Abstract
Extracellular acidification induced by retinal horizontal cells has been hypothesized to underlie lateral feedback inhibition onto vertebrate photoreceptors. To test this hypothesis, the H+-sensitive fluorophore 5-hexadecanoylaminofluorescein (HAF) was used to measure changes in H+ from horizontal cells isolated from the retina of the catfish. HAF staining conditions were modified to minimize intracellular accumulation of HAF and maximize membrane-associated staining, and ratiometric fluorescent imaging of cells displaying primarily membrane-associated HAF fluorescence was conducted. Challenge of such HAF-labeled cells with glutamate or the ionotropic glutamate receptor agonist kainate produced an increase in the fluorescence ratio, consistent with an alkalinization response of +0.12 pH units and +0.23 pH units, respectively. This alkalinization was blocked by the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), the L-type calcium channel blocker nifedipine, and lanthanum. The alkalinization reported by HAF was consistent with extracellular alkalinizations detected in previous studies using self-referencing H+-selective microelectrodes. The spatial distribution of the kainate-induced changes in extracellular H+ was also examined. An overall global alkalinization around the cell was observed, with no obvious signs of discrete centers of acidification. Taken together, these data argue against the hypothesis that glutamatergic-induced efflux of protons from horizontal cells mediates lateral feedback inhibition in the outer retina.
Keywords: retina, feedback, horizontal cell
electrical activity in the nervous system can elicit rapid changes in intracellular and extracellular H+, and alterations in both compartments can have major influences on neuronal activity (Chesler 2003). Increases in extracellular H+ depress synaptic transmission from photoreceptors to second-order neurons by binding to calcium channels on the photoreceptor synaptic terminal, altering the voltage activation threshold and the peak conductance of calcium channels and thereby reducing the influx of calcium and the amount of photoreceptor neurotransmitter released (Barnes et al. 1993). The potent effect that changes in extracellular H+ have on retinal signaling have led to the hypothesis that horizontal cells may induce lateral feedback inhibition by raising extracellular H+ levels with subsequent reduction of neurotransmitter release from photoreceptors (for review see Hirasawa et al. 2012; Thoreson and Mangel 2012).
Attempts to test this hypothesis by measuring extracellular H+ changes adjacent to the extracellular surface of horizontal cells with the pH-sensitive dye 5-hexadecanoylaminofluorescein (HAF) have proven to be problematic in past studies. Confocal microscopy revealed that HAF distribution in dissociated horizontal cells treated with the standard dye application protocol was not localized exclusively to the extracellular surface of cells as previously assumed but rather was present throughout the intracellular compartment (Jacoby et al. 2012). HAF loading within the intracellular compartment led to the conclusion that the acidification observed in previous HAF studies was intracellular, rather than extracellular, in origin (Jacoby et al. 2012; Jouhou et al. 2007).
In the present work, conditions were sought to restrict HAF to the plasma membrane, allowing it to be effectively used to monitor changes in extracellular H+ concentrations adjacent to the membrane of isolated horizontal cells. The concentration and the exposure time of HAF staining solutions were varied, and the subsequent distribution of dye in isolated catfish cone-driven horizontal cells was explored with confocal microscopy. Reducing the concentration and time of exposure to HAF significantly enhanced staining associated with the plasma membrane compared with staining levels within the intracellular compartment. Epifluorescent imaging of cells displaying primarily membrane-associated fluorescence revealed that glutamate and the ionotropic glutamate receptor agonist kainate produced a change in HAF fluorescence consistent with an extracellular alkalinization. The glutamate- and kainate-induced alkalinizations are consistent with previous findings obtained with extracellular self-referencing H+-selective microelectrodes that also report an extracellular alkalinization upon glutamatergic stimulation of horizontal cells. The spatial distribution of the kainate-induced changes in extracellular H+ was also examined, revealing an overall global alkalinization around the cell with no obvious smaller centers of acidification. Interpretation of these findings leads to the conclusion that, with appropriate labeling conditions, the H+-sensitive dye HAF can be used successfully to measure alterations in extracellular pH and that these observations are consistent with recordings obtained from self-referencing H+-selective electrodes. Both sets of data argue against the H+ hypothesis of horizontal cell-mediated lateral feedback inhibition in the outer retina.
MATERIALS AND METHODS
Ethical approval.
All experiments were performed with protocols approved by the Institutional Animal Care and Use Committee (IACUC) and Animal Care Committee (ACC) at both the University of Illinois at Chicago and the Marine Biological Laboratory and comply with federal guidelines listed in the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Cell dissociation.
The retinal dissociation protocol used in this study was similar to that reported by Jacoby et al. (2012). Channel catfish (Ictalurus punctatus) between 8 and 12 in. were obtained from Osage Catfisheries (Osage Beach, MO) or Keystone Fish Hatcheries (Richmond, IL). Fish were housed at room temperature for up to 3 mo and prior to dissection were dark-adapted for at least 1 h. Catfish were then transferred to 3.8 liters of water containing 1 g of tricaine methanesulfonate (MS-222, Argent) and 2.5 g of sodium bicarbonate (S233, Fisher Scientific) for 5 min of anesthetization. Fish were then cervically transected and double-pithed. Eyes were then removed and hemisected, and the posterior eyecup was immersed in catfish Ringer solution (in mM: 126 NaCl, 4 KCl, 3 CaCl2, 1 MgCl2, 10 HEPES, 15 glucose) supplemented with 1.2 mg/ml papain (LS003119, Worthington) and 0.5 mg/ml cysteine (C7755, Sigma) for 5 min. The retinas were gently peeled off and returned to the papain-cysteine dissociation solution and agitated for 30 min. Whole retinas were rinsed with fresh Ringer solution and mechanically triturated with a graduated 5-ml pipette, generating a retinal cell suspension; 1 drop of this suspension was placed in 35-mm culture dishes (Falcon 3001; Ibidi 81156 for confocal imaging) preloaded with 3 ml of catfish Ringer solution. Recordings were made from isolated cone-driven horizontal cells, identified by their morphological characteristics (Dong et al. 1994), whose nearest neighbor was at least 1 mm distant, and bathed in Ringer solution containing 1 mM HEPES. Dishes were maintained at 14°C for up to 12 h. All experiments on isolated cells were conducted at room temperature (∼18–21°C).
Laser-scanning confocal microscopy protocol.
Experiments to determine the anatomical localization of HAF (mol wt = 585.7; Invitrogen Molecular Probes), an H+-sensitive fluorescent dye, were performed with a Zeiss LSM-710 laser-scanning confocal microscope equipped with an inverted ×40 water immersion objective (C-Apochromat 40x/1.1) at the Marine Biological Laboratory in Woods Hole, MA. Isolated horizontal cells were plated on Ibidi 81156 culture dishes having thin microscopy-grade plastic bottoms (180 μm thick) and were illuminated from below. Images were acquired with Zeiss Zen software and analyzed further with ImageJ.
Cells exposed to various concentrations and durations of HAF were examined to find conditions in which intracellular staining was minimized and membrane-associated staining was prominent—that is, circumstances in which the dye should report primarily changes in extracellular H+. A stock solution was created by dissolving 1 mg of HAF in 500 μl of ethanol (100%) or DMSO. Fresh HAF stock solutions were diluted in catfish Ringer solution to 5 μM, 2.5 μM, or 500 nM working concentrations. Isolated horizontal cells were incubated in these different concentrations of HAF-containing Ringer solution for various times and temperatures. After HAF staining and incubation, dishes loaded with dye were washed with dye-free Ringer solution for subsequent imaging.
Epifluorescent imaging protocol.
Epifluorescent imaging was used to examine changes in light intensity of fluorescent dyes sensitive to alterations in pH and calcium. An important difference between the confocal imaging described above and the epifluorescent protocol is that epifluorescence will collect all the light from the cell, increasing the sensitivity to changes in ion concentrations. Cells that have a fluorescent dye completely restricted to the plasma membrane will nonetheless appear uniformly labeled in an epifluorescent photomicrograph—there is no optical slicing of the image that is performed here. All epifluorescent images were collected with a pco. edge sCMOS camera (PCO, Kelheim, Germany) mounted on a compound microscope (Olympus BX50WI) equipped with an Olympus LUMPlanFL ×40 long-working-distance water immersion objective lens. A mercury/xenon light source and a fast, switchable Lambda 10-2 filter wheel (Sutter Instruments) were used to stimulate dye fluorescence. Cells typically were stained with 500 nM HAF for 10 min in the dark at room temperature (∼18°C). Cells were then alternately excited with 488- and 440-nm excitation filters (XF1011 and XF1071, respectively, Omega Optical) and imaged every 4 s. Ratio images were produced by subtracting the background and dividing the 488-nm image by the 440-nm image. Cells loaded with the single-wavelength intracellular calcium indicator Oregon Green 488 BAPTA-1 AM (Invitrogen Molecular Probes) were incubated in 5 μM Oregon-Green-containing Ringer solution in the dark for 14 min at 18°C. Images were acquired 20–30 min after wash-off to allow for dye loading to reach equilibrium and then imaged at 488 nm every 4 s. Both HAF- and Oregon Green-stained cell images were filtered by a high-pass dichroic (>530 nm collected) at a resolution of 1,280 × 1,080 pixels (binning 2 × 2) with MicroManager software and further analyzed with ImageJ. A 10-s pressure micropuff delivered drug-containing Ringer solution via a micropipette (resistance 5–7 MΩ) to the cell of interest. All solutions were carefully maintained at a pH of 7.4.
Calibration of HAF.
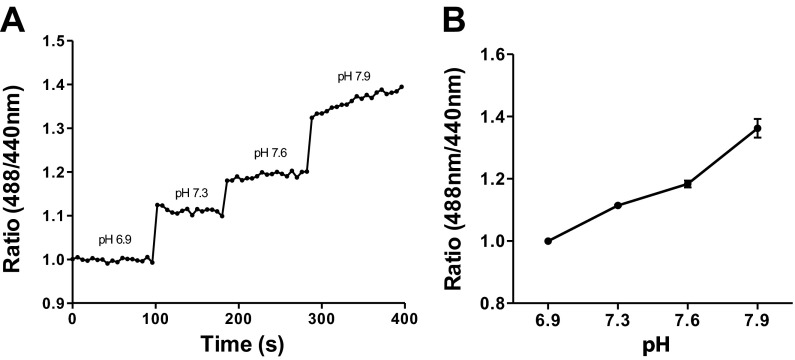
Calibration was performed on live cells with the optimized staining protocol (500 nM HAF at 18°C for 10 min), and full fluid exchanges of solutions of known pH values were applied to the dish. The emitted fluorescence ratio was acquired to determine the relative sensitivity and polarity of HAF to incremental increases in H+. Epifluorescent imaging of a central portion of the cell soma (∼20-μm diameter) was selected as the region of interest, and the ratio of images (488 nm/440 nm) was plotted. Incremental increases in pH of the extracellular bath solution resulted in a stepwise increase in the fluorescence ratio (see Fig. 3).
Fig. 3.
Calibration curve for membrane-associated HAF fluorescence. A: HAF calibration via epifluorescent imaging; cells were stained with the 500 nM HAF staining protocol, and Ringer solutions of known pH values were applied to the bath. Cells were alternately stimulated with 488- and 440-nm light; fluorescence emitted at 530 nm and beyond was collected. An area of ∼20 μm in the central portion of the soma was selected as the region of interest and the ratio of light emitted at 488/440 nm plotted. B: average calibration of 7 cells exhibiting membrane-associated HAF fluorescence.
Data treatment and statistical analysis.
Student's paired t-tests were used throughout to determine statistical significance, with a criterion of P < 0.01 selected as indicating significantly different distributions. Data are presented throughout as means ± SE. In experiments that quantified the peak amplitude fluorescence change after drug application, the values reported reflect the difference between the prestimulus average and point of greatest value after drug application (normalized ΔF/F). In experiments in which a fluorescence response was blocked by drug application, the values reported reflect the difference between the prestimulus and poststimulus average. All data were statistically analyzed and graphically displayed with Prism 5 (GraphPad Software).
RESULTS
Anatomical localization of HAF in isolated horizontal cells.
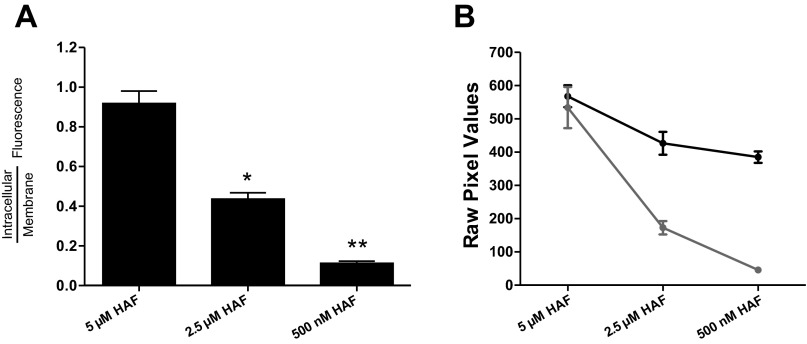
Catfish cone horizontal cells were initially subjected to the same staining protocol used by Jouhou et al. (2007) and Jacoby et al. (2012), which exposed cells to 5 μM HAF for 20 min at 4°C. As revealed by confocal microscopy, this staining protocol resulted in a high concentration of HAF within the intracellular compartment (Fig. 1A), consistent with findings from Jacoby et al. (2012). A center optical section of a horizontal cell was used to calculate the ratio of the level of intracellular HAF over the level of membrane-associated HAF. In 10 cells loaded with this protocol, the ratio of intracellular fluorescence to fluorescence adjacent to the membrane averaged 0.91 ± 0.07, indicating that the level of intracellular staining was similar to that of the membrane (Fig. 2). In early experiments, it was noted that incubation of cells at 4°C followed by a warming period to room temperature when dishes were removed from the refrigerator led to increased intracellular levels of HAF. Therefore, to maximize membrane-associated HAF and minimize intracellular infiltration, all subsequent staining protocols were performed at 18°C. As the concentration of HAF and incubation time were decreased to 2.5 μM for 10 min, the level of intracellular fluorescence was reduced and fluorescence near the cell membrane became more prominent (Fig. 1B). In these conditions the ratio of intracellular staining over membrane-associated staining averaged 0.44 ± 0.03 in six cells, a statistically significant difference (Fig. 2). As the concentration was further diminished to 500 nM HAF for 10 min, plasma membrane-associated HAF was maximized and intracellular infiltration was minimized (Fig. 1C). In 16 cells exposed to that staining protocol, the ratio of intracellular fluorescence over membrane-associated fluorescence decreased significantly to 0.11 ± 0.01, illustrating a high level of staining at the membrane of the cell compared with the intracellular space (Fig. 2). This revised protocol was used in all subsequent trials examining extracellular H+ dynamics.
Fig. 1.
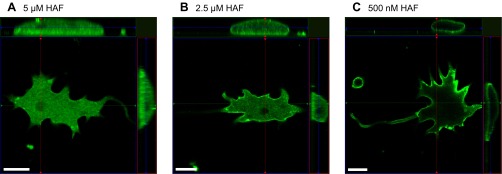
Reducing 5-hexadecanoylaminofluorescein (HAF) staining concentration and incubation time maximizes membrane-associated dye localization. A: confocal slice and orthographic projection from z-stack images of a horizontal cell stained with 5 μM HAF for 20 min at 4°C; heavy infiltration of HAF into the intracellular compartment is evident. B: staining with 2.5 μM HAF for 10 min at 18°C; reduced intracellular HAF is observed. C: staining with 500 nM HAF for 10 min at 18°C; HAF is now localized primarily to the cell membrane. Scale bars, 20 μm. Thin colored lines indicate position of the optical slice in the x-axis (red), y-axis (green), and z-axis (blue).
Fig. 2.
Ratio of intracellular HAF staining vs. plasma membrane-associated staining. A: graph depicting the ratio of intracellular fluorescence to cell membrane fluorescence in the 5 μM, 2.5 μM, and 500 nM HAF staining protocols. Fluorescence in the intracellular space decreases as HAF concentration is decreased compared with the edge of the cell. *Statistically significant difference comparing 5 μM and 2.5 μM; **statistically significant difference between 500 nM and 2.5 μM conditions. B: raw pixel values within the intracellular compartment (gray line) and plasma membrane (black line) as a function of the staining protocol applied.
In prior HAF studies on isolated horizontal cells of fish (Jacoby et al. 2012; Jouhou et al. 2007), DMSO was used as a solvent to prepare HAF stock solutions. In the original manuscript describing the use of HAF as an extracellular H+ sensor, Genz et al. (1999) achieved a two times higher amount of dye loading on the surface of guinea pig colon epithelial cells by using ethanol rather than DMSO as the solvent. It is possible that DMSO might have been responsible for the high degree of intracellular infiltration of HAF because of its ability to cross the plasma membrane (Anchordoguy et al. 1992; Leekumjorn and Sum 2006). However, high intracellular concentrations of HAF were observed regardless of whether ethanol or DMSO was used as the solvent when cells were stained with 5 μM HAF for 20 min at 4°C. Enhanced membrane-associated localization of HAF with the revised staining protocol (incubation in 500 nM HAF for 10 min at 18°C) was also observed regardless of whether DMSO or ethanol was employed. In all of the experiments that follow, HAF stocks were dissolved in 100% ethanol.
Figure 3A shows a calibration from a cell stained with the reduced loading protocol and demonstrates that the membrane-associated dye responds as expected to changes in extracellular H+; the ratio of 488- to 440-nm emitted light increases as the alkalinity of the solution is increased. Figure 3B shows the average calibrations obtained from seven cells stained with the 500 nM HAF protocol, again demonstrating an increase in the ratio of 488- to 440-nm emitted light upon alkalinization of the extracellular solution, consistent with previous HAF calibrations (Genz et al. 1999; Jacoby et al. 2012; Jouhou et al. 2007).
Cell activation by kainate and glutamate increases intracellular calcium.
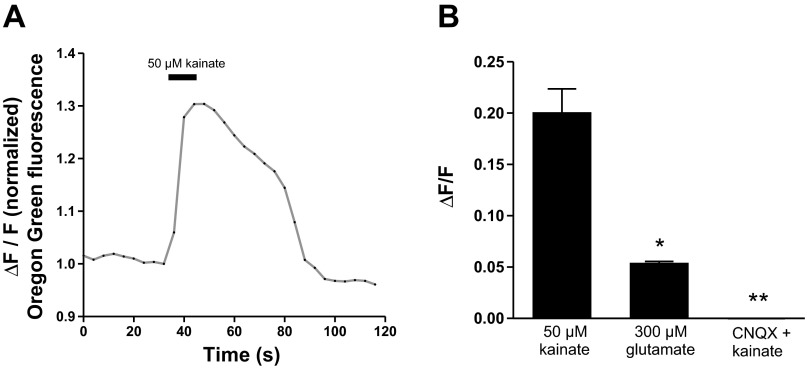
For reasons discussed in greater length in discussion, the manner in which chemical agents were applied to activate or inhibit horizontal cells was modified. In past experiments, either solutions containing activating agents were superfused over cells (Jacoby et al. 2012; Jouhou et al. 2007) or solutions were applied as one-time bolus additions to the bath (Jacoby et al. 2012). The present experiments were conducted in a static bathing solution, and drugs were applied via pressure ejection from a micropipette placed ∼100 μm from the cell. To test this application regimen, we loaded cells with the fluorescent calcium indicator Oregon Green and examined changes in intracellular calcium. Figure 4A shows an example of the change in intracellular calcium observed upon application of a 10-s puff of 50 μM kainate; a robust increase in intracellular calcium was observed. In eight cells, there was an average increase in fluorescence of 0.20 ± 0.02 ΔF/F (Fig. 4B, left; all imaging results in this report were normalized to prestimulus fluorescence). Additionally, a 10-s puff of 300 μM glutamate also produced a calcium rise in isolated horizontal cells; in eight cells there was an average increase in fluorescence of 0.053 ± 0.002 ΔF/F (Fig. 4B, center). The response to glutamate is, as expected, significantly smaller than that induced by kainate, likely because of the rapid desensitization of the glutamate receptors that occurs with glutamate, but not kainate, as the agonist (cf. Kreitzer et al. 2009). Also as expected, the increase in intracellular calcium initiated upon glutamate receptor activation by kainate was readily and consistently blocked with the AMPA/kainate receptor antagonist CNQX. Figure 4B, right, shows the results from 10 cells stained with Oregon Green and challenged with 50 μM kainate with 75 μM CNQX present in both the application pipette and the bath; no change in Oregon Green fluorescence was observed in cells when challenged with kainate in the presence of CNQX (−0.00002 ± 0.002 ΔF/F). These experiments allowed for verification of successful drug delivery to the cell of interest with the pressure injection puff system and confirmed the ability of CNQX to block a kainate-mediated rise in intracellular calcium.
Fig. 4.
Horizontal cells loaded with Oregon Green report an intracellular rise in calcium upon puff of kainate. A: intracellular calcium indicator Oregon Green BAPTA-1 AM ester, which is internalized by isolated horizontal cells, consistently reports an intracellular rise in calcium upon micropuff of 50 μM kainate. B: Oregon Green fluorescence associated with 50 μM kainate (left), 300 μM glutamate (center), and 50 μM kainate in the presence of 75 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, right). *Statistically significant difference between the response to 50 μM kainate and 300 μM glutamate; **statistically significant difference in the presence of CNQX from responses induced by kainate or glutamate.
Kainate applied to horizontal cells with high levels of intracellular HAF induces an intracellular acidification.
Changes in H+ concentration were examined under different staining protocols that altered HAF localization in cells. Cells were first stained with 5 μM HAF, which induced high levels of intracellular HAF; images of isolated horizontal cells were then acquired with epifluorescent imaging. In a static bath (no superfusion), a micropipette loaded with 50 μM kainate was placed ∼100 μm away from the target cell and a 10-s micropuff was delivered. Upon challenge of the cell with 50 μM kainate, cells with high intracellular HAF demonstrated a decrease in the 488-to-440 nm fluorescence ratio, indicative of an acidification response. In six cells, there was an average normalized decrease in fluorescence of −0.147 ± 0.015 ΔF/F. These results are consistent with the original observations of Jouhou et al. (2007) and the follow-up work reported in Jacoby et al. (2012). In control experiments with puffing but no glutamate receptor activation, no change in fluorescence was observed.
Membrane-associated HAF reports an alkalinization following horizontal cell activation with kainate and glutamate.
HAF fluorescence was then examined in cells in which membrane-associated HAF was significantly enhanced relative to intracellular HAF staining. Images of isolated horizontal cells treated with the revised HAF staining protocol (500 nM HAF for 10 min at 18°C), which led to enhanced membrane-associated HAF compared with intracellular HAF, were acquired with epifluorescent imaging. Figure 5A shows an epifluorescent (nonconfocal) image of an isolated catfish horizontal cell stained with the protocol that leads to enhanced levels of membrane-associated HAF. The epifluorescence technique captures all light from the entirety of the cell, and as such the cell appears uniformly labeled, even though, as shown in Fig. 1C, confocal microscopy reveals that HAF staining of cells with this protocol is largely restricted to the plasma membrane.
Fig. 5.
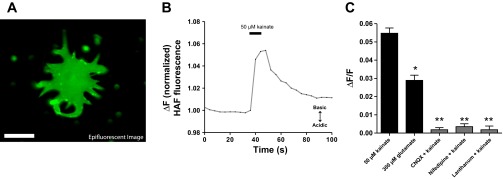
Cells stained with the reduced HAF protocol consistently report an alkalinization when challenged with kainate or glutamate. A: epifluorescent (nonconfocal) derived image of an isolated catfish horizontal cell exhibiting membrane-associated HAF staining via revised 500 nM HAF staining protocol. Scale bar, 25 μm. B: representative response from 1 cell exhibiting robust membrane-associated staining and challenged with 50 μM kainic acid; an extracellular alkalinization (increase in fluorescent ratio) is observed. C: graph showing the peak amplitude fluorescence change when challenged with 50 μM kainate or 300 μM glutamate (black bars); the extracellular alkalinization was blocked when kainate was delivered in the presence of CNQX, nifedipine, or lanthanum (gray bars). *Statistically significant difference between the response to 50 μM kainate and 300 μM glutamate; **statistically significant difference in presence of blockers compared with responses induced by kainate or glutamate.
Figure 5B shows ratiometric epifluorescent imaging from one cell stained with the revised 500 nM HAF staining protocol and challenged with a puff of 50 μM kainate. An increase in the 488-to-440 nm fluorescence ratio was observed, indicative of an extracellular alkalinization. In 11 cells, there was an average normalized increase in fluorescence of 0.054 ± 0.003 ΔF/F (Fig. 5C). On no occasion in the 11 cells examined was a decrease in the fluorescent ratio (acidification) observed in cells stained at 500 nM HAF for 10 min. Furthermore, a 10-s puff of 300 μM glutamate also produced an alkalinization in isolated horizontal cells stained with the revised HAF staining protocol; in seven cells there was an average increase in fluorescence ratio of 0.029 ± 0.002 ΔF/F (Fig. 5C). The alkalinization, while clearly present, was significantly smaller than that induced by kainate, again likely because of the desensitization of the glutamate receptors when glutamate is the agonist, but not when kainate is employed to stimulate the cells.
The alkalinization response was blocked when 75 μM CNQX was present in both the bath solution and with kainate in the microinjection pipette. In six cells analyzed, an average normalized fluorescence change in the presence of CNQX was 0.002 ± 0.001 ΔF/F, significantly different from the response obtained in the control condition. The extracellular alkalinization was also significantly reduced by the L-type voltage-gated calcium channel antagonist nifedipine; seven cells yielded no significant change in the fluorescent ratio (0.004 ± 0.002 ΔF/F) in the presence of 100 μM nifedipine. The extracellular alkalinization was also significantly reduced by 100 μM lanthanum, a compound reported to block plasmalemma Ca2+-H+-ATPase (PMCA) activity (Herscher and Rega 1997; Schatzmann et al. 1986). In seven cells bathed in lanthanum, the average change in fluorescence of cells stimulated with the kainate puff solution was 0.002 ± 0.002 ΔF/F (Fig. 5C). All of these agents have also been shown to block the extracellular alkalinization previously detected from horizontal cells by self-referencing H+-selective electrodes (Jacoby et al. 2012; Kreitzer et al. 2012).
Spatial distribution of the extracellular alkalinization induced by kainate.
A major potential advantage of monitoring alterations in extracellular H+ with HAF fluorescent imaging is the ability to examine the spatial profile of H+ alterations associated with many regions from a single cell. Such a tool allows the possibility of looking for “hot spots” not only of alkalinization but of concurrent acidification as well.
When horizontal cells were stained with the revised HAF staining protocol to maximize membrane-associated HAF, the majority of cells exhibited small punctatelike staining patterns (∼5–10 puncta per cell) on the cell periphery, highlighting areas of increased HAF (Fig. 6A, epifluorescent image with overlaid gray arrowheads). At present, we do not know what these puncta truly represent—they may be bits of pieces from other cells that have adhered to the horizontal cell in question or they might be physiologically distinct regions of a given horizontal cell. Their well-defined appearance led us to wonder whether these sites might differ in the nature of the glutamatergic-induced alteration of extracellular H+. The changes in fluorescence of these punctate regions of interest in response to kainate application were selected and directly compared to the overall HAF fluorescence change when the central soma was selected (∼20-μm diameter). Figure 6B (gray traces) shows the alterations in fluorescence measured at the six puncta labeled with arrowheads in the cell in Fig. 6A, along with the response obtained from the central area of the cell (Fig. 6B, heavy black trace). An increase in the ratio of 488- to 440-nm fluorescence indicative of an alkalinization was observed at all locations. Similar responses were recorded in six cells analyzed. Selection of the central soma reported a change in the fluorescence ratio consistent with an alkalinization response (0.065 ± 0.004 ΔF/F); across these six cells, 29 punctatelike regions of interest were selected and also reported an alkalinization response (0.073 ± 0.005 ΔF/F). Both the polarity of the response (increase in the fluorescence ratio) and the amplitude of response following cell activation were not significantly different; an alkalinization was observed at all locations examined.
Fig. 6.
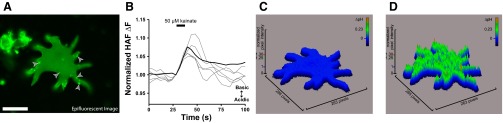
Fluorescence from punctatelike regions of interest and whole cell single-pixel matrix all report extracellular alkalinization upon application of kainate. A: epifluorescent (nonconfocal) derived image of a horizontal cell exhibiting membrane-associated HAF staining and stimulated with 488-nm light; gray arrowheads highlight puncta of apparent high HAF localization. Scale bar, 25 μm. B: alteration in membrane-associated HAF fluorescence from selection of central soma region (black trace) and punctate regions of staining (gray traces); an extracellular alkalinization is observed at all locations examined. C and D: pseudocolor surface intensity plots from the same cell presented in A (which was imaged with epifluorescence microscopy); the whole cell single-pixel matrix reconstruction presented here was derived from baseline fluorescence prior to addition of agonist (C) and again plotted during the peak of the alkalinization in response to addition of agonist (D).
The spatial analysis of the alkalinization was then broadened to address all of the pixels associated with a given cell. The prestimulus ratio of HAF fluorescence prior to application of kainate was first normalized so that each pixel was assigned a value of 1 (Fig. 6C). Alterations in the 488-to-440 nm fluorescence ratio were examined at each pixel during the peak of the alkaline response induced by kainate. The results are shown in Fig. 6D, where the height of each pixel represents the change in 488-to-440 nm ratio (pixel intensity) of the entire pixel matrix that shapes the cell. An increase in the 488-to-440 nm ratio indicative of an alkalinization is colored in green and red; a decrease in the ratio would have been portrayed as a deep blue or black spot. On average each pixel increased in intensity (from normalized baseline value of 1) to 1.25 arbitrary units, with the highest pixel value reaching 1.3 arbitrary units. No pixels responded to cell activation with a decrease in their pixel value, indicating that all pixels analyzed yielded an alkalinization response to the addition of the ionotropic glutamate receptor agonist kainate.
DISCUSSION
The major goal of this study was to determine whether the H+-sensitive fluorophore HAF could be used to selectively measure extracellular alterations in H+ adjacent to retinal horizontal cells. As demonstrated in the present work and in Jacoby et al. (2012), the staining protocol originally employed to assess extracellular changes in H+ from horizontal cells resulted in high amounts of HAF inside cells, which subsequently reported intracellular changes in pH. After the concentration of HAF was reduced by an order of magnitude and the exposure time to the dye was limited, intracellular infiltration was minimized, leaving prominent staining of HAF associated with the cell membrane. Glutamatergic stimulation of cells stained with the original HAF protocol and displaying high amounts of intracellular HAF resulted in a change in HAF fluorescence indicative of an acidification. HAF present in the interior of horizontal cells is capable of reporting changes in intracellular H+ (Jacoby et al. 2012), and, as reported by Dixon et al. (1993), Molina et al. (2004), and Jacoby et al. (2012), depolarization of horizontal cells with glutamatergic agents or high potassium results in an intracellular acidification of these cells. The revised HAF staining protocol resulted in significant HAF staining associated with the cell membrane and markedly less in the interior of the cell. Glutamatergic stimulation of cells stained with the revised HAF protocol elicited a reversal in polarity of the cellular response; an increase in the HAF fluorescence ratio was observed, indicating an alkalinization response by cells exhibiting enhanced membrane-associated HAF labeling. At no point did the cells stained with the revised HAF protocol respond to glutamate or kainate with an acidification (decrease in fluorescence ratio). The changes in fluorescence reported by membrane-associated HAF in response to cell activation are consistent with the glutamate-, kainate-, and high potassium-induced extracellular alkalinization detected from catfish horizontal cells measured with extracellular self-referencing H+-selective microelectrodes (Jacoby et al. 2012; Kreitzer et al. 2007). These results suggest that HAF is indeed capable of reporting changes in extracellular H+ when the staining protocol is adjusted to maximize membrane-associated staining.
Our data from optical confocal microscopic slices clearly demonstrate that the revised HAF staining protocol, which employs a lowered concentration of HAF and a shorter loading time, results in dye that is predominantly associated with the plasma membrane. However, confocal microscopy does not have sufficient resolution to determine which leaflet of the plasma membrane (exterior or interior) the dye is associated with. It is conceivable that some of the dye faces the cytoplasm of the cell. However, the changes in H+ induced by glutamate and kainate from cells stained with the revised protocol were uniformly alkaline in nature. Dixon et al. (1993) and Molina et al. (2004) demonstrated that challenge of cells with extracellular glutamate leads to an intracellular acidification of the cells. If significant amounts of HAF were in the inner leaflet and faced the cytoplasm, it should have reported this intracellular acidification. The lack of such a response suggests strongly that most of the HAF is likely to be in the external leaflet of the plasma membrane and face the extracellular solution.
The extracellular alkalinization detected from horizontal cells has been hypothesized to result from activation of a plasmalemma Ca2+-H+-ATPase antiporter (PMCA). According to this hypothesis, glutamatergic activation of horizontal cells depolarizes the cells, leading to the influx of a significant amount of calcium, largely through voltage-gated channels. The calcium is then extruded from the cell by the PMCA, which draws in protons from the extracellular solution into the cell, leading to extracellular alkalinization and intracellular acidification. Previous studies using self-referencing H+-selective electrodes strongly support a calcium-dependent mechanism in the alterations in extracellular H+ induced by high potassium, glutamate, kainate, and direct depolarization of cells: the alkalinizations are dependent upon the presence of extracellular calcium, are blocked by the L-type calcium channel blocker nifedipine, and are mimicked by photolysis-induced release of calcium from caged compounds (Jacoby et al. 2012; Kreitzer et al. 2007; Molina et al. 2004). Additional support for PMCA involvement includes findings from these same studies showing that the potassium-, glutamate-, and kainate-induced alterations in extracellular H+ are abolished by the PMCA-selective inhibitor 5,6-carboxyeosin and from immunochemical studies showing significant staining of PMCAs on the membranes of horizontal cells. In the experiments reported here, the L-type calcium channel blocker nifedipine was also effective in eliminating the extracellular alkalinization reported by cells exhibiting membrane-bound HAF. In the present study, attempts to measure changes in fluorescence of cells with membrane-associated HAF labeling in the presence of the PMCA inhibitor 5,6-carboxyeosin were thwarted by the fluorescent nature of carboxyeosin itself, elicited by the same excitation wavelengths used to stimulate fluorescence of HAF. No other highly specific agents are known that block the PMCA pump of catfish horizontal cells. However, lanthanum, another compound reported to block PMCA activity (Herscher and Rega 1997; Schatzmann et al. 1986), also abolished the glutamatergic-induced alkalinizations reported by HAF. It is well known that lanthanum can block a number of different channels (including calcium channels) in addition to blocking PMCA activity (Alshuaib and Mathew 2005; Nathan et al. 1988). Nonetheless, the ability of lanthanum to abolish the extracellular alkalinizations reported by both alterations in HAF fluorescence (the present work) and H+-selective microelectrodes (Jacoby et al. 2012) is consistent with the hypothesis that the extracellular alkalinization may result from enhanced PMCA activity.
Direct depolarization of whole cell voltage-clamped retinal horizontal cells of skate and catfish also initiates an extracellular alkalinization as detected with self-referencing H+-selective microelectrodes (Kreitzer et al. 2007; Molina et al. 2004). Attempts to obtain whole cell recordings from HAF-treated cells in the present experiments were unsuccessful. It proved difficult to obtain and maintain gigaohm seals on horizontal cells stained with HAF, while unstained cells were easily patched and readily held in the whole cell patch configuration. It is possible that the lipophilic molecular structure of HAF compromises patch formation between the electrode and the membrane.
The changes in HAF fluorescence ratio induced by puffs of 50 μM kainate resulted in an average 5.4% increase in ΔF/F, and puffs of 300 μM glutamate induced an average 2.9% increase in ΔF/F from the baseline level, both indicative of an extracellular alkalinization immediately adjacent to the cell membrane. When fit to the HAF calibration curve (Fig. 3), it is estimated that the alkaline change taking place adjacent to the external face of the plasma membrane was approximately +0.23 pH units for kainate and approximately +0.12 pH units for glutamate. Gentle puffing of the drugs in a quiet standing solution was the only drug delivery method through which HAF fluorescent imaging could resolve a robust and repeatable alkalinization. Attempts to measure H+ changes with superfusion of solutions did not result in repeatable alterations in HAF fluorescence. This is perhaps not surprising, since similar difficulties in monitoring extracellular H+ changes with self-referencing H+-selective microelectrodes with superfusion are also encountered. It is likely that the extracellular proton cloud surrounding horizontal cells is significantly disturbed and largely washed away by the process of superfusion, precluding accurate measurement of H+ concentrations around the cell (Perlman et al. 1993; Smith et al. 1999).
Recent work by Liu et al. (2013) using a mammalian retinal preparation suggests the possibility that feedback inhibition by horizontal cells onto photoreceptors might involve changes in extracellular H+ mediated by voltage-dependent flow of bicarbonate ions through GABA channels present on the horizontal cells. All of the experiments reported in the present work were done using 1 mM HEPES as the extracellular buffer for protons; no bicarbonate was added to our solutions. HEPES was chosen because of the requirement for a still, quiet solution in which to measure repeatable changes in HAF fluorescence upon stimulation of cells while maintaining a constant overall value of pH for the bulk of the extracellular solution. Bicarbonate solutions are typically bubbled continuously with a known amount of CO2 to maintain pH and are then superfused over tissues and cells, a condition in which changes in extracellular H+ with self-referencing electrodes or HAF fluorescence cannot be measured. Kreitzer et al. (2007) reported that extracellular H+ changes measured from isolated catfish horizontal cells with self-referencing electrodes did not detect a glutamate-induced acidification from cells bathed in a bicarbonate-based pH buffering system; rather, an extracellular alkalinization in this experimental setting was observed. In light of the recent model presented by Liu et al. (2013), a CO2 chamber suitable for bicarbonate-based pH buffering of a static bath solution that can be used for both self-referencing H+-selective electrodes and HAF fluorescent imaging is currently being designed to adequately address this interesting hypothesis.
Both self-referencing H+-selective electrodes and H+ alteration of HAF fluorescence measured with a protocol to enhance membrane-associated staining compared with intracellular staining report an alkalinization in response to ionotropic glutamate receptor activation. This is in direct contrast to the extracellular acidification that would be expected from horizontal cells predicted by the H+ hypothesis of lateral feedback inhibition onto photoreceptors. Yet the evidence for proton-mediated effects in the outer retina is strong (for review see Hirasawa et al. 2012; Thoreson and Mangel 2012). One possible way to reconcile our results with what would be predicted by the horizontal cell's proposed role in proton-mediated feedback inhibition could be the existence of microdomains in horizontal cells that induce different changes in extracellular H+ at different spatial locations along the cell. It is conceivable, for example, that proton extrusion pumps and transporters might be concentrated at horizontal cell dendritic tips associated with the synaptic pedicles and spherules of individual photoreceptors, while PMCA pumps might be active elsewhere. The revised extracellular HAF labeling protocol we have developed has enabled us to examine the spatial distribution of changes in extracellular H+ and probe for the existence of pH-regulatory microdomains on the surface of isolated horizontal cells in a manner that would be difficult to achieve using self-referencing H+-selective microelectrodes. In experiments presented here, small punctate regions of high-intensity HAF fluorescence were observed on the outer periphery of many cells. Analysis of these puncta did not reveal selective pH changes differing from that detected in the central soma. Moreover, an analysis of the spatial changes in HAF fluorescence pixel by pixel did not reveal any points that were associated with an acidification following challenge with kainate. It is possible that pH-regulatory microdomains exist on horizontal cells in the intact retina, but our data examining HAF fluorescence changes in isolated retinal horizontal cells provide no support for this contention.
We believe that our data are more parsimoniously interpreted as indicating that depolarization of horizontal cells leads to extracellular alkalinization mediated by activation of PMCA antiporters in the horizontal cells, and that under physiological conditions in the intact retina this extracellular alkalinization might work to relieve inhibition induced by protons exocytosed from photoreceptors. DeVries (2001) demonstrated that vesicular fusion at the cone terminal results in the release of protons into the synaptic cleft in tandem with the neurotransmitter glutamate. These protons facilitate a local negative feedback loop that induces short-term inhibition of neurotransmitter release from cone photoreceptors due to their block of voltage-dependent calcium channels (DeVries 2001; Hosoi et al. 2005). Depolarizing horizontal cells with glutamate leads to an activation of PMCAs that could in turn facilitate the removal of these protons from the extracellular space, disinhibiting photoreceptor calcium channels. It is notable that a positive feedback model, resulting in increased glutamate release from cones, was presented by Jackman et al. (2011). They demonstrated that AMPA receptor-dependent depolarization of horizontal cells led to an intracellular calcium increase giving rise to amplification of glutamate release from cone photoreceptor terminals. Their work did not directly implicate protons in this positive feedback loop; however, their findings do suggest postsynaptic mechanisms that could potentially act to relieve the H+ block of calcium channels and sensitize the synapses for further release of glutamate. We speculate that PMCA-mediated relief of proton inhibition may be a common feature of synaptic signaling throughout the central nervous system to resensitize synapses for further release of neurotransmitter.
In summary, we have shown that, with appropriate alterations in staining protocol, the H+-sensitive dye HAF can be associated primarily to the plasma membrane of retinal horizontal cells and that alterations of HAF fluorescence now report an alkalinization, which we interpret as a reduction in extracellular acidity adjacent to the membranes of isolated cells upon glutamate receptor activation. These findings are consistent with the extracellular alkalinization previously demonstrated with self-referencing extracellular H+-selective microelectrodes as well as the intracellular acidification reported from cells displaying high levels of intracellular HAF. These data implicate activation of a Ca2+-H+-ATPase antiporter in mediating the observed changes in extracellular H+. The revised HAF protocol also allowed the spatial distribution of kainate-induced changes in extracellular H+ to be examined, demonstrating an overall global alkalinization with no obvious smaller centers of acidification. Taken together, our data argue against the H+ hypothesis of horizontal cell-mediated lateral inhibition in the outer retina.
GRANTS
This work was supported by National Science Foundation Grants 0924372, 0924383, and 0115378, National Eye Institute Grant EY-01792 (University of Illinois at Chicago), a Laura and Arthur Colwin Summer Fellowship from the Marine Biological Laboratory, a Grant-in-Aid of Research Award from Sigma Xi, the W. C. and May Preble Deiss Award for Graduate Research from the University of Illinois at Chicago, a UIC LAS award for Faculty in the Sciences, and funding from the Midwest Eye-Banks.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.J., M.A.K., S.A., and R.P.M. conception and design of research; J.J. performed experiments; J.J., M.A.K., S.A., and R.P.M. analyzed data; J.J., M.A.K., S.A., and R.P.M. interpreted results of experiments; J.J. prepared figures; J.J., M.A.K., and R.P.M. drafted manuscript; J.J., M.A.K., S.A., and R.P.M. edited and revised manuscript; J.J., M.A.K., S.A., and R.P.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Michael Alpert for his assistance and insight into the technical nuances of the epifluorescent imaging rig. In addition, we thank Jim McIlvain and Chris Rieken for assistance with confocal microscopy at the Marine Biological Laboratory in Woods Hole, MA. We are grateful to Keystone Fish Hatcheries and Steve Kahrs at Osage Catfisheries, Inc., for consistently providing us with catfish at all times of the year.
REFERENCES
- Alshuaib WB, Mathew MV. Transient K+ current is blocked by lanthanum in Drosophila neurons. Neurochem Res 30: 1087–1092, 2005 [DOI] [PubMed] [Google Scholar]
- Anchordoguy TJ, Carpenter JF, Crowe JH, Crowe LM. Temperature-dependent perturbation of phospholipid bilayers by dimethylsulfoxide. Biochim Biophys Acta 1104: 117–122, 1992 [DOI] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA 90: 10081–10085, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003 [DOI] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron 32: 1107–1117, 2001 [DOI] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K, Copenhagen DR. l-Glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron 11: 267–277, 1993 [DOI] [PubMed] [Google Scholar]
- Dong CJ, Picaud SA, Werblin FS. GABA transporters and GABAc-like receptors on catfish cone- but not rod-driven horizontal cells. J Neurosci 14: 2648–2658, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genz AK, v Engelhardt W, Busche R. Maintenance and regulation of the pH microclimate at the luminal surface of the distal colon of guinea-pig. J Physiol 517: 507–519, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscher CJ, Rega AF. On the mechanism of inhibition of the PMCa2+-ATPase by lanthanum. Ann NY Acad Sci 834: 407–409, 1997 [DOI] [PubMed] [Google Scholar]
- Hirasawa H, Yamada M, Kaneko A. Acidification of the synaptic cleft of cone photoreceptor terminal controls the amount of transmitter release, thereby forming the receptive field surround in the vertebrate retina. J Physiol Sci 62: 359–375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Arai I, Tachibana M. Group III metabotropic glutamate receptors and exocytosed protons inhibit L-type calcium currents in cones but not in rods. J Neurosci 25: 4062–4072, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Babai N, Chambers JJ, Thoreson WB, Kramer RH. A positive feedback synapse from retinal horizontal cells to cone photoreceptors. PLoS Biol 9: e1001057, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J, Kreitzer MA, Alford S, Qian H, Tchernookova BK, Naylor ER, Malchow RP. Extracellular pH dynamics of retinal horizontal cells examined using electrochemical and fluorometric methods. J Neurophysiol 107: 868–879, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouhou H, Yamamoto K, Homma A, Hara M, Kaneko A, Yamada M. Depolarization of isolated horizontal cells of fish acidifies their immediate surrounding by activating V-ATPase. J Physiol 585: 401–412, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer MA, Birnbaum AD, Qian H, Malchow RP. Pharmacological characterization, location, and regulation of ionotropic glutamate receptors in skate horizontal cells. Vis Neurosci 26: 375–387, 2009 [DOI] [PubMed] [Google Scholar]
- Kreitzer MA, Collis LP, Molina AJ, Smith PJ, Malchow RP. Modulation of extracellular proton fluxes from retinal horizontal cells of the catfish by depolarization and glutamate. J Gen Physiol 130: 169–182, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer MA, Jacoby J, Naylor E, Baker A, Grable T, Tran E, Booth SE, Qian H, Malchow RP. Distinctive patterns of alterations in proton efflux from goldfish retinal horizontal cells monitored with self-referencing H+-selective electrodes. Eur J Neurosci 36: 3040–3050, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekumjorn S, Sum AK. Molecular study of the diffusional process of DMSO in double lipid bilayers. Biochim Biophys Acta 1758: 1751–1758, 2006 [DOI] [PubMed] [Google Scholar]
- Liu X, Hirano AA, Sun X, Brecha NC, Barnes S. Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J Physiol (April 22, 2013). 10.1113/jphysiol.2012.248179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina AJ, Verzi MP, Birnbaum AD, Yamoah EN, Hammar K, Smith PJ, Malchow RP. Neurotransmitter modulation of extracellular H+ fluxes from isolated retinal horizontal cells of the skate. J Physiol 560: 639–657, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan RD, Kanai K, Clark RB, Giles W. Selective block of calcium current by lanthanum in single bullfrog atrial cells. J Gen Physiol 91: 549–572, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman I, Sullivan JM, Normann RA. Voltage- and time-dependent potassium conductances enhance the frequency response of horizontal cells in the turtle retina. Brain Res 619: 89–97, 1993 [DOI] [PubMed] [Google Scholar]
- Schatzmann HJ, Luterbacher S, Stieger J, Wüthrich A. Red blood cell calcium pump and its inhibition by vanadate and lanthanum. J Cardiovasc Pharmacol 8, Suppl 8: S33–S37, 1986 [DOI] [PubMed] [Google Scholar]
- Smith PJ, Hammar K, Porterfield DM, Sanger RH, Trimarchi JR. Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux. Microsc Res Tech 46: 398–417, 1999 [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res 31: 407–441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]