based on the ability of drug transporters to efflux the Hoechst 33342 dye via the ATP-binding cassette (ABC) family of transporter proteins, mainly ABCB1 (P-glycoprotein, MDR1), ABCC1-5 (multidrug-resistant proteins, MRP1-5), and ABCG2 (breast cancer resistance protein, BRCP1). These proteins are specifically expressed within the cell membrane of stem cell populations (Golebiewska et al.; Zhou et al., 2001). Hoechst 33342 dye excluding cells, termed ‘Side Population’ cells (SP cells), have been described in a variety of tumor types, where they have been shown to display increased capacity for self-renewal and tumorigenicity when transplanted into immunocompromised mice (Golebiewska et al.; Wu and Alman, 2008). SP cells were detected in various human NSCLC cancer cell lines and had several in vitro properties typical of stem cells, including clonogenic proliferation, invasive phenotypes, multi-drug resistance, and increased telomerase (hTERT) as well as lower levels of DNA replication associated protein MCM7 as compared to rest of the non-SP (main population, MP) cells (Ho et al., 2007). Similarly, SP cells were detected in primary tumors obtained from lung cancer patients. Like NSCLC cell lines, SCLC cell lines such as NCI-H82, H146 and H526 also demonstrated the presence of SP cells with tumorigenic potential. Gene expression profiling revealed the upregulation of several pluripotency associated genes such as Klf4, Nanog, Numb, Oct4 and Notch1 in SP cells (Salcido et al.). Our recent studies also showed a subset of SP cells in lung cancer cell lines and lung cancer specimens (Singh et al., 2013; Singh et al., 2012). SP cells were found to be expressing higher levels of ABCG2, Oct4, Sox2, Nanog, Twist, Snail, Slug, Vimentin and Fibronectin (Singh et al., 2012). Further, SP cells were mostly accumulated in the G0/G1 phase of cell cycle and were able to trans-differentiate into angiogenic tubules (Singh et al., 2013). Using systems biology approach for the differentially expressed genes among SP and MP cells, we obtained the signature of five genes which is able to predict the prognosis of NSCLCs (Perumal et al., 2012). These lines of evidence support the notion that the side population assay selects for cancer stem cells in lung tumors; however, experimental variables such as incubation time, dye concentration, cell concentrations, and gating variability may produce different frequency of SP cells from the similar type of samples (Golebiewska et al.) Therefore, a common experimental procedure needs to be proposed to avoid the variability from one laboratory to other.
Another method for identifying and isolating stem cell population is based on the high aldehyde dehydrogenase activity of stem cells. Aldehyde dehydrogenase (ALDH) is a family of intracellular enzymes that participates in cellular detoxification, differentiation and drug resistance in stem cells (Moreb et al., 1996). ALDH activity is found to regulate the self-renewal of hematopoietic stem cells by inhibiting the endogenous retinoic acid biosynthesis (Chute et al., 2006). Flow cytometry has been used to detect and isolate cells with elevated ALDH activity; this technique has led to the successful isolation of putative cancer stem cells from a variety of human cancers (Alison et al.; Moreb, 2008). Evidence for ALDH as a relevant cancer stem cell marker for the lung came with the discovery of elevated levels of ALDH-isoforms, ALDH1A1 and ALDH3A1 protein expression in SCC and adenocarcinoma patients (Jiang et al., 2009a; Patel et al., 2008). Additionally, higher expression of these isoforms of ALDH was also detected in putative lung stem cell niche for adenocarcinoma (Patel et al., 2008). In another study, more than 200 NSCLC tumor samples were analyzed for the expression of ALDH-isoforms and high expression of ALDH1A1 was strongly associated with reduced patient survival for Stage I NSCLC (Okudela et al., 2012; Sullivan et al.). Our study has demonstrated the higher ALDH activity in SP cells in NSCLC cell lines (Singh et al., 2013). NSCLC cells with high ALDH activity showed more tumorigenic and clonogenic activity as compared to the cells with low its activity, supporting high ALDH activity as a putative lung cancer stem cell phenotype (Sullivan et al.). However in a recent study with 268 cases of NSCLCs, authors have reported an inverse relationship between ALDH1A1 expression and tumor aggressiveness (Okudela et al., 2013). ALDH1A1 expression was found to be decreased among smokers and least expressed among poorly differentiated adenocarcinomas and large cell carcinomas (Okudela et al., 2013). These observations suggest that the functional properties may not be sufficient as an independent marker for the cells with stem cells characteristics. Similar to ALDH, glycine decarboxylase (GLDC) is proposed as another metabolic enzyme driving tumorigenesis and stem-cell characteristics in NSCLCs (Zhang et al., 2012). Higher expression of GLDC was found in lung cancer stem cells isolated from primary NSCLC tumors with stages I-III and its aberrant expression was associated with poor survival of lung cancer patients (Zhang et al., 2012).
Identification and isolation of putative cancer stem cells is also commonly carried out based on stem cell specific cell surface phenotypic markers. One of such markers successfully used to isolate lung cancer stem cell is CD133 (Prom1), either alone or in combination with other markers; it is a cell surface glycoprotein that consists of five transmembrane domains and two large glycosylated extracellular loops (Mizrak et al., 2008). CD133 and its glycosylated epitope, AC133, have been useful in the selection of normal human hematopoietic and neural stem cells as well as for brain, colon and pancreatic cancer stem cells (Keysar and Jimeno, 2010). Highly tumorigenic, self-renewing CD133+ cells in both NSCLC and SCLC specimens were isolated from single cell suspensions of whole tumor (Eramo et al., 2008). Alternatively, through retrospective approach, all the tumor forming cells from both SCLC and NSCLC were allowed to grow in serum free, stem cell selective media in a non-adherent condition. This strict culture condition allows the expansion of only self-renewing stem cells as spheres. All the sphere forming cells displayed CD133 expression, self-renewal and differentiation to specific lineage as well as recapitulated the hetrogeneous tumor in recipient mice (Eramo et al., 2008). The discovery of putative CD133+ lung cancer stem cells in both SCLC and NSCLC indicate that CD133 may serve as a pan-lung cancer stem cell marker. CD133-expressing stem-like cells isolated from NSCLC patients were found to be resistant to Cisplatin treatment, suggesting the drug resistant phenotype of cancer stem cells (Bertolini et al., 2009). However, the existence of variable CD133 isoforms and CD133 glycosylation states are also reported, which complicates the detection of CD133 and AC133 in whole tumor as well at single cell level (Bidlingmaier et al., 2008; Mizrak et al., 2008). Clinical significance of CD133 expression in human lung cancer still needs to be completely validated (Salnikov et al., 2010). Few recent studies have explored CD133 expression as prognostic markers for NSCLC. Immunohistochemical study using NSCLC tumors reveled that high CD133 expression correlates with poor prognosis in NSCLCs (Mizugaki et al., 2013; Okudela et al., 2012; Shien et al., 2012). In contrast to these findings, a study done on 133 stage I/II NSCLC patients did not find any significant prognostic correlation for the stem cell markers CD133, ABCG2 or CD117 (Herpel et al., 2011). Owing to the inter- and intra-tumor heterogeneity in cancer, these results suggest that the independent stem cell markers may not have the prognostic value in all types of lung cancer. This notion is supported by the observation that the expression of CD133 and ALDH1A1 is correlated with shortest recurrence free survival and overall survival among 205 stage-I NSCLC patients following surgical resection (Alamgeer et al., 2013). Similarly, CD133 coexpression with Oct4A transcription factor is associated with shortest disease free intervals among 64 adenocarcinoma patients (Cortes-Dericks et al., 2012).
4. Molecular targeting of lung cancer stem cells
In the stem cell model of cancer, the initiation and progression of cancer depends on the deregulated self-renewal of cancer stem cells. A number of genes like notch, wnt and shh, which are involved in maintenance and self-renewal of normal tissue stem cells, are found to be oncogenes in various cancers. These observations suggested that the pathways that govern normal stem cell self-renewal could also govern stem cell self-renewal in cancer as well. Therefore, identification of the developmental pathways involved in self-renewal of cancer stem cells for specific tumors has become an appealing strategy for finding the suitable target for treatment (Dalerba et al., 2007). There is only a handful of information available for the mechanism of self-renewal of normal or cancer stem cells for lung, which is summarized below.
The Wnt protein-mediated activation of Frizzled receptors leads to β-catenin accumulation and nuclear translocation. This Wnt/β-catenin pathway regulates the self-renewal of hematopoietic stem cells (Kirstetter et al., 2006; Reya et al., 2003); however, its role in lung epithelial stem cells is less understood (Stripp and Reynolds, 2008). Recently, activated Wnt/β-catenin signaling has been correlated with lung epithelium regeneration. Expression of constitutively active form of β-catenin, specifically in Clara cells revealed the expansion of BASCs upon naphthalene injury for lung epithelium regeneration (Reynolds et al., 2008). However in another study, deletion of β-catenin specifically in Clara cells had no impact on repair of naphthalene-injured airways and BASC expansion (Zemke et al., 2009). These contrasting studies suggested a context dependent role of Wnt/β-catenin signaling in lung stem cell self-renewal and need further analysis. Higher expression of Wnt proteins and aberrant Wnt signaling has been reported in lung cancer progression (Lemjabbar-Alaoui et al., 2006; Uematsu et al., 2003a; Uematsu et al., 2003b). Inhibition of Wnt signaling by a Wnt-2 monoclonal antibody induced cell death in NSCLC cells (You et al., 2004). However, mRNAs analysis for multiple Wnt in NSCLC cell lines and primary lung tumors revealed markedly decreased expression of Wnt-7a, compared to normal bronchial epithelial cell lines and normal lung tissue. Ectopic expression of Wnt-7a in NSCLC cell lines reversed cellular transformation, decreased anchorage-independent growth, and induced epithelial differentiation in a subset of the NSCLC cell lines, suggesting a tumor suppressor role of Wnt-7a (Winn et al., 2005; Winn et al., 2006). The possible association of Wnt signaling with stem cell self-renewal and lung tumorigenesis suggests its importance; however, further studies will be necessary to confirm the involvement of aberrant Wnt/β-catenin signaling in lung cancer stem cell self-renewal (Daniel et al., 2006; Reya and Clevers, 2005). Further, in a study using 200 lung adenocarcinoma samples, the coexpression of insulin-like growth factor I receptor (IGF-IR) with β -catenin and POU5F1 was found to be associated with poor prognosis. IGF-IR mediated signal induction resulted in activation of PI3K/AKT/GSK3β signaling, leading to the co-localization of β-catenin, Sox2 and POU5F1 with increased frequency of CD133 and ALDH-positive cells (Xu et al., 2013).
The Hedgehog (Hh) signaling pathway is a key developmental pathway during embryogenesis (Litingtung et al., 1998). The Hh signaling pathway is activated by sonic hedgehog (shh), a mammalian Hh ligand involved in pulmonary cell fate determination and branching morphogenesis (Bellusci et al., 1997; Pepicelli et al., 1998). In response to naphthalene injury, activated Hh signaling was observed in airway repair and epithelial regeneration and increased the numbers of neuroendocrine cells in PNECs niches (Watkins et al., 2003). Aberrations in expression and activation of this pathway also led to the development SCLCs (Goodrich and Scott, 1998; Nilsson et al., 2000; Taipale and Beachy, 2001). Suppression of aberrant Hh signaling in some SCLCs resulted in a dramatic drop in cell viability and tumorigenicity, therefore representing a suitable therapeutic target against SCLC (Vestergaard et al., 2006). However, a recent study did not find any significant correlation between Hh signaling pathway proteins with recurrence free or overall survival in 248 cases of early stage NSCLCs (Raz et al., 2012). Thus, there appears to be a certain amount of ambiguity regarding the contribution of the Hh pathway to the stemness of NSCLC initiating cells and the utility of targeting this pathway to combat this disease.
The Notch signaling pathway is one of the important cell fate determinants during tissue homeostasis. Upon the binding of Notch ligands to receptors on adjacent cells, the intracellular domain of the receptor is cleaved by a γ-secretase, allowing for the activation of downstream targets, such as the inhibitory basic helix-loop-helix transcription factor Hes1 (Artavanis-Tsakonas et al., 1999). Notch signaling appears to be required for determining proximal and distal lung epithelial cell fates during lung development (Collins et al., 2004). The indirect effect of Notch signaling has been demonstrated during lung development in a Hes1 knockout mouse model where inhibition of Notch signaling resulted in premature differentiation of pulmonary neuroendocrine stem cells (Ito et al., 2000). In other studies, activation of Notch signaling, either through the ectopic expression of intracellular Notch domains or through γ-secretase activation resulted in an increased number of distal airway stem cells, through reduced differentiation of neuroendocrine and alveolar stem cells (Dang et al., 2003; Guseh et al., 2009; Tsao et al., 2008). These results suggested that activated Notch signaling preserves the undifferentiated state of pulmonary stem cells. In lung cancer, while elevated Notch signaling transcripts have been described in NSCLC, the role of Notch in tumor maintenance remains poorly understood. Utilizing a Notch-GFP-reporter construct, a subset of cells with higher expression of Notch (GFP bright) is found to demonstrate stem-cell like properties in NSCLC cell lines (Hassan et al., 2013). Poor clinical outcome was observed for lung-adenocarcinoma patients with higher Notch ligand expression (Hassan et al., 2013). Suppression of Notch signaling in some NSCLC cells by treatment with a γ-secretase inhibitor induced cell death and decreased tumor growth in mice (Haruki et al., 2005; Konishi et al., 2007). Recently, elevated expression of Notch signaling responsive transcripts was reported in putative lung cancer stem cells with high ALDH activity. Using a γ-secretase inhibitor to suppress Notch signaling or ShRNA-mediated knockdown of NOTCH3 in lung cancer cells led to a significant reduction in ALDH+ cells (Sullivan et al., 2010). It suggests that Notch signaling may be activated in putative lung cancer stem cell populations is required for tumor initiation capacity (Bertolini et al., 2009; Jiang et al., 2009b; Levina et al., 2008). Activation of Notch signaling is also associated with resistance against platinum based chemotherapy and concurrent increase in CD133-positive cells in cell line based studies (Liu et al., 2013). Both γ-secretase inhibitor and shRNA against Notch1 remarkably reduced cisplatin-induced enhancement of CD133-positive cells and increased the sensitivity against doxorubicin and paclitaxel in lung cancer (Liu et al., 2013).
Transcription factors Oct4, Sox2 and Nanog have been identified as core transcription factors that maintain embryonic stem cell self-renewal (Kim et al., 2008). Several lines of evidences suggest the involvement of Sox2 in normal lung development (Gontan et al., 2008; Que et al., 2009). Sox2 depletion in developing lung result in significant decrease in basal, ciliated and Clara cells as well as increased numbers of mucus-secreting cells, suggesting its role in normal differentiation during embryonic lung development (Tompkins et al., 2009). In adult lung, Sox2 expression was found to be crucial for proper repair of airway epithelium upon SO2 induced injury (Tompkins et al., 2009). Sox2 depletion in basal stem cells resulted in suppressed undifferentiated proliferation in vitro, suggesting the role of Sox2 in self-renewal of basal stem cells in lung (Tompkins et al., 2009). Several studies have demonstrated the amplification of Sox2 in SCCs of lung (Bass et al., 2009). Further, high Sox2 expression was correlated with in vitro cell proliferation and anchorage independent growth of SCC cell lines, signifying its role as an oncogene (Bass et al., 2009). The oncogenic role of Sox2 was demonstrated using Sox2 overexpressing in vivo mouse model of lung cancer (Lu et al., 2010). Sox2 was specifically expressed in SP-C-positive developing lung or scgb1a1-positive adult lung airway stem cells which resulted in epithelial hyperplasia and adenocarcinoma development (Lu et al., 2010). Further, immunohistochemical study on stage-I lung adenocarcinoma patient samples revealed a strong prognostic correlation (Sholl et al., 2010). Higher Sox2 expression was significantly associated with decreased overall survival for both male and female patients (Sholl et al., 2010). These studies strongly suggest the role of Sox2 transcription factor for normal lung stem cells maintenance and lung development as well as correlated with the lung cancer progression. Studies from our laboratory had found a role for Sox2 in lung cancer stem cells maintenance and self-renewal (Singh et al., 2012). We observed that that SP cells from NSCLC cell lines express higher levels of Sox2. Depletion of Sox2 resulted in decreased side population frequency, indicating its direct role in maintaining the self-renewal of SP cells in NSCLCs (Singh et al., 2012). Under hypoxic conditions, Oct4 and Sox2 induced by HIF1α and HIF2α was found to up-regulate CD133 promoter in NSCLC cell lines (Iida et al., 2012) and overexpression of Oct4 and Nanog in NSCLC cell lines induces stem cell properties like self-renewal, tumorigenesis, invasion and metastasis (Chiou et al., 2010). Astudy using 64 tumor and non-tumor biopsies of lung-adenocarcinoma patients found short disease free survival correlating with high expression of Oct4A and CD133 (Cortes-Dericks et al., 2012). Depletion of Oct4 in CD133+ cells resulted in decreased self-renewal of these cells (Chen et al., 2008). Immunohistochemical studies support the role of Oct4 and Nanog in lung adenocarcinoma progression. The high levels of Oct4 and Nanog was positively associated with moderate and poorly differentiated grade of adenocarcinoma as well as poor overall survival of the patients (Chiou et al., 2010). However, lack of Oct4 and Nanog expression was reported in low grade as well as lower stage adenocarcinoma (Chiou et al., 2010), whereas Sox2 was positively expressed irrespective of the stage or grade of lung cancer (Sholl et al., 2010). These observations strongly raise the possibility that Sox2 may regulate self-renewal of cancer stem cells independently of Oct4 and Nanog in lung cancer.
Resistance to chemotherapy is partly correlated with the presence of stem-like cancer cells. It has been suggested that prolonged exposure of cisplatin resulted in stem cell like characters including decreased proliferation, accumulation of resistant cells in G0/G1 phase of cell cycle, enhanced colony formation ability, higher ALDH activity, increased frequency of CD133 and CD44 positive cells and higher expression of Oct4/ Sox2/ Nanog and EMT markers like C-Met and β-catenin (Barr et al., 2013). Exposure to 5-FU was also found to enrich stem-like cancer cells in lung adenocarcinoma cells (Shi et al., 2013). Resistance against DNA damaging agents was tested by examining the alteration of DNA-damage repair proteins in stem-like cells from a panel of NSCLC cell lines (Lundholm et al., 2013). Increased basal γH2AX (H2A histone family, member X) expression and diminished DNA damage-induced phosphorylation of DNA-dependent protein kinase (DNA-PK), ataxia telangiectasia-mutated (ATM), Krüppel-associated protein 1 (KAP1) and monoubiquitination of Fanconi anemia, complementation group D2 (FANCD2) after Irradiation or Cisplatin treatment (Lundholm et al., 2013).
Aberrant oncogenic signaling responsible for deregulated self-renewal growth may offer effective therapy specific to stem-like cancer cells. We have found that the self-renewal of SP cells among NSCLCs is regulated through activated EGFR/Src/Akt oncogenic Signaling (Singh et al., 2012). Treatment with Erlotinib, Gefitinib, Dasatinib and PI3K inhibitor LY294002 resulted in marked decrease in self-renewal of SP cells in our cell line based study (Singh et al., 2012). Recently, resistance to anti-EGFR therapy is correlated with enrichment of stem-like cancer cells with overexpression of ALDH1, increase in SP cells and enhanced self-renewal growth (Shien et al., 2013). However, these resistant cells were found to be sensitive against histone deacetylase and proteasome inhibitors (Shien et al., 2013). Similarly, in our study we also have found that higher levels of Mcl-1 were expressed in SP cells compared to MP cells at both transcriptional and translational levels. Irrespective to the EGFR-inhibitor sensitivity or resistance, Obatoclax, a pharmacological inhibitor of Mcl-1, could effectively prevent the self-renewal of SP cells among NSCLCs (Singh et al., 2013). Interestingly, it has been reported recently that endogenous inhibitor of angiogenesis known as tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) can modulate SP phenotype and function, and suggests that TIMP-2 may act as an endogenous suppressor of the SP cells in human lung cancer cells, independent of its activity to inhibit matrix metalloproteinases (Han et al., 2013). Thus, it appears that there are mutliple signaling molecules and pathways that contribute to the functioning of lung cancer stem cells, and many of these might be targeted to combat this disease.
5. Conclusions
The cancer stem cell model has gained considerable support recently in context of lung cancers and stem-like cells are associated with aggressive cancer behavior, metastatic progression, resistance to therapy and relapse. These stem-like cells have been characterized from primary tumors, human-tumor xenografts in mouse or established cell lines using several diverse markers. This ambiguity in the nature of specific stem-cell markers for non-small cell lung cancer might be due to cellular inter- as well as intratumoral heterogeneity among stem-like cancer cells, depending on the histological subtype of NSCLC. This lack of specific identification markers for the diverse pool of stem-like cancer cells may represent a major hurdle for translating the cancer stem cell concept into improving the therapeutic strategies against lung cancer. Further, approaches have been taken to target stem-like cancer cells by controlling developmental pathways involved in self-renewal of cancer stem cells, even though these pathways are not exclusive to only stem-like cancer cells. There are not many studies yet which describe a specific mutation driving an oncogenic signaling pathway is correlated with aberrant developmental signaling and self-renewal growth of these cancer stem cells. Thus, further understanding drivermutation driven cellular signaling in the context of aberrant self-renewal and differentiation of cancer stem like cells would facilitate targeting of these cells for cancer therapy. Moreover, since lung cancer stem cells are thought to consist of a heterogeneous population depending on the histology and site of tumors, multiple signaling pathways might have to be targeted to effectively eliminate them for therapeutic benefit. It can be imagined that the multidisciplinary efforts currently under way to characterize and target stem-like cells in lung cancer will reap significant therapeutic benefits in the future.
Acknowledgements
Studies in the Chellappan Lab are supported by the grants CA127725 and 136912 from the NCI. Support from the National Functional Genomics Center is acknowledged. SS is supported by the grant from the Wellcome Trust/DBT India Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamgeer M, Ganju V, Szczepny A, Russell PA, Prodanovic Z, Kumar B, Wainer Z, Brown T, Schneider-Kolsky M, Conron M, Wright G, Watkins DN. The prognostic significance of aldehyde dehydrogenase 1A1 (ALDH1A1) and CD133 expression in early stage non-small cell lung cancer. Thorax. 2013 doi: 10.1136/thoraxjnl-2012-203021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alison MR, Guppy NJ, Lim SM, Nicholson LJ. Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose? J Pathol. 2010;222:335–344. doi: 10.1002/path.2772. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Barr MP, Gray SG, Hoffmann AC, Hilger RA, Thomale J, O'Flaherty JD, Fennell DA, Richard D, O'Leary JJ, O'Byrne KJ. Generation and characterisation of cisplatin-resistant non-small cell lung cancer cell lines displaying a stem-like signature. PLoS One. 2013;8:e54193. doi: 10.1371/journal.pone.0054193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth PJ, Koch S, Muller B, Unterstab F, von Wichert P, Moll R. Proliferation and number of Clara cell 10-kDa protein (CC10)-reactive epithelial cells and basal cells in normal, hyperplastic and metaplastic bronchial mucosa. Virchows Arch. 2000;437:648–655. doi: 10.1007/s004280000316. [DOI] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, Ramos AH, Woo MS, Weir BA, Getz G, Beroukhim R, O'Kelly M, Dutt A, Rozenblatt-Rosen O, Dziunycz P, Komisarof J, Chirieac LR, Lafargue CJ, Scheble V, Wilbertz T, Ma C, Rao S, Nakagawa H, Stairs DB, Lin L, Giordano TJ, Wagner P, Minna JD, Gazdar AF, Zhu CQ, Brose MS, Cecconello I, Jr, Marie SK, Dahl O, Shivdasani RA, Tsao MS, Rubin MA, Wong KK, Regev A, Hahn WC, Beer DG, Rustgi AK, Meyerson M. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. UR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med (Berl) 2008;86:1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Bottinger EP, Jakubczak JL, Haines DC, Bagnall K, Wakefield LM. Transgenic mice overexpressing a dominant-negative mutant type II transforming growth factor beta receptor show enhanced tumorigenesis in the mammary gland and lung in response to the carcinogen 7,12-dimethylbenz-[a]-anthracene. Cancer Res. 1997;57:5564–5570. [PubMed] [Google Scholar]
- Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R, Berns A. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell. 2011;19:244–256. doi: 10.1016/j.ccr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Carney DN, Gazdar AF, Bunn PA, Jr, Guccion JG. Demonstration of the stem cell nature of clonogenic tumor cells from lung cancer patients. Stem Cells. 1982;1:149–164. [PubMed] [Google Scholar]
- Carney DN, Gazdar AF, Minna JD. Positive correlation between histological tumor involvement and generation of tumor cell colonies in agarose in specimens taken directly from patients with small-cell carcinoma of the lung. Cancer Res. 1980;40:1820–1823. [PubMed] [Google Scholar]
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, Arendt LM, Kuperwasser C, Bierie B, Weinberg RA. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D'Alessio AC, Young RA, Weinberg RA. Poised Chromatin at the ZEB1 Promoter Enables Breast Cancer Cell Plasticity and Enhances Tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, Ku HH, Chiou SH. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, McDonnell DP. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Clevers H. Stem cells, asymmetric division and cancer. Nat Genet. 2005;37:1027–1028. doi: 10.1038/ng1005-1027. [DOI] [PubMed] [Google Scholar]
- Collins BJ, Kleeberger W, Ball DW. Notch in lung development and lung cancer. Semin Cancer Biol. 2004;14:357–364. doi: 10.1016/j.semcancer.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Cortes-Dericks L, Galetta D, Spaggiari L, Schmid RA, Karoubi G. High expression of octamer- binding transcription factor 4A, prominin-1 and aldehyde dehydrogenase strongly indicates involvement in the initiation of lung adenocarcinoma resulting in shorter disease-free intervals. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012;41:e173–181. doi: 10.1093/ejcts/ezs170. [DOI] [PubMed] [Google Scholar]
- Curtis SJ, Sinkevicius KW, Li D, Lau AN, Roach RR, Zamponi R, Woolfenden AE, Kirsch DG, Wong KK, Kim CF. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell. 2010;7:127–133. doi: 10.1016/j.stem.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Dang TP, Eichenberger S, Gonzalez A, Olson S, Carbone DP. Constitutive activation of Notch3 inhibits terminal epithelial differentiation in lungs of transgenic mice. Oncogene. 2003;22:1988–1997. doi: 10.1038/sj.onc.1206230. [DOI] [PubMed] [Google Scholar]
- Daniel VC, Peacock CD, Watkins DN. Developmental signalling pathways in lung cancer. Respirology. 2006;11:234–240. doi: 10.1111/j.1440-1843.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- DeMayo FJ, Finegold MJ, Hansen TN, Stanley LA, Smith B, Bullock DW. Expression of SV40 T antigen under control of rabbit uteroglobin promoter in transgenic mice. Am J Physiol. 1991;261:L70–L76. doi: 10.1152/ajplung.1991.261.2.L70. [DOI] [PubMed] [Google Scholar]
- Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt A, Wong KK. Mouse models of lung cancer. Clin Cancer Res. 2006;12:4396s–4402s. doi: 10.1158/1078-0432.CCR-06-0414. [DOI] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27:401–415. doi: 10.1080/019021401300317125. [DOI] [PubMed] [Google Scholar]
- Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF, Carney DN, Nau MM, Minna JD. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985;45:2924–2930. [PubMed] [Google Scholar]
- Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci U S A. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med. 2007;175:547–553. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol. 2008;317:296–309. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Scott MP. Hedgehog and patched in neural development and disease. Neuron. 1998;21:1243–1257. doi: 10.1016/s0896-6273(00)80645-5. [DOI] [PubMed] [Google Scholar]
- Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern MT, Warner KE. Motivations for smoking cessation: a comparison of successful quitters and failures. J Subst Abuse. 1993;5:247–256. doi: 10.1016/0899-3289(93)90066-k. [DOI] [PubMed] [Google Scholar]
- Han H, Bourboulia D, Jensen-Taubman S, Isaac B, Wei B, Stetler-Stevenson WG. An endogenous inhibitor of angiogenesis inversely correlates with side population phenotype and function in human lung cancer cells. Oncogene. 2013 doi: 10.1038/onc.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Haruki N, Kawaguchi KS, Eichenberger S, Massion PP, Olson S, Gonzalez A, Carbone DP, Dang TP. Dominant-negative Notch3 receptor inhibits mitogen-activated protein kinase pathway and the growth of human lung cancers. Cancer Res. 2005;65:3555–3561. doi: 10.1158/0008-5472.CAN-04-3132. [DOI] [PubMed] [Google Scholar]
- Hassan KA, Wang L, Korkaya H, Chen G, Maillard I, Beer DG, Kalemkerian GP, Wicha MS. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1972–1980. doi: 10.1158/1078-0432.CCR-12-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Billups LH, Avery MD, Rude TH, Dansie DR, Lopez A, Sass B, Whitmire CE, Kouri RE. Lung cancer model system using 3-methylcholanthrene in inbred strains of mice. Cancer Res. 1981;41:5027–5032. [PubMed] [Google Scholar]
- Herpel E, Jensen K, Muley T, Warth A, Schnabel PA, Meister M, Herth FJ, Dienemann H, Thomas M, Gottschling S. The cancer stem cell antigens CD133, BCRP1/ABCG2 and CD117/c-KIT are not associated with prognosis in resected early-stage non-small cell lung cancer. Anticancer research. 2011;31:4491–4500. [PubMed] [Google Scholar]
- Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Iida H, Suzuki M, Goitsuka R, Ueno H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. International journal of oncology. 2012;40:71–79. doi: 10.3892/ijo.2011.1207. [DOI] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008a;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008b;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremy George P, Banerjee AK, Read CA, O'Sullivan C, Falzon M, Pezzella F, Nicholson AG, Shaw P, Laurent G, Rabbitts PH. Surveillance for the detection of early lung cancer in patients with bronchial dysplasia. Thorax. 2007;62:43–50. doi: 10.1136/thx.2005.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, Mahmood U, Mitchell A, Sun Y, Al-Hashem R, Chirieac LR, Padera R, Bronson RT, Kim W, Janne PA, Shapiro GI, Tenen D, Johnson BE, Weissleder R, Sharpless NE, Wong KK. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009a;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Collins BJ, Jin N, Watkins DN, Brock MV, Matsui W, Nelkin BD, Ball DW. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res. 2009b;69:845–854. doi: 10.1158/0008-5472.CAN-08-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysar SB, Jimeno A. More than markers: biological significance of cancer stem cell-defining molecules. Mol Cancer Ther. 2010;9:2450–2457. doi: 10.1158/1535-7163.MCT-10-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- Konishi J, Kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, Dang TP. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67:8051–8057. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lemjabbar-Alaoui H, Dasari V, Sidhu SS, Mengistab A, Finkbeiner W, Gallup M, Basbaum C. Wnt and Hedgehog are critical mediators of cigarette smoke-induced lung cancer. PLoS One. 2006;1:e93. doi: 10.1371/journal.pone.0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Song H, Huang C, Yao E, Gacayan R, Xu SM, Chuang PT. Alveolar type II cells possess the capability of initiating lung tumor development. PLoS One. 2012;7:e53817. doi: 10.1371/journal.pone.0053817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT, Lee YC, Lai TC, Lee CH, Hsiao YW, Lu J, Shen CN, Lu PJ, Hsiao M. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer research. 2013;73:406–416. doi: 10.1158/0008-5472.CAN-12-1733. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL, Onaitis MW. Evidence that SOX2 overexpression is oncogenic in the lung. PLoS One. 2010;5:e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm L, Haag P, Zong D, Juntti T, Mork B, Lewensohn R, Viktorsson K. Resistance to DNA-damaging treatment in non-small cell lung cancer tumor-initiating cells involves reduced DNA-PK/ATM activation and diminished cell cycle arrest. Cell death & disease. 2013;4:e478. doi: 10.1038/cddis.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema JP. Cancer stem cells: the challenges ahead. Nature cell biology. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- Meuwissen R, Berns A. Mouse models for human lung cancer. Genes Dev. 2005;19:643–664. doi: 10.1101/gad.1284505. [DOI] [PubMed] [Google Scholar]
- Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- Minna JD, Kurie JM, Jacks T. A big step in the study of small cell lung cancer. Cancer Cell. 2003;4:163–166. doi: 10.1016/s1535-6108(03)00221-6. [DOI] [PubMed] [Google Scholar]
- Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- Mizugaki H, Sakakibara-Konishi J, Kikuchi J, Moriya J, Hatanaka KC, Kikuchi E, Kinoshita I, Oizumi S, Dosaka-Akita H, Matsuno Y, Nishimura M. CD133 expression: a potential prognostic marker for non-small cell lung cancers. International journal of clinical oncology. 2013 doi: 10.1007/s10147-013-0541-x. [DOI] [PubMed] [Google Scholar]
- Moreb J, Schweder M, Suresh A, Zucali JR. Overexpression of the human aldehyde dehydrogenase class I results in increased resistance to 4-hydroperoxycyclophosphamide. Cancer Gene Ther. 1996;3:24–30. [PubMed] [Google Scholar]
- Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Curr Stem Cell Res Ther. 2008;3:237–246. doi: 10.2174/157488808786734006. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, Toftgard R. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438–3443. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Okudela K, Woo T, Mitsui H, Suzuki T, Tajiri M, Sakuma Y, Miyagi Y, Tateishi Y, Umeda S, Masuda M, Ohashi K. Downregulation of ALDH1A1 expression in non-small cell lung carcinomas--its clinicopathologic and biological significance. International journal of clinical and experimental pathology. 2013;6:1–12. [PMC free article] [PubMed] [Google Scholar]
- Okudela K, Woo T, Mitsui H, Tajiri M, Masuda M, Ohashi K. Expression of the potential cancer stem cell markers, CD133, CD44, ALDH1, and beta-catenin, in primary lung adenocarcinoma--their prognostic significance. Pathol Int. 2012;62:792–801. doi: 10.1111/pin.12019. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer. 2008;59:340–349. doi: 10.1016/j.lungcan.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Perumal D, Singh S, Yoder SJ, Bloom GC, Chellappan SP. A novel five gene signature derived from stem-like side population cells predicts overall and recurrence-free survival in NSCLC. PLoS One. 2012;7:e43589. doi: 10.1371/journal.pone.0043589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RN. Tobacco and the global lung cancer epidemic. Nat Rev Cancer. 2001;1:82–86. doi: 10.1038/35094091. [DOI] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Raz G, Allen KE, Kingsley C, Cherni I, Arora S, Watanabe A, Lorenzo CD, Edwards VD, Sridhar S, Hostetter G, Weiss GJ. Hedgehog signaling pathway molecules and ALDH1A1 expression in early-stage non-small cell lung cancer. Lung Cancer. 2012;76:191–196. doi: 10.1016/j.lungcan.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani T, Di PY, Taketo MM, Stripp BR. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcido CD, Larochelle A, Taylor BJ, Dunbar CE, Varticovski L. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br J Cancer. 2010;102:1636–1644. doi: 10.1038/sj.bjc.6605668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010;126:950–958. doi: 10.1002/ijc.24822. [DOI] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- Shi MM, Xiong YL, Jia XS, Li X, Zhang L, Li XL, Wang EH. Fluorouracil selectively enriches stem-like cells in the lung adenocarcinoma cell line SPC. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:1503–1510. doi: 10.1007/s13277-013-0675-5. [DOI] [PubMed] [Google Scholar]
- Shien K, Toyooka S, Ichimura K, Soh J, Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, Tsukuda K, Yamane M, Oto T, Kiura K, Miyoshi S. Prognostic impact of cancer stem cell-related markers in non-small cell lung cancer patients treated with induction chemoradiotherapy. Lung Cancer. 2012;77:162–167. doi: 10.1016/j.lungcan.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Shien K, Toyooka S, Yamamoto H, Soh J, Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H, Tsukuda K, Takigawa N, Kiura K, Gazdar AF, Lam WL, Miyoshi S. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 2013;73:3051–3061. doi: 10.1158/0008-5472.CAN-12-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl LM, Barletta JA, Yeap BY, Chirieac LR, Hornick JL. Sox2 protein expression is an independent poor prognostic indicator in stage I lung adenocarcinoma. Am J Surg Pathol. 2010;34:1193–1198. doi: 10.1097/PAS.0b013e3181e5e024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Singh S, Bora-Singhal N, Kroeger J, Laklai H, Chellappan SP. betaArrestin-1 and Mcl-1 modulate self-renewal growth of cancer stem-like side-population cells in non-small cell lung cancer. PLoS One. 2013;8:e55982. doi: 10.1371/journal.pone.0055982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Trevino J, Bora-Singhal N, Coppola D, Haura E, Altiok S, Chellappan SP. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Molecular cancer. 2012;11:73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003;3:832–844. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc. 2008;5:328–333. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, Honorio S, Xie Y, Scaglioni PP, DiMaio JM, Gazdar AF, Shay JW, Wistuba II, Minna JD. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday ME, Haley KJ, Sikorski K, Graham SA, Emanuel RL, Zhang F, Mu Q, Shahsafaei A, Hatzis D. Calcitonin driven v-Ha-ras induces multilineage pulmonary epithelial hyperplasias and neoplasms. Oncogene. 1999;18:4336–4347. doi: 10.1038/sj.onc.1202810. [DOI] [PubMed] [Google Scholar]
- Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19:754–764. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS One. 2009;4:e8248. doi: 10.1371/journal.pone.0008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Spitz MR, Fueger JJ, Amos CA. Lung carcinoma in former smokers. Cancer. 1996;78:1004–1010. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1004::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tsao PN, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J, Cardoso WV. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 2008;283:29532–29544. doi: 10.1074/jbc.M801565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene. 2003a;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- Uematsu K, Kanazawa S, You L, He B, Xu Z, Li K, Peterlin BM, McCormick F, Jablons DM. Wnt pathway activation in mesothelioma: evidence of Dishevelled overexpression and transcriptional activity of beta-catenin. Cancer Res. 2003b;63:4547–4551. [PubMed] [Google Scholar]
- Vestergaard J, Pedersen MW, Pedersen N, Ensinger C, Tumer Z, Tommerup N, Poulsen HS, Larsen LA. Hedgehog signaling in small-cell lung cancer: frequent in vivo but a rare event in vitro. Lung Cancer. 2006;52:281–290. doi: 10.1016/j.lungcan.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Z, Yan Y, Lemon WJ, LaRegina M, Morrison C, Lubet R, You M. A chemically induced model for squamous cell carcinoma of the lung in mice: histopathology and strain susceptibility. Cancer Res. 2004;64:1647–1654. doi: 10.1158/0008-5472.can-03-3273. [DOI] [PubMed] [Google Scholar]
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- Wikenheiser KA, Clark JC, Linnoila RI, Stahlman MT, Whitsett JA. Simian virus 40 large T antigen directed by transcriptional elements of the human surfactant protein C gene produces pulmonary adenocarcinomas in transgenic mice. Cancer Res. 1992;52:5342–5352. [PubMed] [Google Scholar]
- Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, Bren-Mattison Y, Van Raay TJ, Nemenoff RA, Heasley LE. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280:19625–19634. doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- Winn RA, Van Scoyk M, Hammond M, Rodriguez K, Crossno JT, Jr, Heasley LE, Nemenoff RA. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281:26943–26950. doi: 10.1074/jbc.M604145200. [DOI] [PubMed] [Google Scholar]
- Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Xu C, Xie D, Yu SC, Yang XJ, He LR, Yang J, Ping YF, Wang B, Yang L, Xu SL, Cui W, Wang QL, Fu WJ, Liu Q, Qian C, Cui YH, Rich JN, Kung HF, Zhang X, Bian XW. beta-Catenin/POU5F1/SOX2 transcription factor complex mediates IGF-I receptor signaling and predicts poor prognosis in lung adenocarcinoma. Cancer Res. 2013;73:3181–3189. doi: 10.1158/0008-5472.CAN-12-4403. [DOI] [PubMed] [Google Scholar]
- Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, Hogan BL, Onaitis MW. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci U S A. 2012;109:4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T, Hirao F, Sakatani M, Nishikawa H, Ogura T. Induction of squamous cell carcinoma in the lung of C57BL/6 mice by intratracheal instillation of benzo[a]pyrene with charcoal powder. Gann. 1977;68:343–352. [PubMed] [Google Scholar]
- Yoshimoto T, Inoue T, Iizuka H, Nishikawa H, Sakatani M, Ogura T, Hirao F, Yamamura Y. Differential induction of squamous cell carcinomas and adenocarcinomas in mouse lung by intratracheal instillation of benzo[a]pyrene and charcoal powder. Cancer Res. 1980;40:4301–4307. [PubMed] [Google Scholar]
- You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F, Jablons DM. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23:6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- Zemke AC, Teisanu RM, Giangreco A, Drake JA, Brockway BL, Reynolds SD, Stripp BR. beta-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am J Respir Cell Mol Biol. 2009;41:535–543. doi: 10.1165/rcmb.2008-0407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259–272. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]