Abstract
Parietal and frontal cortex are central to controlling forelimb movements. We previously showed that movements such as reach, grasp, and defense can be evoked from primary motor (M1), premotor (PMC), and posterior parietal (PPC) cortex when 500-ms trains of electrical pulses are delivered via microelectrodes. Stimulation sites that evoked a specific movement clustered into domains, which shared a topographic pattern in New World monkeys and prosimian galagos. Matched functional domains in parietal and frontal cortex were preferentially interconnected. We reasoned that matched functional domains form parallel networks involved in specific movements. To test the roles of domains in M1, PMC, and PPC, we systematically inactivated with muscimol domains in one region and determined if functional changes occurred in matching domains in other regions. The most common changes were higher current thresholds for stimulation-evoked movements and shorter, not fully developed, trajectories of movements. Inactivations of an M1 functional domain greatly reduced or abolished movements evoked from the matching domains in PMC or PPC, whereas movements evoked from nonmatching domains remained mostly unaffected. In contrast, inactivating PMC or PPC domains did not consistently abolish the ability to evoke movements from matching M1 domains. However, inactivation of PMC domains suppressed or altered the movements evoked from PPC domains. Thus movement sequences evoked from PMC depend on M1 and movement sequences evoked from PPC depend on both M1 and PMC. Overall, the results support the conclusion that PPC, PMC, and M1 are subdivided into functional domains that are hierarchically related within parallel networks.
Keywords: inhibition, GABA agonist, microstimulation, behavior, primate
electrical stimulation with 500-ms trains of current pulses delivered via microelectrodes (microstimulation) to motor regions of the cortex evokes behaviorally meaningful movements in monkeys (Cooke and Graziano 2004; Gharbawie et al. 2011a; Graziano 2006; Graziano et al. 2002a,b; Stepniewska et al. 2006a), and prosimian galagos (Stepniewska et al. 2005, 2008a, 2009a,b, 2011). Similar patterns of complex movements such as reaching, grasping, or defensive movements can be evoked from parts of motor cortex (M1), premotor cortex (PMC; dorsal PMD and ventral PMV), and posterior parietal cortex (PPC) that are also strongly interconnected. We hypothesized that neurons involved in distinct behaviors cluster into restricted behavior-specific regions, which we refer to as functional domains. Such domains are located within M1, PMD, PMV, and PPC and they form a series of specialized networks working in parallel and are at least partially segregated. Similar, but less specific proposals for parietal-frontal networks have been previously suggested, based on connection patterns and neurophysiological results (Caminiti et al. 1996, 1999; Johnson et al. 1996; Lewis and van Essen 2000; Luppino et al. 1999; Marconi et al. 2001; Tanne-Gariepy et al. 2002; for review, see Matelli and Luppino 2001 and Rizzolatti et al. 1998). Under the framework proposed for the organization of the parietal-frontal network, complex movements evoked by long trains of electrical pulses delivered into a cortical site likely activate a wide neuronal network that includes cortical and subcortical structures. However, the most important network nodes are likely in PPC, PMC, and M1. This hypothesis is supported by results of our most recent optical imaging studies demonstrating that long-train stimulation of PPC produces somatotopically and functionally appropriate, focal activations of M1-PMC (Stepniewska et al. 2011).
The concept of parietal-frontal networks for specialized movements can be further tested by selective inactivation. Damage or inactivation of nodes involved in the same network should produce behavioral changes consistent with the proposed functions of the network. Indeed, in macaques muscimol injections into the M1 hand representation (Brochier et al. 1999; Fogassi et al. 2001; Kermadi et al. 1997; Kubota 1996; Matsumura et al. 1991; Rouiller et al. 1998; Schieber and Poliakov 1998), rostral PMV (Fogassi et al. 2001; but see also Schieber 2000), or anterior intraparietal area (AIP) (Gallese et al. 1994; Kermadi et al. 1997) strongly impair hand movements, especially those demanding fine control of the digits, and this is consistent with the role proposed for these areas in the control of the manipulative abilities of the hand (Gallese et al. 1994). Thus the functions of one area involved in a network depend in part on the other areas of the same network, but also may be somewhat independent, since all these areas (M1, PMC, and PPC) project separately to the spinal cord and brain stem (Dum and Strick 1991; Galea and Darian-Smith 1994; He et al. 1993; Nudo and Masterton 1990; Wu and Kaas 2000). The extent of this independent control, however, is not fully understood.
The goal of the present study was to determine the roles of sensorimotor areas (M1, PMD, PMV, and PPC) in mediating complex motor responses. To achieve this goal, we performed a series of experiments that combined long trains of microstimulation with focal cortical inactivation. Thus we used muscimol to inactivate individual functional domains in M1, PMD, PMV, or PPC during stimulation of functionally matched domains in other areas or regions to determine the dependence of each area on others in mediating the behavior. The duration of each train of electrical stimulation pulses (500 ms) approximates the time course of the behavior being studied (e.g., reaching and grasping; Georgopoulos et al. 1986; Reina et al. 2001). Stimulation over these long time periods evokes movements similar or identical to those made voluntarily (Cooke et al. 2003; Graziano et al. 2002a,b; Stepniewska et al. 2009a; Thier and Andersen 1998). In the present study, changes in responsiveness to microstimulation across M1, PMD, PMV, and PPC were observed after selective inactivation of portions of these cortices. We used the terms stimulation, microstimulation, and intracortical microstimulation (ICMS), interchangeably throughout the article. Some of these results have been summarized in a preliminary report (Stepniewska et al. 2008b).
MATERIALS AND METHODS
The experiments were on New World owl monkeys (Aotus trivirgatus) and squirrel monkeys (Saimiri sciureus) and prosimian galagos (Otolemur garnetti) of either sex that were 1–8 yr old and weighed 0.8–1.4 kg. The accessibility of M1, PMC, and PPC on the brain surface in these primates offers a considerable advantage over macaques for muscimol injection and microstimulation. In addition, their motor systems are organized in a manner similar to that of macaque monkeys, although the greater direct input from M1 to spinal motoneurons in macaques enhances manual dexterity (Rathelot and Strick 2009). We used long-train microstimulation in combination with cortical inactivation to investigate parietal-frontal cortical circuitry in six New World monkeys (4 owl monkeys and 2 squirrel monkeys) and three prosimian galagos. In each animal microstimulation results were collected in the same way before and after inactivation to allow each animal to serve as its own control.
Surgery and Intracortical Microstimulation
All surgeries were conducted in compliance with the standards set forth by the National Institutes of Health and with the approval of the Vanderbilt Animal Care and Use Committee. Each animal was initially anesthetized with ketamine hydrochloride (10–30 mg/kg im), so it could be placed in a stereotaxic frame, and then anesthesia was maintained with 2% isoflurane. The skull was opened to expose parietal and frontal cortex of the left or right cerebral hemispheres. (For clarity and easier comparison across cases, results in all cases are illustrated on left hemispheres.) After the dura was retracted, the motor cortical areas M1, PMD, PMV, and the posterior parietal areas were mapped with electrical stimulation using low impedance (0.5–1 MΩ) microelectrodes. Trains of pulses were generated by a Master 8 stimulator (AMPI; www.ampi.co.il) with biphasic stimulus isolation (Bak Electronics; www.bakelectronicsinc.com). Stimuli consisted of short (60 ms) and long (500 ms) trains of 0.2-ms biphasic pulses delivered at 300 Hz. Current amplitudes were at levels 1–40 μA for M1 and up to 400 μA (in one case 500 μA) for PMC and PPC. Differences in current thresholds for evoking movements and the type of movements evoked allowed us to define the borders that separate M1, PMD, and PMV (see Stepniewska et al. 1993; Preuss et al. 1996). The microelectrode was lowered perpendicular to the cortical surface to a depth of 1.5–1.8 mm for the lowest thresholds of stimulation. Penetration sites were marked on a high-resolution photograph of the exposed cortex. During microstimulation anesthesia was maintained with ketamine hydrochloride (30–50 mg·kg−1·h−1) diluted with physiological saline (1:4) and delivered intravenously with an infusion pump, supplemented every 2–4 h with 0.2–0.5 mg/kg im of xylazine.
Inactivation Procedure
After functional domains in forelimb and face representations within M1, PMD, PMV, and PPC were located by ICMS, we inactivated one of these domains by focal injection of the GABA agonist muscimol (Sigma-Aldrich; www.sigmaaldrich.com) in a concentration 5 μg/1 μl of buffered physiological saline and microstimulated domains in other areas. The effectiveness of muscimol for inactivating a restricted volume of cortex has been previously reported (Kermadi et al. 1997; Schmidlin et al. 2004). The volumes of muscimol injected at each site, the number and position of sites, and the distance between them to cover the desired territory were determined from our preliminary data and published reports (Arikan et al. 2002; Brochier et al. 1999; Kubota 1996; Martin 1991; Schieber 2000; Schieber and Poliakov 1998; Sommer and Tehovnik 1997; Tehovnik and Sommer 1997). Muscimol injections (1–4 μl) in different locations within frontal and posterior parietal regions were guided by the ICMS maps. In a given penetration, 1 μl of the muscimol solution was injected at two different depths (2 × 0.5 μl) at the approximate rate of 0.1 μl/min. Our injection parameters were designed to inactivate a restricted cortical territory (1–2 mm radius around the injection core), which allowed us to target specific functional domains within M1, PMD, PMV, and PPC. Although the effects of a muscimol inactivation can last as long as 24 h (Collins et al. 2005; Martin and Ghez 1993), all data were collected within 4 h after muscimol injections. We retested sites after muscimol injections using the same current thresholds that evoked movements before muscimol treatment. If the site was unresponsive on three retesting attempts over the course of 1–2 min, then we continued testing with incremental increases in current amplitude up to 250 μA for M1 and up to 500 μA for PPC. Sites that remained unresponsive were considered “suppressed.” We occasionally retested such sites at later times throughout the duration of the experiment to ensure that the result was due to muscimol inactivation and not an inadvertent effect of anesthesia.
Microstimulation of the injected region and its surroundings confirmed the extent of effective inactivation. The estimated borders of the inactivation zone were placed between sites that remained responsive and sites that were not (suppressed sites). In addition, the expression of cytochrome oxidase (CO) was reduced after hours of inactivation, and a decrease in histochemical staining of CO was used to estimate size and location of the inactivated domain produced by muscimol injection (Fig. 1). The existence of sites responsive to stimulation identified regions that were clearly outside the inactivation zone. Blocked sites that sometimes occurred outside the estimated inactivation zones suggested a fringe of reduced effectiveness. In most cases the same or very closely situated sites were stimulated before and after muscimol injections. At the end of microstimulation, several sites were marked by electrolytic lesions throughout the depth of the cortex. Although microlesions were readily apparent in histological preparations, there was no evidence of visible damage from the biphasic stimulation.
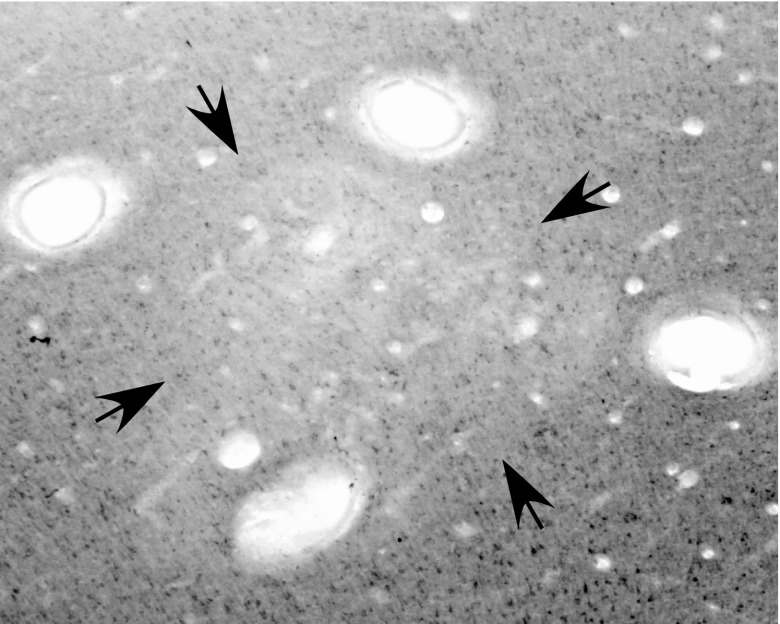
Fig. 1.
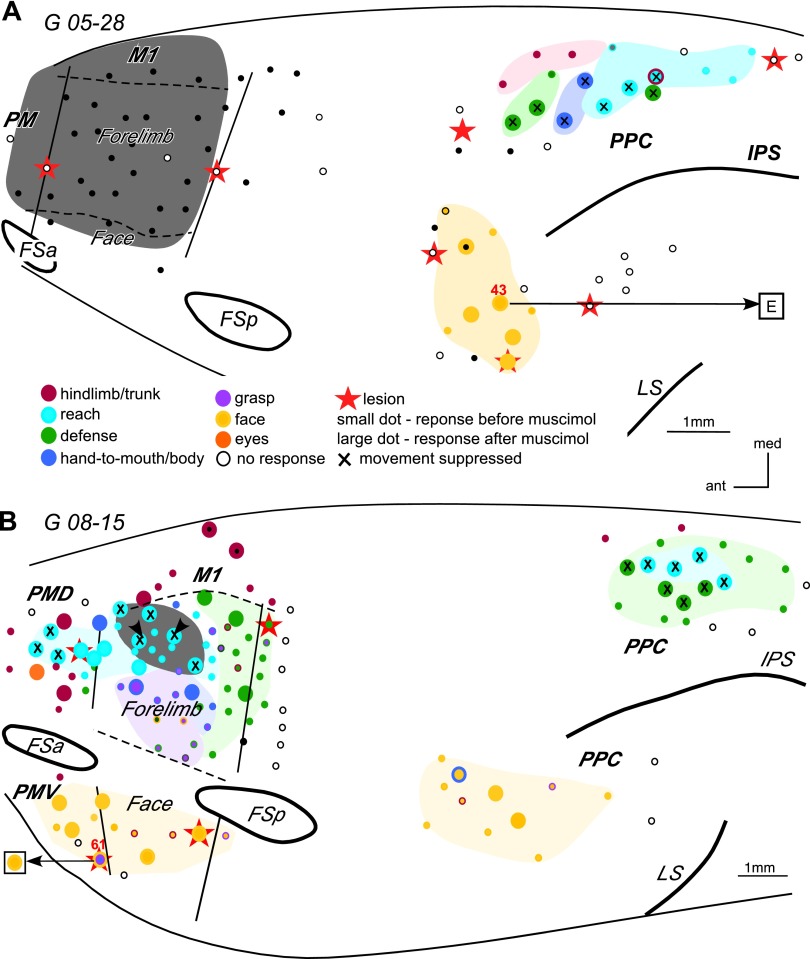
A cortical section from PMD-M1 border region cut parallel to the surface and stained for cytochrome oxidase (CO) in G 08–01. Note the reduced CO level within the area marked by arrows, and the four electrolytic lesions (open ovals) placed at the functional borders of the reach domain. Muscimol was injected at three sites within the domain (see Fig. 7B).
Analysis of Evoked Movements
Movements evoked by microstimulation were observed by two or three investigators and classified into categories outlined in our previous studies of galagos (e.g., hand-to-mouth, reaching, grasp, or defensive; Stepniewska et al. 2005, 2009a). While one investigator moved and placed the stimulating electrode in various cortical locations, the other investigators observed and classified the movements without direct knowledge about the cortical location of the electrode. There was nearly complete agreement on the type of evoked movements across investigators, and the stimulation was sometimes repeated to establish consistency of the movement. Evoked movements were distinguished from the occasional spontaneous movements that sometimes occur under ketamine anesthesia, by the consistency and the latency of the evoked movement sequence relative to onset of electrical stimulus. Movements were classified during the course of experiment to guide mapping strategy. In addition most movements were recorded on videotape to verify movement classification and to determine complexity, latency, and kinematics of movements. A digital video camera (Sony, DCR-HC65) was positioned in front of the animal, and a mirror next to the animal at a 45° angle to the line of sight of the camera, to provide two nearly orthogonal viewpoints for motion tracking. The camera recorded the forelimb and its reflection in the mirror at 30 frames/s. For each stimulation site, movement sequences were synchronized with the onset of pulse trains, which were visualized by a light-emitting diode (LED) that was captured in the same video recording frame. Thus the start of the stimulation train could be determined to the nearest video frame (33 ms). Movement sequences, especially those evoked from the same site before and after muscimol injection, were traced from video frames, and their trajectories compared.
Perfusion and Histology
After the effects of muscimol were tested with microstimulation, each animal was killed with an overdose of sodium pentobarbital (80 mg/kg or more) and perfused through the heart with buffered physiological saline followed by 2% formaldehyde and then 2% formaldehyde with 10% sucrose. The brain was removed, and the cortex was separated from the brainstem, unfolded, flattened between two glass plates, and submerged in 30% sucrose for cryoprotection. One to three days later, the cortical tissue was frozen and cut parallel to the surface into two series of 40- to 50-μm sections. Alternate sections were stained for cytochrome oxidase (Wong-Riley 1979) or myelinated fibers (Gallyas 1979) to allow us to align the ICMS maps and muscimol injections with the architectonic borders of cortex.
RESULTS
The roles of specific sensorimotor domains in M1, PMC, and PPC in the performance of complex motor behaviors were determined by inactivating one of these domains with muscimol during stimulation of the other domains. Within minutes muscimol effectively rendered injected domains unresponsive to ICMS. For most injections, the inactivated region was limited to one functional domain within each area. Domains in PMC or PPC with strong connections to the inactivated domain in M1 were also suppressed, whereas the functional properties of unconnected functional domains were largely preserved. Changes in responsiveness to microstimulation, observed after selective cortical inactivation of M1, PMD, PMV, and PPC, are described below. We start with a brief description of the organization of functional domains.
Organization of Complex Movement Domains in Sensorimotor Regions
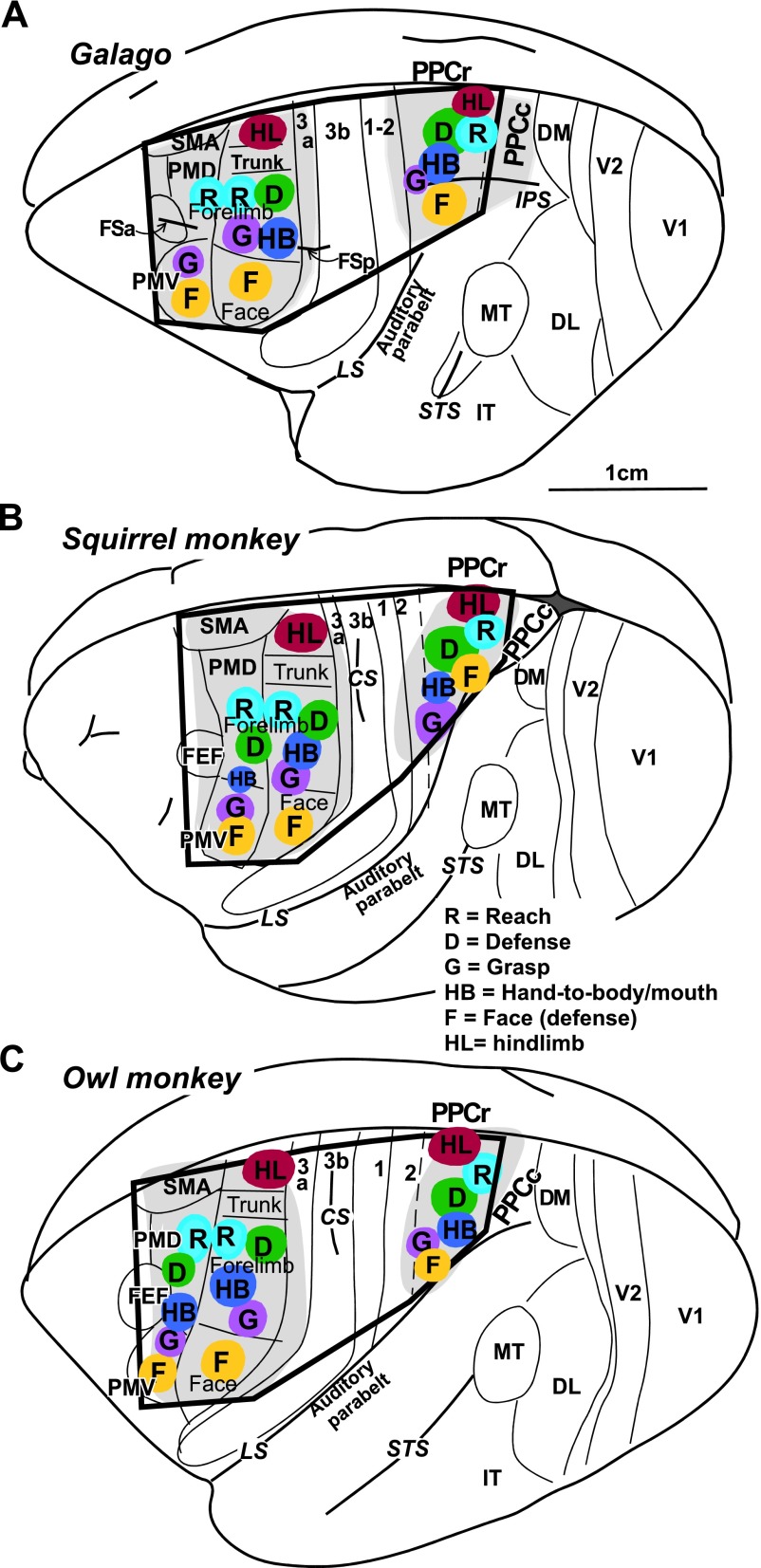
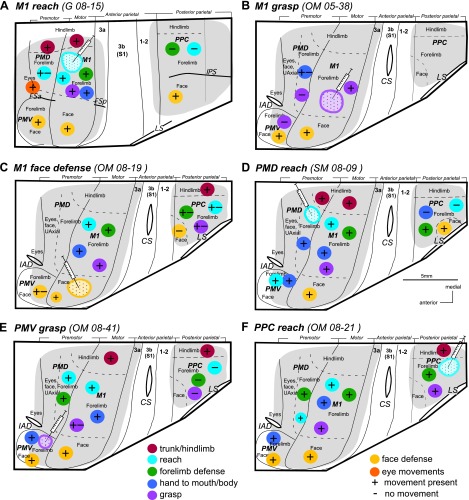
The locations of M1, PMD, PMV, and PPC in galagos and New World monkeys are shown in Fig. 2. For all cases these regions were first mapped with long (occasionally also with short) trains of current pulses to establish locations of functional domains within the regions. M1, PMV, and PMD were responsive to short- and long-train stimulation, with PMD evoking movements usually at much higher current intensity (up to 200 μA) than M1 (below 40 μA). Stimulation of PMV evoked movements at slightly higher thresholds than M1. PPC was responsive to long-, but not short-, train stimulation at current intensities of 100–400 μA. For some cases (e.g., OM 08–21, OM 08–19), more complete maps were obtained than for others (e.g., OM 05–38, G 08–01). In all studied species, long-train stimulation evoked similar complex movements from sites in M1, PMC, and PPC regions. Clusters of sites that evoked a particular movement defined behavior-specific domains. Small differences in the relative location of functional domains were noticed between monkeys and galagos (see Kaas et al. 2011), and smaller differences were seen across individuals of the same species.
Fig. 2.
Schematic illustration of functional domains in frontal and parietal cortex in the left hemisphere of prosimian galago (A), squirrel monkey (B), and owl monkey (C). Territories investigated with microstimulation are depicted in light grey and the approximate locations of functional domains are depicted in color. The black frame corresponds to outlines of schematics in summary Fig. 14. Motor cortical areas: FEF, frontal eye field; M1, primary motor; PMD, dorsal premotor; PMV, ventral premotor; SMA, supplementary motor. Somatosensory cortical areas: 3a, 3b, 1–2. Visual cortical areas: DL, dorsolateral; DM, dorsomedial; FST, fundal superior temporal; IT, inferotemporal; MT, middle temporal; MTc, middle temporal crescent; MST, middle superior temporal; V1, primary visual; V2, secondary visual; V3, third visual. Sulci: CS, central sulcus; FSa, anterior frontal; FSp, posterior frontal; IPS, intraparietal; LS, lateral; STS, superior temporal; PPCr, rostral region of the posterior parietal cortex.
Frontal motor region.
The frontal region responsive to ICMS lies between two frontal fissures (FSa and FSp) in galagos (Fig. 2A) and anterior to central sulcus (CS) in monkeys (Fig. 2, B and C). In both galagos and New World monkeys, the general topography of the movements evoked from M1-PMC by short- or long-train ICMS indicates that movements are represented in an approximately mediolateral somatotopic fashion, with hindlimb movements represented medially, forelimb centrally, and face laterally (Fig. 2). As shown previously (Stepniewska et al. 2009a; Gharbawie et al. 2011a) and in this study, several functional domains can be identified within M1-PMC forelimb representation using long-train ICMS. A reach domain was consistently identified in caudal PMD and was contiguous with the matching domain in rostral M1. In most cases, one or two additional clusters of sites that evoked reaching movement were identified in M1. Multiple (up to 3) separate domains for defense, hand-to-mouth or hand-to-body, and grasp were identified in M1 in all animals. The relative locations of those domains varied somewhat across cases. Defensive (protective or withdrawing) and hand-to-mouth or to body movement domains covered most of the M1 forelimb representation (and in some cases PMD, e.g. SM 08–09, OM 08–21). Hand-to-mouth movements were typically evoked from more lateral aspects of the M1 forelimb representation. Grasping, concurrent grasping and hand-to-mouth, and forelimb supination movements were evoked from M1 forelimb region near the M1 face representation and extended caudally into area 3a. A few more medial clusters of such movement sites were also found. Grasping movements, and occasionally other forelimb movements (e.g., hand-to mouth), were also evoked from PMV of monkeys (e.g. SM 08–09, G 08–01). Similar to lateral M1, PMV represented mostly face movements that included grimace, ear retraction, and less frequently mouth opening (or closing) and tongue movements. Downward and upward eye movements were evoked from a region near or in the frontal eye field (FEF) or rostral PMD (G 08–15, OM 08–21). Detailed descriptions of the evoked movements have been presented elsewhere (see Gharbawie et al. 2011a and Stepniewska et al. 2009a).
Posterior parietal cortex.
Long-train electrical stimulation (500 ms) evoked movements from domains that paralleled the somatotopic organization of the frontal cortex domains. In galagos only the rostral region of PPC (PPCr) was responsive to ICMS, which includes cortex medial and lateral to the anterior half of intraparietal sulcus (IPS; Fig. 2A). In owl and squirrel monkeys, which do not have an IPS, movements were evoked from a region dorsal to lateral sulcus (LS) and caudal to somatosensory cortex (Fig. 2, B and C). In both galagos and monkeys, most of the responsive PPC region was devoted to forelimb movements, which cluster in cortical domains emphasizing different types of complex movements, similar to those evoked from M1 and PMC.
In galagos, the domain for reaching movements was located most caudal in PPCr, whereas defensive and hand-to-mouth movement domains were in central PPCr. Defensive and hand-to-mouth movement domains were contiguous, with hand-to-mouth movements usually evoked from more rostrolateral sites than defensive movements. We previously reported a small grasping domain in rostral PPC near the border with area 1–2 (see Fig. 6 in Stepniewska et al. 2009a), but it was not identified in the present cases. Eye movements could be evoked from lateral PPC (see Figs. 2–4 in Stepniewska et al. 2009a), but this movement domain was not consistently revealed in previous cases and was not identified in the present cases. Ventral PPC (lateral to the IPS) included a domain for defensive face movements (grimace), sometimes correlated with mouth opening and teeth exposure. Occasionally, defensive face movements were concurrently evoked with forelimb movements.
Fig. 6.
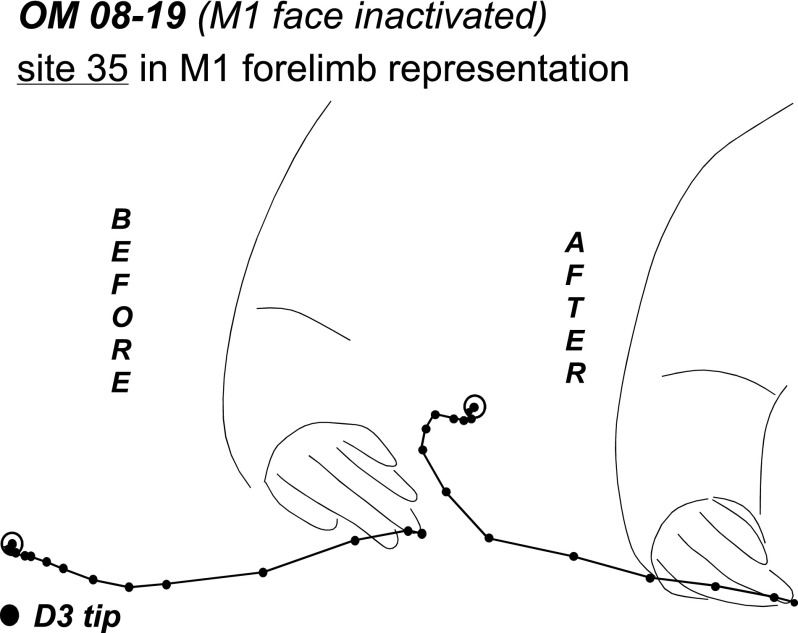
Trajectories of lateral defensive movements evoked from site (35) in the M1 forelimb representation in OM 08–19. Trajectories traced from video records show the original movement (“before” muscimol injection) and the movement evoked during retesting (“after” muscimol injection). Black lines show the path of the hand during stimulation, where each dot depicts the tip of digit 3 in successive video frames (33 ms). The end-point of each trajectory is circled for transparency. The forearm is illustrated in a starting position.
Fig. 4.
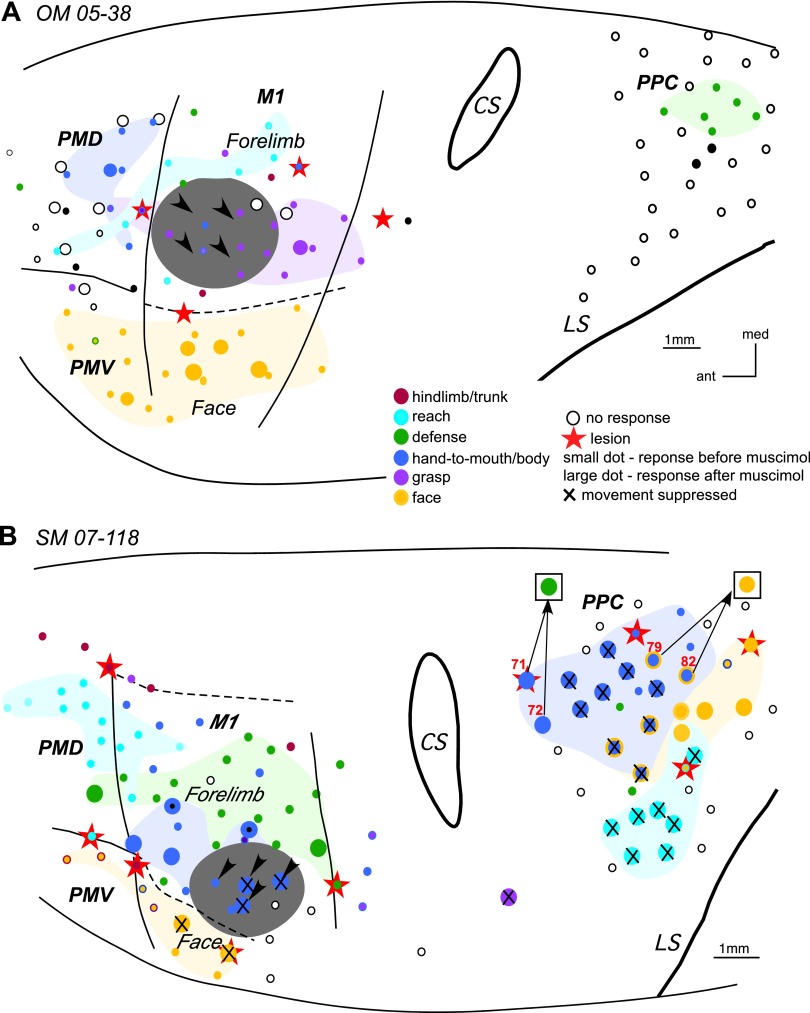
The effect of inactivation of M1 grasp domain in OM 05–38 (A) and inactivation of M1 hand-to-mouth domain in SM 07–118 (B). In A large circles depict retested sites that were close, but did not overlap the original sites, which are depicted as small dots. Black dots in PMD and PPC depict sites that evoked ear movements. In B note the change in movement sequences evoked from PPC sites (71, 72, 79, 82) during retesting. Arrows from a stimulation sites point to the responses evoked during retesting (shown in squares). For detailed description of results, see the text. Other conventions as in Figs. 2 and 3.
Much as in galagos, a mediolateral series of movement domains was revealed in PPC of monkeys, but the movement region was tilted mediocaudally. Thus reach, defense, and grasp domains were organized in PPC in a caudomedial to rostrolateral progression (Gharbawie et al. 2011a; see also cases OM 08–19 and OM 08–21, see Figs. 5 and 11B). A reach domain was identified in caudomedial PPC in all monkeys except monkey SM 07–118 (see Fig. 4B). A defense domain was lateral and slightly rostral to the PPC reach domain. A grasp domain was lateral and slightly rostral to the defense domain and directly caudal to area 1. A hand-to-body domain was not always identified (e.g. OM 08–21, see Fig. 11B), but when present, it was in central PPC near the defense domain (e.g. OM 08–19, see Fig. 5). Face movements (grimace or others) and eye movements were evoked, respectively, from sites in rostrolateral PPC and sites directly medial to the lateral sulcus (Stepniewska et al. 2006a). In squirrel monkeys, evoked face movements were usually accompanied by defensive forelimb movements. Only a few sites evoked face movements alone, and they were typically caudal to the mixed forelimb/face domain (SM 07–118, see Fig. 4B; SM 08–09, see Fig. 7A). Face movement sites were found only in one owl monkey in this study (OM 08–19, see Fig. 5). In this case the rostrolateral PPC region was mapped more extensively than in the other three owl monkeys.
Fig. 5.
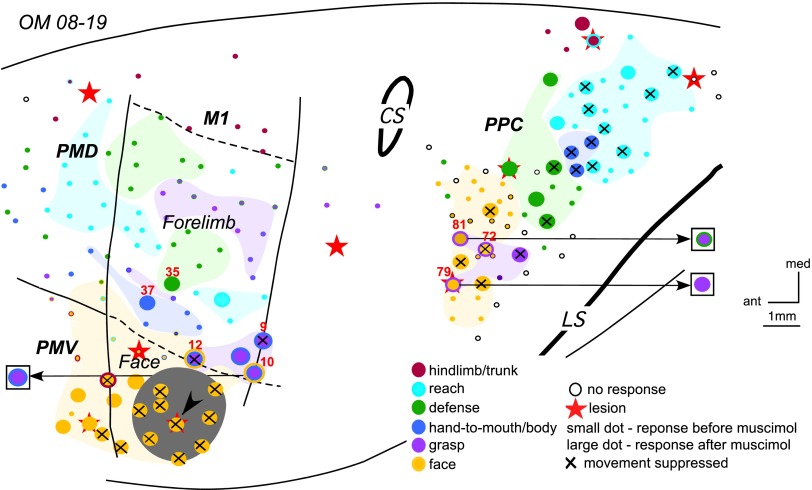
The results of inactivation of the M1 face defense domain in OM 08–19. Note significant changes in movement sequences evoked from site 10 in M1 and sites 79 and 81 in PPC after muscimol injection. Arrows from a stimulation sites point to the responses evoked during retesting (shown in squares). For detailed description of results see the text. Conventions as in Figs. 2–4.
Fig. 11.
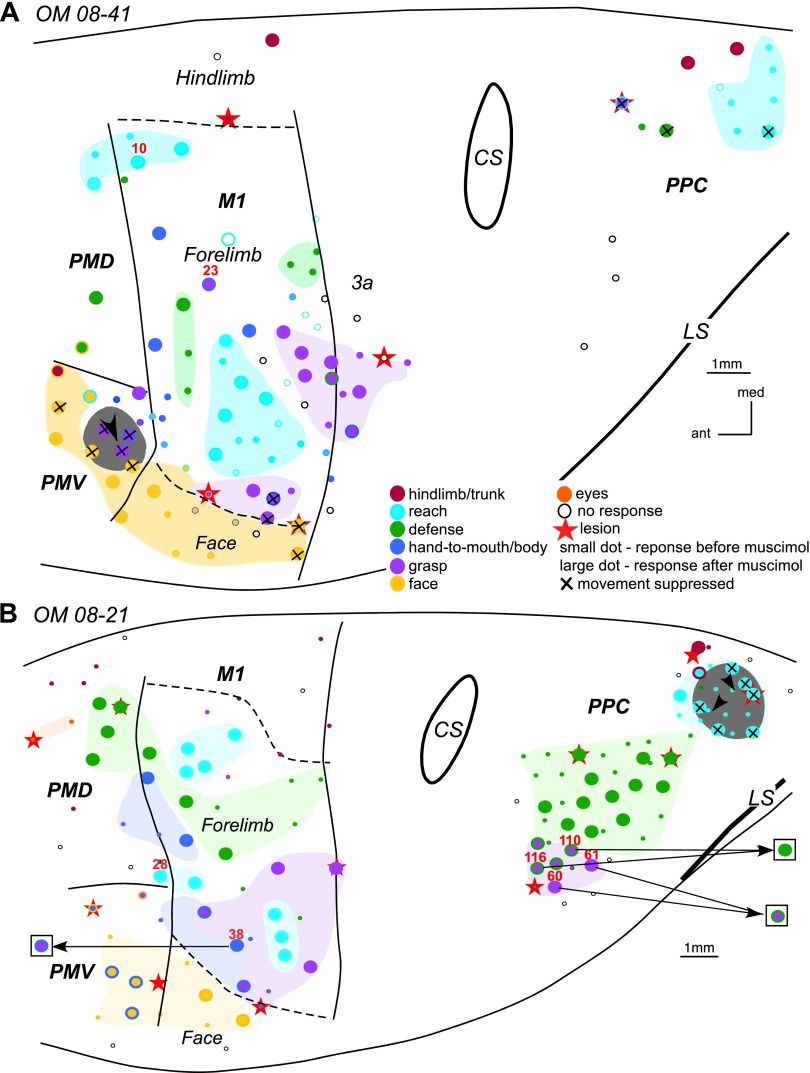
The effects of inactivation of grasp domain in PMV in OM 08–41 (A) and reach domain in PPC in OM 08–21 (B). In A stimulation of sites 10 and 23 in M1 evoked movements which trajectories are shown in Fig. 12. In B note the change in movement sequences evoked from 4 sites (60, 61, 110, and 116) in PPC and site 38 in M1 during retesting. Arrows from a stimulation sites point to the responses evoked during retesting (shown in squares). For detailed description of results see the text. Conventions as in Figs. 2 and 3.
Fig. 7.
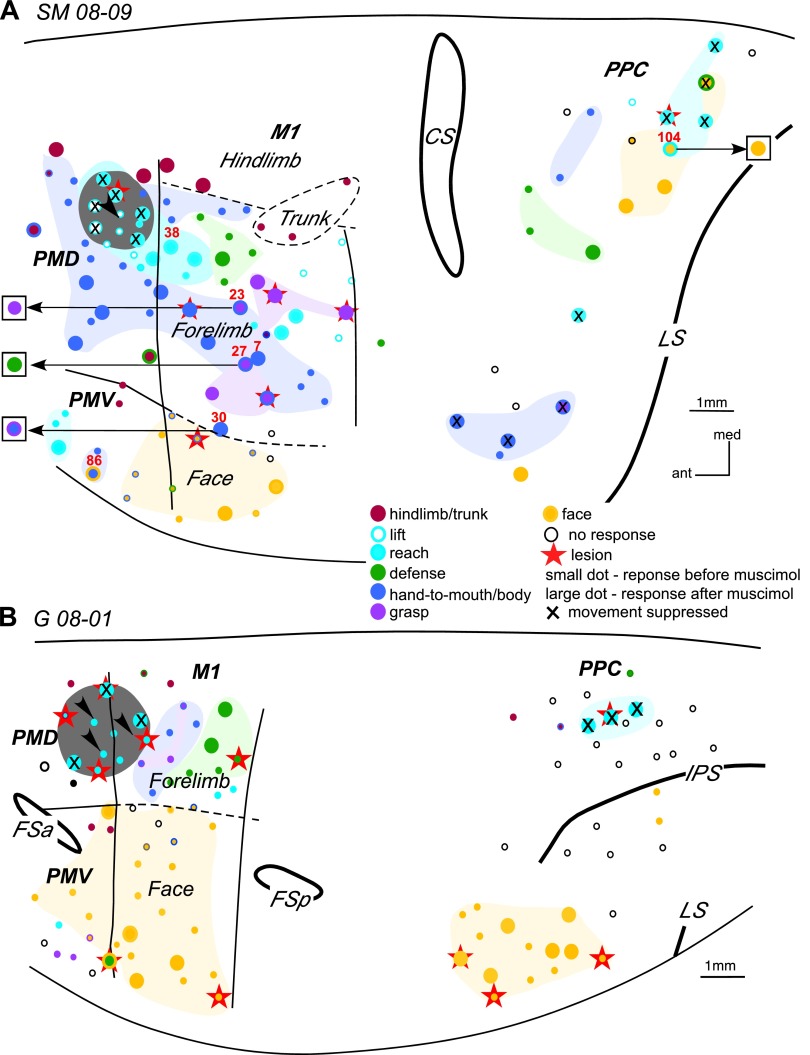
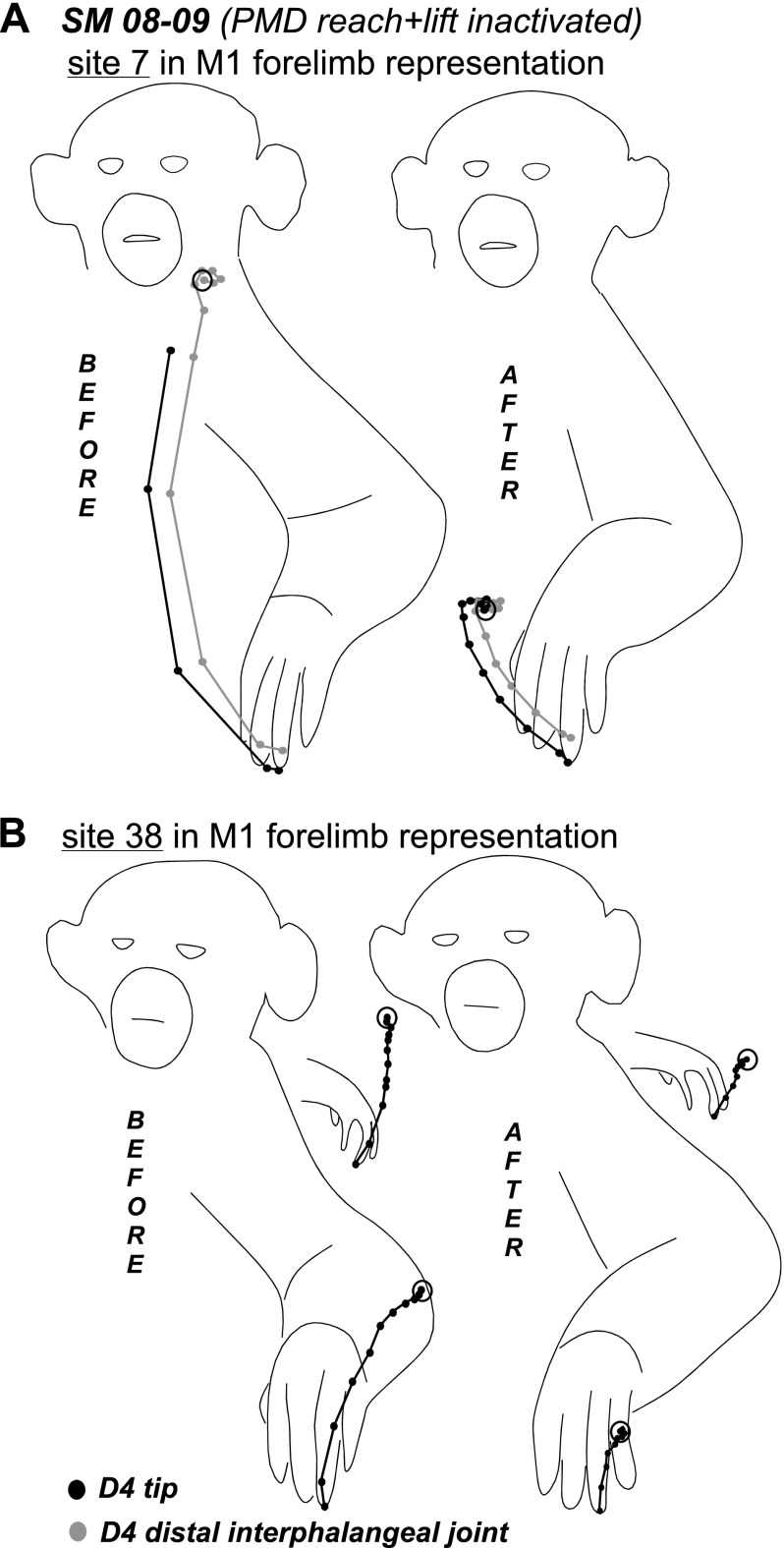
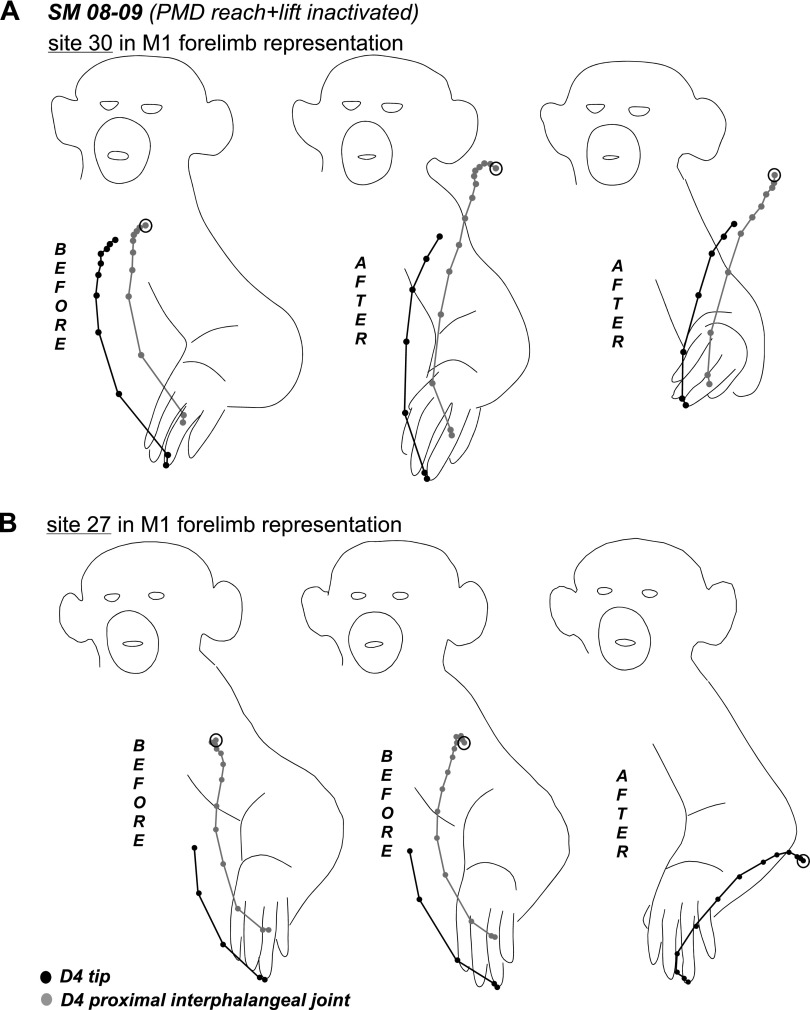
The results of inactivation of the PMD reaching domain in SM 08–09 (A) and PMD-M1 reach domain in G 08–01 (B). In A notable changes in movement sequences occurred during retesting of 3 sites (23, 27, and 30) in M1 and 1 site (104) in PPC. Arrows from a stimulation sites point to the responses evoked during retesting (shown in squares). The effects of inactivation on movement trajectories in SM 08–09 are illustrated in Figs. 8–10. For detailed description of results see the text. Other conventions as in Figs. 2–4.
M1 Inactivation
Inactivating parts of M1 abolished movements that were represented in that part of M1, whereas other parts of M1 remained responsive to microstimulation. Moreover, the types of movements blocked in M1 could no longer be evoked from matching domains in PMC or PPC.
The effects of inactivating parts of M1 were studied in three galagos, two owl monkeys, and one squirrel monkey (Table 1). To study the effects of inactivation of the entire M1 forelimb representation, we placed a small piece of gelfoam soaked in muscimol on the cortical surface after it was sized to the M1 forelimb representation as defined by short ICMS mapping (galago G 05–28, Fig. 3A). The estimated region of inactivation (marked in grey in Fig. 3A) possibly extended into parts of the M1 hindlimb and face representations, as well as part of the PMD forelimb representation. Before muscimol treatment, long-train ICMS evoked complex forelimb movements, including defensive, reaching and hand-to-mouth movements, from numerous sites in dorsal PPC. Eight of these sites were retested during M1 inactivation, and none were responsive to microstimulation. In contrast, stimulation sites in ventral PPC evoked face (and ear) movements that were largely unaffected by muscimol injection in the M1 forelimb representation. Nevertheless, higher currents were necessary for evoking movements from some of those sites. For example, site 43 in ventral PPC evoked face grimace and ear movements before muscimol application, but it evoked only ear movement after M1 inactivation, and the current threshold for this movement increased from 100 to 300 μA. Lack of face movements at this site likely reflects the inhibition of a small part of face representation of M1.
Table 1.
Number of cortical sites stimulated after muscimol inactivation of motor domains
| Case No. | Inactivated Domain | All | M1 | PMD | PMV | PPC |
|---|---|---|---|---|---|---|
| Galago | ||||||
| G 05–28 | M1 forelimb | 13 | — | — | — | 8FL + 5F |
| G 08–01 | M1 + PMD reach | 19 | 3FL + 3F | 2FL | 1FL + 1F | 3FL + 6F |
| G 08–15 | M1 reach | 41 | 3HL + 12FL + 3F | 6FL + 2T + 1E | 2F + 1F/FL | 8FL + 2F + 1F/FL |
| Squirrel monkey | ||||||
| SM 07–118 | M1 hand-to-body | 37 | 8FL + 2F | 1FL | — | 16FL + 5F + 5FL/F |
| SM 08–09* | PMD reach | 50 | 2HL + 17FL + 2F | 2HL + 12FL | 2FL | 8FL + 3F + 2FL/F |
| Owl monkey | ||||||
| OM 05–38 | M1 grasp | 16 | 3FL + 4F | 7FL | 1FL + 1F | — |
| OM 08–19* | M1 face defense | 50 | 5FL + 12F + 2FL/F | — | 4F + 1F/T | 1HL + 1HL/FL + 18FL + 3F + 3FL/F |
| OM 08–21 | PPC reach | 56 | 19FL + 2F | 5FL | 3F/FL | 1HL + 1HL/FL + 25FL |
| OM 08–41* | PMV grasp | 44 | 1HL + 20FL + 4F | 2FL + 1FL/F | 4FL + 5F + 1F/FL + 1F/T | 3FL + 2HL |
M1, primary motor cortex; PMV, ventral premotor cortex; PMD, dorsal premotor cortex; PPC, posterior parietal cotex; E, eye movements; F, face movements; FL, forelimb movements; HL, hindlimb movements; T, trunk movements.
Cases with illustrated movements trajectories.
Fig. 3.
Schematic illustration of the effects of inactivating the entire M1 forelimb representation in G 05–28 (A), and the M1 reaching domain in G 08–15 on other functional domains (B). The grey area in A depicts the approximate location of the muscimol application with gelfoam. Arrowheads in B depict the location of 2 muscimol injections. In A and B, dots represent microstimulation sites. Small dots represent sites that were stimulated before muscimol injections. Large dots represent sites that were stimulated before and after muscimol injections. Large dots with a small black dot in the center represent sites that were stimulated after musimol injection, but not before. Areas highlighted in light colors depict the approximate extent of a functional domain or a cluster of sites that evoked similar movements. Thin solid lines mark approximate areal borders and dashed lines mark borders between body representations within M1. Arrows from a stimulation sites point to the responses evoked during retesting (shown in squares). For example, in A stimulation of all sites in ventral PPC evoked concurrent face grimace and ear movements. Inactivation of the M1 forelimb representation suppressed the face movements evoked from PPC, but the ear movements could still be evoked (site 43). In B stimulation of site 61 in PMV evoked grasp followed by hand-to-mouth and mouth opening. Inactivation of the M1 reach domain suppressed the forelimb movements evoked from PMV, but mouth opening was still evoked. Other conventions as in Fig. 2.
More specific changes were observed in the effectiveness of stimulation when particular functional domains or parts of functional domains were inactivated. In galago G 08–15 (Fig. 3B), two small injections of muscimol were placed in the reach domain of M1. Forty one sites in M1, PMD, PMV, and PPC were retested over the course of 3.5 h after muscimol injection. The inactivated region (marked in grey in Fig. 3B) included more than half of the M1 reach domain. Five sites retested within the inactivated region were unresponsive to stimulation. Lateral to the inactivated region, two sites continued to evoke reaching movements, although stimulation thresholds were significantly higher (10 vs. 150 μA). Such results indicate that the effects of muscimol injections in M1 can be very specific, blocking some but not other sites in an individual domain. M1 sites retested caudal and lateral to injected domain were unaffected as they continued to evoke defensive, hand-to-mouth, and grasping/hand-to-mouth movements. Face movements were evoked from all retested sites in lateral M1. However, the movements evoked from these sites were weaker compared with movements evoked from the same sites before inactivation of M1 reach domain. As expected, sites retested in PMV that evoked face movements remained effective. However, one site (61) in PMV where both face and forelimb movements were evoked before inactivation lost the capacity to evoke forelimb movements, but not the face movements. In PMD, five sites for reaching movements were retested. Three of these sites were unresponsive, and the two responsive sites while near the PMD-M1 border evoked reaching movements but at higher current thresholds (20 vs. 150 μA and 40 vs. 200 μA). Another site at the PMD-M1 border evoked hand-to-body movement before muscimol treatment and continued to do so after muscimol treatment. Two sites for trunk and hindlimb movements also remained effective, as did a nearby site for eye movements in the FEF. Thus inactivation of the M1 reach domain either inhibited reaching movements from PMD or increased the threshold for evoking movements. The loss of function was less specific in PPC after inactivation of the M1 reach domain. The four sites retested in the PPC reach domain were unresponsive. However, four nearby sites for defensive forelimb movements were retested and no movements were evoked as well, even with higher current intensity (500 μA), than before inactivation (150–250 μA). Sites retested in ventral PPC remained effective in evoking face movements.
The effects of selective M1 inactivation were further investigated in owl monkey OM 05–38 (Fig. 4A). The M1 grasp domain was inactivated with four small injections of muscimol (black arrowheads in Fig. 4A). Sixteen cortical sites in M1, PMD, and PMV were retested over the course of 70 min after muscimol injection. The inactivated region encompassed more than half of the M1 grasp domain, but sites that used to evoke hand-to-body movement (either alone or concurrent with grasp) were also involved. Grasping movements could still be evoked from a site caudal to the muscimol-injected region, and face movements could be evoked from M1 sites lateral to the same region. The thresholds for both types of movements remained comparable to those before inactivation.
Six sites in PMD that represented grasping, concurrent grasping, and hand-to-body or just hand-to-body movements were unresponsive to microstimulation during retesting. The loss of those movements may be explained by the fact that the region inactivated by muscimol involved sites for grasping as well as for hand-to-body movements and that these behaviors are mediated by M1. Only one site representing hand-to-body movement remained effective, although the movement was much weaker after inactivation. Retesting a site in dorsal PMV for grasping failed to evoke movements. Inactivation did not interfere with face defensive movement evoked from a lateral PMV site, but movement strength was reduced and threshold increased. PPC was not tested after muscimol injections in this monkey. The results indicate that muscimol effectively blocked much of the grasping domain of M1 and that this inhibited sites in premotor cortex (PMV and PMD) for grasping and partially inhibited sites in PMD for hand-to-body movements.
The effects of inactivation of a single functional domain in M1 on the responsiveness to microstimulation of domains in other sensorimotor areas (especially PPC) was also investigated in squirrel monkey SM 07-118. The M1 hand-to-mouth domain was inactivated with four muscimol injections (Fig. 4B). Thirty-seven sites distributed throughout M1, PMD, and PPC were retested over the course of 4 h after muscimol injections. Some dorsal and rostral sites within the hand-to-mouth M1 domain were unaffected, so inactivation was only partial. Another M1 site caudal to the inactivated territory continued to evoke defensive forelimb movements, but two face movement sites lateral to the inactivated region were unresponsive when stimulated with current up to 150 μA even though their movement thresholds before inactivation were 30 and 90 μA. The single site retested in PMD evoked defensive forelimb movement at an unchanged current threshold. Retesting was more comprehensive in PPC with 26 sites. Most hand-to-mouth (or to body) sites in PPC, which were responsive to current amplitudes 150–250 μA, were unresponsive after inactivation of M1 hand-to-mouth domain. Hand-to-mouth sites that included concurrent ear movements (e.g., site 82) or face grimace (e.g., site 79) were devoid of the hand movement during retesting, but the ear and face movements were spared. However, in three other sites hand and face movements were eliminated. Interestingly, two most rostral PPC sites (71 and 72) that evoked hand-to-body movements during retesting evoked defensive forelimb movements. The lateral direction of the defensive movements were almost the diametric opposite of the original hand-to-mouth movement. All other forelimb sites retested in PPC, including reaching and grasping, were suppressed. Other face sites retested in caudal PPC remained responsive, albeit to higher current thresholds. Increased current thresholds likely reflected partial inactivation of face sites in M1. The results demonstrate a more widespread suppression of PPC function than would have been expected from the size and location of the inactivated M1 territory. PMV was not retested after muscimol injection in this case.
The effects of inactivating a large part of the face representation in M1 on the responsiveness of sensorimotor areas was investigated in owl monkey OM 08–19. The M1 face defensive domain was inactivated with one large injection of muscimol (Fig. 5). Fifty sites distributed across M1, PMV, and PPC were retested over the course of 3.5 h after injection. Most of the M1 face representation was inactivated, including a distant site near the PMV border. However, one site on the rostral edge of the inactivated domain remained responsive. A more rostral site at the border with PMV also remained responsive, but its current threshold increased from 20 to 80 μA during retesting. Two M1 sites at the face/forelimb border that evoked concurrent forelimb and face movements were retested as well. The site closest to the inactivation territory (12) was completely suppressed. Another site (10) no longer evoked face movements but continued to evoke forelimb movements (grasp + supination), albeit with current amplitudes that increased from 40 to 200 μA during retesting. Forelimb sites retested in M1 were not suppressed after inactivation of face M1 (except for site 9 at the border with area 3a) and their thresholds remained the same. In addition, the movement trajectories traced from video recordings were conserved for most of such sites during retesting. For example, site 35 evoked defensive forelimb movements that were consistent across trials before and during inactivation. During inactivation only subtle differences were detected in the latter part of the movement, which included a slightly longer trajectory (Fig. 6). A site near the M1 face domain (37) evoked a weak forearm supination followed by hand-to-body movement. During inactivation, stimulation of this site evoked the same movement, albeit with an additional forelimb lift that resulted (as for site 35) in slightly longer trajectory (not illustrated).
Face sites in PMV also required higher current levels during retesting to evoke movements. Two sites near the M1/PMV border were totally suppressed. Also, all face sites in PPC were suppressed during retesting. The most rostral sites in PPC that evoked a facial grimace and concurrent grasping were either suppressed during retesting (e.g., site 72), or devoid of the face movement with forelimb movement remaining the same (e.g., site 79), or turning out to be more complex (e.g., site 81). During retesting, microstimulation of site 81 evoked a grasp followed by a retraction of the hand towards the body. Some forelimb sites in PPC were suppressed during retesting, but hindlimb sites in medial PPC and their thresholds were largely unchanged. PMD was not retested after the M1 face inactivation.
In summary, muscimol injections into forelimb or face domains in M1 effectively inactivated a territory localized near the injection sites, whereas forelimb and face sites more distant from the injection remained functional but in some instances at higher current thresholds. M1 inactivation suppressed the responsiveness of functionally matched domains in PMC, while effecting a more widespread and less specific suppression in PPC that included functionally matched domains and numerous sites in neighboring (unmatched) domains. However, more experiments are needed to confirm the preservation of function for unmatched domains in PMC.
PMD Inactivation
In another set of experiments, we investigated the effects of PMD inactivation on responsiveness in other sensorimotor areas in one squirrel monkey and one galago. Inactivating the PMD reach domain in both cases altered the responses and thresholds at nearby stimulation sites in PMD but had little effect on other frontal cortex sites. Functionally matched sites in PPC were suppressed, and some of the related sites were altered as well.
In squirrel monkey SM 08-09 (Fig. 7A), one muscimol injection was used to inactivate the PMD reach domain. The injection site also included several sites from which forelimb lifting was evoked. Fifty sites were retested over the course of 3 h from muscimol injections. PMD reach domain inactivation did not affect forelimb or hindlimb sites within PMD, including sites at the M1 border that evoked reaching. However, for most of these sites, higher current thresholds were necessary to evoke movements. Forelimb sites retested in M1 (17 sites) and PMV (2 sites), including those representing reaching, were also responsive to stimulation. Sequences of movements evoked from the M1 sites during retesting were mostly unaltered, but movements were truncated in some instances (e.g., sites 7 and 38, Fig. 8, A and B). In other retesting instances, the complexity of forelimb movements evoked from M1 was altered. For example, stimulation of site 23 after inactivation evoked a grasp only, whereas the original movement included grasp followed by hand-to-mouth movement. In contrast, stimulation of site 30 at the forelimb/face border originally evoked hand-to-mouth movement, but the same movement included a grasp component after PMD inactivation. In addition, movement trajectories revealed different end-point positions for the hand before and after inactivation (Fig. 9A), and the end-point positions after inactivation were consistent irrespective of the starting position (Fig. 9A), supporting the previous observations of Graziano et al. (2002a,b). A site in lateral forelimb M1 (27) originally evoked grasping and concurrent hand-to-body movements, but during inactivation it evoked defensive forelimb movements that followed a completely different trajectory (Fig. 9B). Face sites in M1 were not affected by PMD inactivation and their thresholds remained the same.
Fig. 8.
Trajectories of hand-to mouth (A) and lateral reach movements (B) evoked from sites 7 and 38, respectively, in the M1 forelimb representation of SM 08–09. Trajectories show the original movement and the movement evoked during inactivation of the PMD reach domain. Black dots indicate the positions of the tip of digit 4 and gray dots indicate the position of distal interphalengeal joint in successive video frames. Note the truncated trajectories of both movements evoked after muscimol inactivation. For site 38, trajectories of hand movements reflected in the mirror are also shown. Other conventions as in Fig. 6.
Fig. 9.
Trajectories of hand-to-mouth (A) and hand-to-body movements (B) evoked from sites 30 and 27, respectively, in M1 forelimb representation of SM 08–09. Trajectories show the original movement and the movement evoked during inactivation of the PMD reach domain. Black dots indicate the positions of the tip of digit 4 and gray dots indicate the position of proximal interphalengeal joint in successive video frames. Note that the final position of the hand in A during retesting was quite consistent irrespective of the starting position. In B the original hand-to-body movement changed to lateral defensive movement during retesting. Conventions as in Figs. 6 and 8.
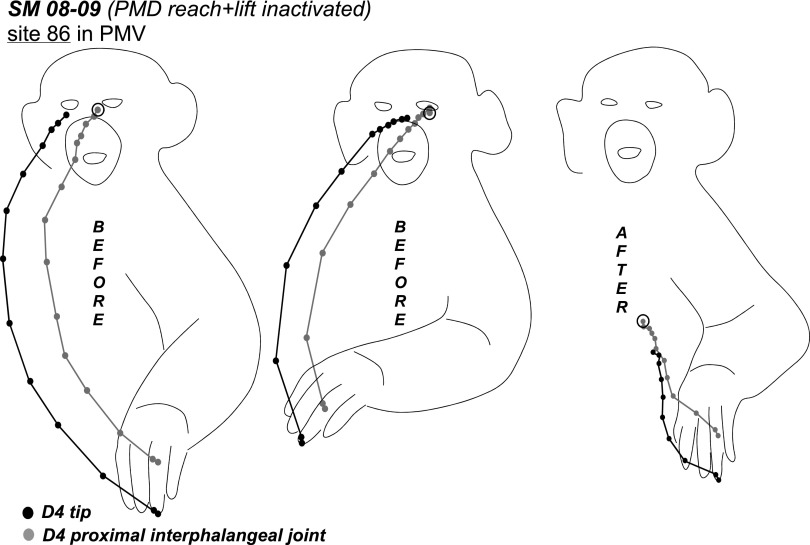
One site retested in PMV during PMD inactivation continued to evoke reaching movements, albeit with current amplitudes that increased from 40 to 300 μA. The movement was also much weaker and truncated (not illustrated). Similarly, another PMV site (86) continued to evoke hand-to-mouth movement with simultaneous mouth opening during PMD inactivation but with current amplitudes that increased from 40 to 60 μA. The trajectory of this movement was also shorter and the final spatial position was much lower than for the original movement (Fig. 10). As for site 30, end-point positions of the original movement evoked from site 86 were consistent regardless of the starting position.
Fig. 10.
Trajectories of hand-to-mouth movement evoked from site (86) in PMV of SM 08–09. Trajectories show the original movement and the movement evoked during inactivation of the PMD reach domain. As in Fig. 9A final position of the hand in original movement was consistent irrespective of the starting position. Conventions as in Figs. 6 and 9.
Forelimb sites retested in PPC (reach and other domains) were suppressed, whereas face sites remained responsive to the original current amplitudes. PPC site 104 originally evoked reaching and concurrent face movements, but PMD inactivation suppressed the capacity to evoke reaching from this site.
The PMD reach domain was also inactivated in galago G 08-01 (Figs. 1 and 7B). Three muscimol injections covered caudal PMD and spread into rostral M1 where reaching was also represented. Nineteen sites across PMD, M1, PMV, and PPC were retested over the course of 2 h of muscimol injections. Although fewer cortical sites were retested in this case compared with SM 08–09 (Table 1), the results for both cases were similar. Thus forelimb and face sites in M1 remained responsive after PMD inactivation. Forelimb and face sites in PMV also remained responsive during retesting. All PPC sites that originally evoked reaching were suppressed during retesting but face sites remained responsive.
PMV Inactivation
We investigated the effects of partial PMV inactivation in a single case. Only mild alterations of evoked movements were observed for sites in M1, whereas widespread suppression of movements was found for sites in PPC.
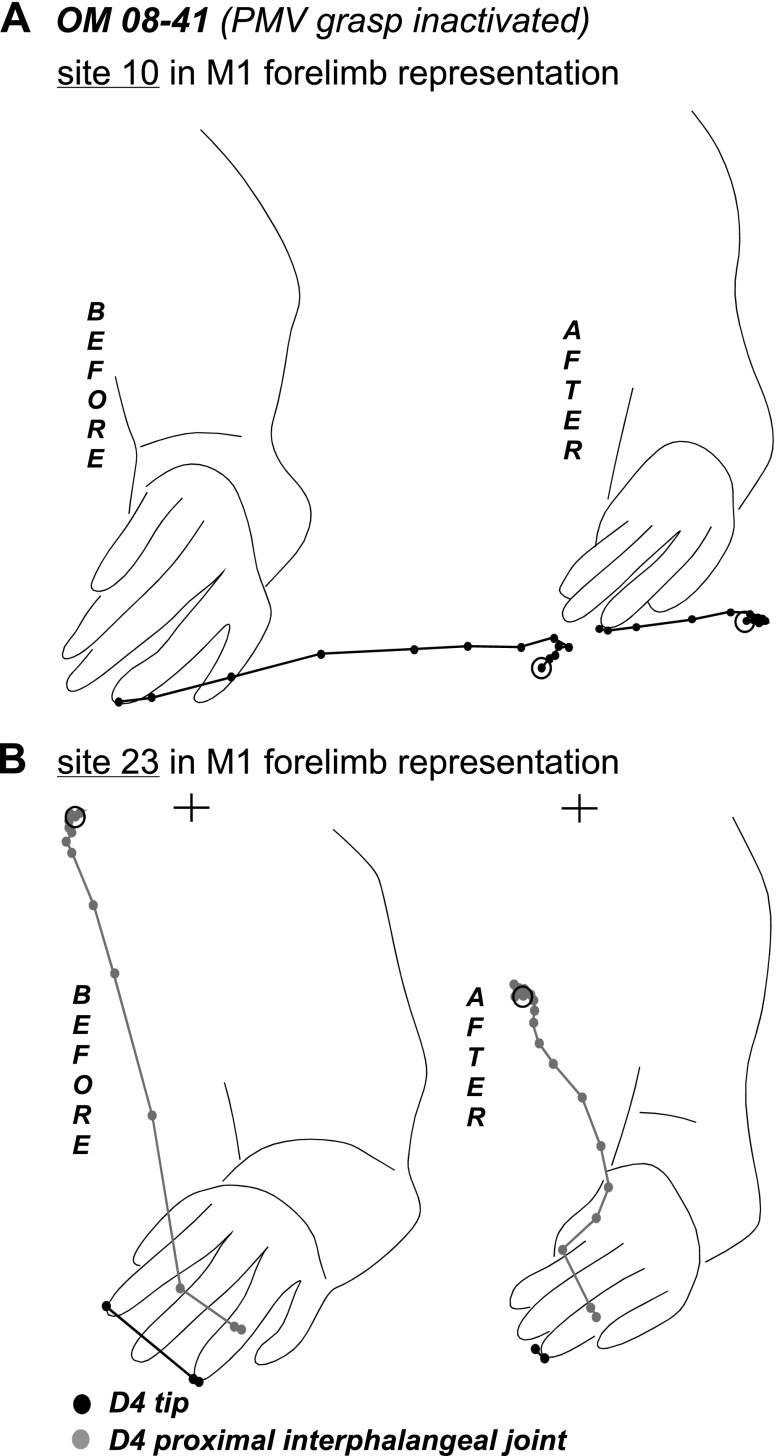
In owl monkey OM 08-41 (Fig. 11), a single muscimol injection inactivated the PMV grasp domain. Forty-four sites distributed across PMV, PPC, PMD, and M1 were retested over the course of 3 h after muscimol injections. A PMV site that was dorsal and caudal to the inactivated territory evoked grasping and concurrent arm lifting, suggesting only partial inactivation of the PMV grasp domain. Although the muscimol injection may have encroached into the face representation, most face sites in PMV were fully responsive during retesting. Other PMV sites were also unaltered. The three sites retested in PMD continued to evoke reaching and defensive forelimb movements albeit with slightly higher current amplitudes.
Only mild effects of PMV inactivation were observed in M1, and these were largely exclusive to M1 grasping sites. Originally, grasping movements were evoked from two clusters of sites in M1. One cluster was in caudocentral aspects of the M1 forelimb representation and may have overlapped area 3a. The second cluster of sites was more lateral, near the face representation. None of the grasping sites retested in caudocentral M1 were suppressed, but two of three lateral grasping sites were unresponsive even with current amplitudes up to 250 μA. Although the type of movements evoked from other forelimb sites in M1 (reaching, defensive, or hand-to-body) was unchanged during retesting, these movements were weaker and in some instances required higher current amplitudes. Tracings of the trajectories of the reaching movements evoked from site 10 near the M1/PMD border showed that movements were shorted during retesting (Fig. 12A). Shorter movement trajectories were also observed during retesting of site 23 in the center of the M1 forelimb representation. More specifically, site 23 originally evoked a grasp with hand-to-mouth movements, which was truncated during retesting as the hand failed to reach the mouth (Fig. 12B). Four face sites were retested in M1. The two sites in rostral M1 near the PMV border were not suppressed, whereas the two sites near border with 3a were unresponsive.
Fig. 12.
Trajectories of reaching movement evoked from site 10 and hand-to-mouth movement evoked from site 23 in M1 forelimb representation of OM 08–41 (A). Trajectories show the original movement and the movement evoked during inactivation of the PMV grasp domain. Note the truncated trajectories of both movements evoked after muscimol inactivation. In B “+” marks the mouth position. Other conventions as in Figs. 6 and 9.
As in other cases with partial inhibition of frontal motor regions, suppression was widespread in PPC. Thus all forelimb sites, representing grasping, but also reaching, and defensive movements were unresponsive. In contrast, hindlimb sites in PPC were not suppressed.
PPC Inactivation
The effects of PPC inactivation on other sensorimotor areas were investigated in owl monkey, OM 08-21. Changes at other sites in PPC were observed, but sites in PMC and M1 were unchanged.
The PPC reach domain was inactivated with two muscimol injections (Fig. 11B). Fifty-six sites distributed across PPC, PMD, PMV, and M1 were retested over the course of 3 h after inactivation. The reach domain was not completely inactivated since reaching movements could still be evoked from at least two sites in rostral aspects of the domain. The other 18 forelimb sites retested in PPC remained responsive during retesting, and their current thresholds were not much different from the original testing results. However, the sequence of the motor responses were altered for some of these sites during retesting, particularly for sites within grasping domain, which is closely associated with the reach domain (Gharbawie et al. 2011a). For example, the grasping movements originally evoked from two lateral sites 60 and 61, changed during retesting such the forelimb was withdrawn immediately following the grasp. In contrast, the grasping followed by forelimb withdrawal originally evoked from sites 110 and 116 changed during retesting such that the evoked movement was reduced to forelimb withdrawal.
Forelimb defensive sites in PMD as well as hand-to-mouth and face sites in PMV were unaltered during retesting and their thresholds remained the same. All M1 sites including grasping, reaching, defensive, hand-to-mouth, and face were also unaltered during retesting. Only 1 site (38) of the 21 sites retested in M1 evoked a different movement sequence during retesting. This forelimb site was near the border of the face representation in M1, and its original forelimb to body movement changed into a grasp followed by hand-to-body movement.
Trajectories of Evoked Movements
We investigated the possibility that sites not suppressed during muscimol inhibition evoked movements that were qualitatively different from the original movements. Thus we traced trajectories from our video records of the original movements and movements evoked during retesting. A video camera positioned in front of the animal allowed us to trace movement trajectories in two dimensions, and the position of the mirror (see materials and methods) facilitated three-dimensional tracing of movement trajectories for some of the recorded movements. The entire video arrangement was consistent for the duration of each stimulation session for each case, so the original and retested trajectories could be reliably compared.
Hand trajectories traced before and after muscimol for eight sites (7 in M1 and 1 in PMV) are shown in Figs. 6, 8–10, and 12. Movements evoked from these sites drove the hand toward various spatial locations. In those examples, sites that evoked the consistent movements before muscimol during retesting produced a movement sequence that was truncated relative to the movements originally evoked. More significant effects were obvious for sites that evoked a completely different sequence of movements after muscimol, which was reflected in the trajectories of the hand (e.g., SM 08–09, site 27, Fig. 9B).
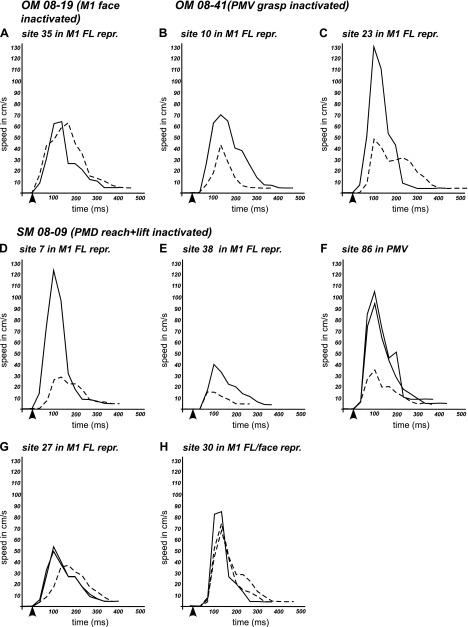
Frame-by-frame comparisons showed that the speeds of the evoked movements were in some instances reduced during retesting after muscimol, even though spatial trajectories may have been comparable to the original movements. Figure 13 shows speed profiles for each stimulation site before (continuous lines) and after (dashed lines) muscimol injections, which followed an approximate Gaussian distribution. Speed profiles recorded for multiple trials from the same site were very similar or even identical before or after muscimol (e.g., SM 08–09, site 27, Fig. 13G). Slightly different speed profiles were obtained for trials before muscimol for which start position of hand differed (SM 08–09, site 86, Figs. 10 and 13F). Overall speed profiles were reduced after muscimol (Fig. 13). Typically greater peak speeds characterized longer movement trajectories (e.g., hand-to-mouth). Thus the truncated movements recorded during retesting had lower peak speeds. For some stimulated sites, such as OM 08–19 site 35 and SM 08–09 site 30, peak speeds after muscimol remained similar (Fig. 13, A and H). For most movements, the peak speed was achieved 100–133 ms after stimulus onset, and this time profile was comparable for movements evoked before and after muscimol injections. Stimulation-evoked movements were initiated during the first two video frames (0–66 ms) from the start of electrical stimulation. This movement initiation profile was generally conserved during retesting. Only in two instances (OM 08–41, site 23 and SM 08–09, site 7; Fig. 13, C and D) was a delay detected and it amounted to a single video frame.
Fig. 13.
Speed profiles of hand movements trajectories traced from selected sites in M1 and PMV in cases with muscimol inactivation of M1 face domain (A), PMV grasp domain (B and C), and PMD reach domain (D–H). Each graph includes the speed profile of the original movement (solid line) and movement evoked during retesting (dashed line). Black arrowheads indicate the start of microstimulation train. Note the lower peak speeds for most movements evoked after muscimol application.
Summary
The results of the experiments described above are schematically summarized in Fig. 14. Inactivation of the M1 forelimb domains (Fig. 14, A and B) completely suppressed most forelimb domains in PPC and partially suppressed the matching domains in PMD and PMV, but face and hindlimb domains remained responsive during retesting in all areas. Inactivation of the M1 face domain (Fig. 14C) totally suppressed face movements in PPC, and higher current amplitudes were necessary to evoke face movements from PMV. Forelimb domains in M1 and most forelimb as well as hindlimb domains in PPC remained responsive during retesting. Inactivation of the PMD reach domain (Fig. 14D) suppressed most forelimb domains (reach and others) in PPC, whereas face movements were preserved. All domains in M1, PMV, and other domains of PMD were responsive during retesting after muscimol. Inactivation of the PMV grasp domain (Fig. 14E) suppressed forelimb domains in PPC but spared the hindlimb domains (the PPC face domain was not tested). Other forelimb domains sites in PMV remained responsive during retesting, as did forelimb domains in PMD. A more complex effect of PMV inactivation was observed in M1, where a couple of grasp sites were suppressed and other movements became weaker. Thus the contribution of PMV seems to be important for some of the movements represented in M1. Inactivation of the PPC reach domain (Fig. 14F) produced the most subtle effects as it did not suppress any domains beyond the inactivated territory, although it altered some forelimb sites in PPC. Importantly, reaching movements could still be evoked from M1 and PMD.
Fig. 14.
Summary results of inactivation of functional domains in frontal (M1, PMD, and PMV) and posterior parietal (PPC) cortex. Inactivation of reach and grasp domains in M1 (A and B), face domain in M1 (C), the PMD reach domain (D), the PMV grasp domain (E), and the PPC reach domain (F). Hamilton syringes depict the approximate spatial center of the muscimol injections and the surrounding circle with dots depicts the inactivated territory. Presence or lack of movements after cortical inactivation are indicated by “+” or “-” shown on color circles corresponding to particular movement domains (see Figs. 2 and 3). Solid lines mark areal borders, and dashed lines mark borders between body representations. Other conventions as in Figs. 2 and 3.
The trajectories of movements evoked from some M1, PMD, and PPC forelimb sites (especially those matching inactivated domain) during retesting were truncated and they were slower relative to the original sequence, or a different movement was evoked altogether. Movements from PPC, M1, PMD, and PMV during retesting were otherwise comparable to the original.
DISCUSSION
We previously demonstrated that matching functional domains in M1, PMC, and PPC are interconnected and form parallel networks that mediate specific ethologically relevant behaviors (Gharbawie et al. 2011a; Stepniewska et al. 2009a). Our concept of parietal-frontal networks for specialized actions is consistent with the results of others (e.g., Johnson et al. 1996; Matelli and Luppino 2001; Rizolatti et al. 1998; Tanne-Gariepy et al. 2002) and predicts that damage to a network would specifically affect the movements relevant to the action represented by this network. To test this hypothesis, we investigated the effects of inactivations with muscimol of particular movement domains in M1, PMC, and PPC on complex movements evoked by intracortical microstimulation. We assumed that the immediate effects of deactivating sensorimotor domains more clearly reveal the normal roles of these domains in more extensive networks compared with permanent lesions. Permanent lesions are usually followed by reorganization of local and other brain networks, behavioral compensations, and other mechanisms of recovery (e.g. Dancause et al. 2005; Frackowiak et al. 1991; Frost et al. 2003; Jeannerod et al. 1994; Liu and Rouiller 1999; Nudo et al. 1996; Padberg et al. 2010; Ward and Frackowiak 2006) that may obscure the normal roles of domains in networks. We favored electrical stimulation in anesthetized primates over behavioral testing in awake primates to keep the movement patterns under experimental control and again minimize the possibility of voluntary compensatory strategies that could complicate interpretation of the results.
This study produced several important findings. First, the results were consistent with our model of hierarchically related domains in functionally distinct, parallel parietal-frontal networks. Evoked movements from PPC domains were eliminated by blocking matching M1 domains, whereas blocking PPC domains had no notable effects on stimulating matching frontal cortex M1 or PMC domains. Second, our results provide evidence that domains of different functional classes interact. This was particularly apparent in experiments in which the movement patterns evoked from a given M1 domain were altered in response to inactivation of an adjacent, but functionally distinct, M1 domain. This alteration could simply be the result of a slight decrease in excitation within the adjacent domain caused by diffusion of the muscimol. Alternatively, altered movements could reflect the blocking of lateral intrinsic projections from the inactivated M1 domain. These lateral connections would likely be excitatory, but they could be terminating mainly on inhibitory neurons (see Douglas and Martin 2004). However, inactivation of a specific domain in M1 had more widespread effects in PMC and even wider in PPC, where the stimulation of unmatched domains produced altered movements or failed to produce movements. This suggests that even a partial depression of activity caused by the diffusion of muscimol in M1 to nearby domains may counter the effects of the activation of many PPC-M1 connections. Alternatively, these wide spread effects in PPC, and to a lesser extent in PMC, could be the product of alterations in less feedback from M1 (Reed et al. 2011). Third, direct projections from PMC and from PPC to motor neurons in the brainstem and spinal cord are not sufficient to evoke complex movements independently of M1, as stimulating domains in these regions was ineffective when the matching domains in M1 was blocked. Collectively, our findings support a framework in which the connections of PPC and PMC with each other and with M1 are critical in producing complex movements.
Connections Between Posterior Parietal and Frontal Motor Regions
The present results support our hypothesis that PPC, PMC, and M1 consist of a number of domains that are hierarchically related and form functionally distinct, mostly segregated parietal-frontal networks. M1 seems to be a critical link in these networks, since the outputs of M1 are essential to the behavior, whereas PPC and PMC mediate complex movements via their connections with M1. Such results are consistent with known patterns of connections between PPC, PMC, and M1.
Our previous connectional studies showed that in galagos and New World monkeys PMC and PPC have numerous reciprocal corticocortical connections, and both regions are also connected with M1 (Fang et al. 2005; Stepniewska et al. 1993, 2006b). Dorsal and ventral divisions of PMC have direct dense and topographical connections with M1. The connections of PPC with M1 are less dense and are limited to the most anterior parts of PPC. However, PPC may communicate with M1 indirectly via PMC. Our recent studies confirmed that the pathways that connect PPC-PMC-M1 are domain specific. By injecting tracers into functional domains in M1-PMC and PPC identified with ICMS in galagos (Stepniewska et al. 2005, 2009a,b) and New World monkeys (Gharbawie et al. 2011a), we determined that domains in the PPCr and frontal motor cortex (M1-PMC), where different classes of complex movements can be evoked, are preferentially interconnected. In line with these findings, stimulation of PPC domains selectively activated matching domains in M1-PMC as shown by our optical imaging studies (Stepniewska et al. 2011).
Our combined neurophysiological and neuroanatomical approach allowed us to identify a number of the parietal-frontal networks that support different actions in both galagos and New World monkeys (Gharbawie et al. 2011a; Stepniewska et al. 2005, 2009a,b). For example, a dorsal parietal-frontal network interconnecting caudomedial PPC with PMD and M1 is involved in reaching, and a more ventral parietal-frontal network interconnecting rostrolateral PPC, PMV, and M1 is involved in grasping. All networks are further characterized by inputs from other cortical and subcortical structures, but M1, PMC, and PPC form the core of these networks. These regions combine visual signals with other sensory and motor signals and build motor commands for reaching, grasping, and other behaviors. Commands from PMC and from PPC (mostly via PMC) are passed onto the motor cortex, which sends long axons down to the brainstem and the spinal cord to synapse directly or indirectly (via interneuron circuitry) onto the motor neurons for movement execution. Thus suppression or alteration of movements evoked from PMC and PPC after muscimol injections in M1 domains and suppression of movements evoked from PPC domains after muscimol injections in PMC are in agreement with the expected roles of well established anatomical connections. Altogether our results suggest a hierarchical relationship of cortical areas and their domains, with M1 domains being central to movement completion (see also Kakei et al. 2001). This hierarchical organization is also in agreement with results of our previous ICMS studies that demonstrated longer latencies for movements evoked from PPC than from M1 and PMC (Stepniewska et al. 2009a).
A main finding of the present study is that inactivations of M1 functional domains suppressed functionally matched domains in PMC and PPC but had much milder effects on the functions of nonmatching domains. This finding provides further evidence for neuronal clusters (domains) in M1, PMC, and PPC that are interconnected in largely segregated networks specialized for specific behaviors. There is considerable evidence for existence of similar, partially independent, parietal-frontal networks involved in specific motor actions in macaques (see Johnson et al. 1996; Luppino et al. 1999; Matelli and Luppino 2001; Rizzolatti et al. 1998; Wise et al. 1997). Physiological studies have shown that different classes of neurons encoding information relevant to reaching, grasping, or other behaviors are common to parietal and frontal regions, which are also linked by reciprocal connections (Battaglia-Mayer et al. 2001; Caminiti et al. 1991, 1999; Hepp-Reymond et al. 1994; Jeannerod et al. 1995; Kalaska 1996; Marconi et al. 2001; Mushiake et al. 1999; Shipp et al. 1998; Rizzolatti et al. 1988; Sakata et al. 1999; Stanton et al. 1995; Tanne-Gariepy et al. 2002). Most recently, connections between parietal and frontal cortical areas representing grasping (Gharbawie et al. 2011b) or defensive behavior (Cooke et al. 2003; Cooke and Graziano 2004; Graziano et al. 2002b), as defined by long-train ICMS, have been described as well.
The segregation of functional parietal-frontal networks, however, is not complete. Our present results show that although inactivation of a particular domain in a sensorimotor area (e.g., M1) does not suppress the movements evoked from adjacent domains within the area, it may alter their trajectories. Thus domains of different classes interact with each other. The interactions between functional motor networks (e.g., reach and grasp networks) are supported by the existence of common inputs from some parietal areas, as well as by intrinsic connections within PPC, PMC, and M1 (Gharbawie et al. 2011b). Such interlinks between networks may represent channels for coordinating different functions of the forelimb (e.g., reach to grasp). We previously suggested that the excitatory connections between functional domains within a region largely terminate onto inhibitory neurons as a way to reduce the probability of generating competing behaviors (Kaas et al. 2011, 2012). Reducing intraregional inhibition by blocking the outputs of any specific domain would be expected to reduce activation thresholds for other domains, but this possibility has not been systematically evaluated. However, our present results provide new evidence for the interaction of the parallel networks in ways that modify the outputs of each other.
Corticospinal Projections
The corticospinal (pyramidal) system is critical for motor functions in primates. About half of the corticospinal fibers arise in M1, but other frontal areas such as PMD and PMV, as well as parietal areas, also contribute axons to the corticospinal tract (Dum and Strick 1991, 1996; Galea and Darian-Smith 1994; He et al. 1993; Nudo and Masterton 1990; Wise 1996; Wu and Kaas 2000). Each population of corticospinal neurons has terminals in the intermediate zone of spinal segments, whereas direct projections onto motoneurons of the ventral horn originate primarily from M1 (Rathelot and Strick 2009). More numerous corticospinal projections from M1 are reflected in its higher excitability and lower stimulation thresholds for evoking movements compared with PMC or PPC (Kuypers 1981; Porter and Lemon 1993). Although the contributions of premotor and parietal areas to corticospinal pathways are less than that of M1, these areas have the potential to influence the generation and control of movements independently, but the extent of this control is not understood. Our results show that direct projections from PMC and PPC to motor neurons in the spinal cord (and brainstem) are not sufficient for evoking complex movements when stimulated, since stimulation of domains in these regions is ineffective during the inactivation of matching M1 domains. Thus corticospinal projections from nonprimary motor areas (including PPC) are not normally of critical importance to complex movements. The cortical connections of PPC with PMC and M1, as well as the PMC connections with M1, appear to be more important for the initiation and guidance of complex motor behaviors.
Relation to Previous Studies
To examine the role of the motor cortical regions in the acquisition, control, and execution of specific movements, researchers have studied behavioral deficits after permanent lesions of these regions as well as after their local inactivation with muscimol. Here, using muscimol to inactivate a functional domain, we show that the functions of a domain in a cortical region that belongs to the parietal-frontal network is dependent on the integrity of matched domains in other cortical regions of the same network. This result is consistent with earlier findings in macaques, where muscimol injections into any node of the manual dexterity network such as the M1 hand representation (Brochier et al. 1999; Kubota 1996; Kermadi et al. 1997; Matsumura et al. 1991; Rouiller et al. 1998; Schieber and Poliakov 1998; Fogassi et al. 2001), rostral PMV (Fogassi et al. 2001, but see also Schieber 2000), or AIP (Gallese et al. 1994; Kermadi et al. 1997) impair hand movements, especially those demanding fine control of the digits.
In the present study, we also show that movements produced by stimulating PMC or PPC depend on the integrity of matching domains in M1. This is in agreement with the results of Schmidlin et al. (2008) who have shown that inactivation of the M1 hand area in macaque monkeys greatly reduced or completely blocked the motor effects evoked by microstimulation of PMV. Thus motor functions of PMV were dependent on the integrity of M1 and were likely mediated by connections between the PMV and M1 (Dum and Strick 2005), as suggested by the short latency and brief time course of the PMV-M1 interaction, and the blockade of this interaction by muscimol injections in M1 (Cerri et al. 2003; Shimazu et al. 2004). Similarly, permanent lesions of M1 in macaque monkeys blocked the motor effects that are typically evoked from nonprimary motor areas (Amassian et al. 1987; Wiesendanger et al. 1987). Moreover, it has been shown that stimulation of these nonprimary motor areas can activate M1 corticospinal neurons indirectly (Amassian et al. 1987; Maier et al. 2002; Shimazu et al. 2004). In a study by Shimazu et al. (2004), stimulation of PMV, which by itself evoked little or no detectable corticospinal output, produced a robust modulation of motor outputs from M1. These studies collectively imply that the roles of nonprimary motor areas in generation of complex movements are largely dependent on the integrity of M1.
Although our inactivation of domains in PMC did not block the movements evoked from M1 (it blocked movements from PPC), it rendered them weaker with higher stimulation thresholds than before the muscimol injection. Other notable changes for most movements evoked from M1 sites retested after muscimol injections in PMC were decreased velocity of movements and shortened hand trajectories. Similar decreases in amplitude and velocity of forelimb movements were found in behaving macaques following local muscimol injections into PMV, and to a lesser degree into PMD, as well as M1 (Kurata and Hoffman 1994; Matsumura et al. 1991). Trajectory errors in reaching (over-reach, under-reach, and increased path curvature) (Martin and Ghez 1999) and in movements of the wrist in different directions (step-tracking) (Hoffman and Strick 1995) were reported also in monkeys after M1 partial inactivation or permanent lesion, respectively.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-164464 (to J. H. Kaas), NS-055843 (to I. Stepniewska), and NS-079471 (to O. A. Gharbawie) and a Postdoctoral Fellowship from the Canadian Institutes of Health (to O. A. Gharbawie).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.S. and J.H.K. conception and design of research; I.S., O.A.G., and M.J.B. performed experiments; I.S. analyzed data; I.S. interpreted results of experiments; I.S. prepared figures; I.S. drafted manuscript; I.S., O.A.G., and J.H.K. edited and revised manuscript; I.S., O.A.G., M.J.B., and J.H.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mary Feurtado and Laura Trice for technical assistance.
Present address of M. J. Burish: Department of Neurology, University of California, San Francisco, CA 94143.
REFERENCES
- Amassian VE, Cracco RQ. Human cerebral cortical responses to contralateral transcranial stimulation. Neurosurgery 20: 148–155, 1987 [PubMed] [Google Scholar]
- Arikan R, Blake NM, Erinjeri JP, Woolsey TA, Giraud L, Highstein SM. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. J Neurosci Meth 118: 51–57, 2002 [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex 11: 528–544, 2001 [DOI] [PubMed] [Google Scholar]
- Brochier T, Boudreau MJ, Paré M, Smith AM. The effects of muscimol inactivation of small regions of motor and somatosensory cortex on independent finger movements and force control in the precision grip. Exp Brain Res 128: 31–40, 1999 [DOI] [PubMed] [Google Scholar]
- Caminiti R, Johnson PB, Galli C, Ferraina S, Burnod Y. Making arm movements within different parts of space: the premotor and motor cortical representation of a coordinate system for reaching to visual targets. J Neurosci 11: 1182–1197, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Ferraina S, Johnson PB. The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cereb Cortex 6: 319–328, 1996 [DOI] [PubMed] [Google Scholar]
- Caminiti R, Genovesio A, Marconi B, Mayer AB, Onorati P, Ferraina S, Mitsuda T, Giannetti S, Squatrito S, Maioli MG, Molinari M. Early coding of reaching: frontal and parietal association connections of parieto-occipital cortex. Eur J Neurosci 11: 3339–3345, 1999 [DOI] [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol 90: 832–842, 2003 [DOI] [PubMed] [Google Scholar]
- Collins CE, Xu X, Khaytin I, Kaskan PM, Casagrande VA, Kaas JH. Optical imaging of visually evoked responses in the middle temporal area after deactivation of primary visual cortex in adult primates. Proc Natl Acad Sci USA 102: 5594–5599, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DF, Graziano MS. Sensorimotor integration in the precentral gyrus: Polysensory neurons and defensive movements. J Neurophysiol 91: 1648–1660, 2004 [DOI] [PubMed] [Google Scholar]
- Cooke DF, Taylor CSR, Moore T, Graziano MS. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci USA 100: 6163–6168, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci 25: 10167–10179, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci 27: 419–451, 2004 [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: 667–689, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci 16: 6513–6525, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci 25: 1375–1386, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol 490: 305–333, 2005 [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: A reversible inactivation study. Brain 124: 571–586, 2001 [DOI] [PubMed] [Google Scholar]
- Frackowiak RS, Weiller C, Chollet F. The functional anatomy of recovery from brain injury. Ciba Found Symp 163: 235–244, 1991 [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol 89: 3205–3214, 2003 [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations and connections. Cereb Cortex 4: 166–194, 1994 [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Sakata HN. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport 5: 1525–1529, 1994 [DOI] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res 1: 203–209, 1979 [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science 233: 1416–1419, 1986 [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Kaas JH. Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in New World monkeys. Cereb Cortex 21: 1981–2002, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Qi H, Kaas JH. Multiple parietal-frontal pathways mediate grasping in macaque monkeys. J Neurosci 31: 11660–11677, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS. The organization of behavioral repertoire in motor cortex. Annu Rev Neurosci 29: 105–134, 2006 [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851, 2002a [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T, Cooke DF. The cortical control of movement revisited. Neuron 36: 349–362, 2002b [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci 13: 952–980, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Hüsler EJ, Maier MA, Ql HX. Force-related neuronal activity in two regions of the primate ventral premotor cortex. Can J Physiol Pharmacol 72: 571–579, 1994 [DOI] [PubMed] [Google Scholar]
- Hoffman DS, Strick PL. Effects of a primary motor cortex lesion on step-tracking movements of the wrist. J Neurophysiol 73: 891–895, 1995 [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Decety J, Michel F. Impairment of grasping movements following a bilateral posterior parietal lesion. Neuropsychologia 32: 369–380, 1994 [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18: 314–320, 1995 [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119, 1996 [DOI] [PubMed] [Google Scholar]
- Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Front Neuroanat 5: 1–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Stepniewska I, Gharbawie O. Cortical networks subserving upper limb movements in primates. Eur J Phys Rehabil Med 48: 299–306, 2012 [PMC free article] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Direction of action is represented in the ventral premotor cortex. Nat Neurosci 4: 1020–1025, 2001 [DOI] [PubMed] [Google Scholar]
- Kalaska JF. Parietal cortex area 5 and visuomotor behavior. Can J Physiol Pharmacol 74: 483–498, 1996 [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Tempini A, Rouiller EM. Effects of reversible inactivation of the supplementary motor area (SMA) on unimanual grasp and bimanual pull and grasp performance in monkeys. Somat Mot Res 14: 268–280, 1997 [DOI] [PubMed] [Google Scholar]
- Kubota K. Motor cortical muscimol injection disrupts forelimb movement in freely moving monkeys. Neuroreport 7: 2379–2384, 1996 [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoffman DS. Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J Neurophysiol 71: 1151–1164, 1994 [DOI] [PubMed] [Google Scholar]
- Kuypers HG. Anatomy of the descending pathways. In: Handbook of Physiology. The Nervous System, Motor Control. Bethesda, MD: Am Physiol Soc, sect 1, vol II, 1981, p. 597–666 [Google Scholar]
- Lewis J, Van Essen D. Corticocortical connections of visual sensorimotor, multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428: 112–137, 2000 [DOI] [PubMed] [Google Scholar]
- Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res 128: 149–159, 1999 [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp Brain Res 128: 181–187, 1999 [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex 12: 281–296, 2002 [DOI] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex 11: 513–527, 2001 [DOI] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127: 160–164, 1991 [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 86: 145–159, 1999 [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Differential impairments in reaching and grasping produced by local inactivation within the forelimb representation of the motor cortex in the cat. Exp Brain Res 9: 429–443, 1993 [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage 14: S27–S32, 2001 [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Oishi T, Ueki K, Kubota K. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor and premotor cortex. J Neurophysiol 65: 1542–53, 1991 [DOI] [PubMed] [Google Scholar]
- Mushiake H, Fujii N, Tanji J. Microstimulation of the lateral wall of the intraparietal sulcus compared with the frontal eye field during oculomotor tasks. J Neurophysiol 81: 1443–1448, 1999 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord, III sites of origin of the corticospinal tract. J Comp Neurol 296: 559–583, 1990 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75: 2144–2149, 1996 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, Sifuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272: 1791–1794, 1996 [DOI] [PubMed] [Google Scholar]
- Padberg J, Recanzone G, Engle J, Cooke D, Goldring A, Krubitzer L. Lesions in posterior parietal area 5 in monkeys result in rapid behavioral and cortical plasticity. J Neurosci 30: 12918–12935, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal function and voluntary movement. In Monographs of the Physiological Society, No 45 Oxford, UK: Oxford Univ Press, 1993 [Google Scholar]
- Preuss TM, Stepniewska I, Kaas JH. Movement representation in the dorsal and ventral premotor areas of owl monkey: a microstimulation study. J Comp Neurol 371: 649–676, 1996 [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA 106: 918–923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Qi H, Kaas JH. Spatiotemporal properties of neuron response suppression in owl monkey primary somatosensory cortex when stimuli are presented to both hands. J Neurosci 31: 3589–3601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina GA, Moran DW, Schwartz AB. On the relationship between joint angular velocity and motor cortical discharge during reaching. J Neurophysiol 85: 2576–2589, 2001 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106: 283–296, 1998 [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Yu XH, Moret V, Tempini A, Wiesendanger M, Liang F. Dexterity in adult monkeys following early lesion of the motor cortical hand area: the role of cortex adjacent to the lesion. Eur J Neurosci 10: 729–740, 1998 [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Kusunoki M, Murata A, Tsutsui K, Tanaka Y, Shein WN, Miyashita Y. Neural representation of three-dimensional features of manipulation objects with stereopsis. Exp Brain Res 128: 160–169, 1999 [DOI] [PubMed] [Google Scholar]
- Schieber MH. Inactivation of the ventral premotor cortex biases the laterality of motoric choices. Exp Brain Res 130: 497–507, 2000 [DOI] [PubMed] [Google Scholar]
- Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci 18: 9038–9054, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin E, Wannier T, Bloch J, Rouiller EM. Progressive plastic changes in the hand representation of the primary motor cortex parallel incomplete recovery from a unilateral section of the corticospinal tract at cervical level in monkeys. Brain Res 1017: 172–183, 2004 [DOI] [PubMed] [Google Scholar]
- Schmidlin E, Brochier T, Maier MA, Kirkwood PA, Lemon RN. Pronounced reduction of digit motor responses evoked from macaque ventral premotor cortex after reversible inactivation of the primary motor cortex hand area. J Neurosci 28: 5772–5783, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci 24: 1200–1211, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S, Blanton M, Zeki S. A visuo-somatomotor pathway through superior parietal cortex in the macaque monkey: cortical connections of areas V6 and V6A. Eur J Neurosci 10: 3171–3193, 1998 [DOI] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ. Reversible inactivation of macaque frontal eye field. Exp Brain Res 116: 229–249, 1997 [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol 353: 291–305, 1995 [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol 330: 238–271, 1993 [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci USA 102: 4878–4883, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Collins CE, Burish MJ, Kaas JH. Intracortical microstimulation uncovers map of complex movements in the posterior parietal cortex of New World owl monkeys. Soc Neurosci Absts 36: 2006a [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Ipsilateral cortical connections of dorsal and ventral premotor areas in New World owl monkeys. J Comp Neurol 495: 691–708, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Burish MJ, Gharbawie OA, Kaas JH. Mapping the frontal motor region in New World monkeys with long train intracortical microstimulation. Soc Neurosci Absts 38: 2008a [Google Scholar]
- Stepniewska I, Gharbawie OA, Burish MJ, Kaas JH. Deactivation of primary motor cortex abolishes movements evoked from premotor and posterior parietal areas in primates. FENS Absts 4: 2008b [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Organization of the posterior parietal cortex in prosimian galago: I. Functional zones identified by microstimulation. J Comp Neurol 517: 765–782, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Cerkevich C, Kaas JH. Organization of the posterior parietal cortex in prosimian galago: II. Ipsilateral cortical connections of sensori-motor region. J Comp Neurol 517: 783–807, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Friedman RM, Gharbawie OA, Cerkevich CM, Roe AW, Kaas JH. Optical imaging in galagos reveals parietal-frontal circuits underlying motor behavior. Proc Natl Acad Sci USA 108: E725–732, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Bello A, Fadiga L, Rizzolatti G. Parietal inputs to dorsal vs. ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res 145: 91–103, 2002 [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and time course of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Meth 74: 17–26, 1997 [DOI] [PubMed] [Google Scholar]
- Thier P, Andersen RA. Electrical microstimulation distinguishes saccade-related areas in the posterior parietal cortex. J Neurophysiol 80: 1713–1735, 1998 [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. The functional anatomy of cerebral reorganisation after focal brain injury. J Physiol (Paris) 99: 425–436, 2006 [DOI] [PubMed] [Google Scholar]
- Wiesendanger M, Hummelsheim H, Bianchetti M, Chen DF, Hyland B, Maier V, Wiesendanger R. Input and output organization of the supplementary motor area. Ciba Found Symp 132: 40–62, 1987 [DOI] [PubMed] [Google Scholar]
- Wise SP. Corticospinal efferents of the supplementary sensorimotor area in relation to the primary motor area. Adv Neurol 70: 57–69, 1996 [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997 [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 171: 11–28, 1979 [DOI] [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol 423: 140–177, 2000 [DOI] [PubMed] [Google Scholar]