Abstract
Epidural stimulation (ES) of the lumbosacral spinal cord has been used to facilitate standing and voluntary movement after clinically motor-complete spinal-cord injury. It seems of importance to examine how the epidurally evoked potentials are modulated in the spinal circuitry and projected to various motor pools. We hypothesized that chronically implanted electrode arrays over the lumbosacral spinal cord can be used to assess functionally spinal circuitry linked to specific motor pools. The purpose of this study was to investigate the functional and topographic organization of compound evoked potentials induced by the stimulation. Three individuals with complete motor paralysis of the lower limbs participated in the study. The evoked potentials to epidural spinal stimulation were investigated after surgery in a supine position and in one participant, during both supine and standing, with body weight load of 60%. The stimulation was delivered with intensity from 0.5 to 10 V at a frequency of 2 Hz. Recruitment curves of evoked potentials in knee and ankle muscles were collected at three localized and two wide-field stimulation configurations. Epidural electrical stimulation of rostral and caudal areas of lumbar spinal cord resulted in a selective topographical recruitment of proximal and distal leg muscles, as revealed by both magnitude and thresholds of the evoked potentials. ES activated both afferent and efferent pathways. The components of neural pathways that can mediate motor-evoked potentials were highly dependent on the stimulation parameters and sensory conditions, suggesting a weight-bearing-induced reorganization of the spinal circuitries.
Keywords: human, epidural spinal stimulation, spinal-cord injury, evoked potentials
in humans, lumbosacral epidural electrical stimulation can facilitate standing and voluntary movement after complete motor paralysis (Harkema et al. 2011). It has also been shown that nonpatterned epidural stimulation (ES), applied to the lumbar spinal cord, can induce stepping-like patterns of electromyography (EMG) activity in leg muscles in individuals with complete spinal-cord injury (SCI) (Dimitrijevic et al. 1998; Gerasimenko et al. 2003; Harkema et al. 2011; Minassian et al. 2007b). We have begun to examine how the potentials evoked via an epidural electrode array are modulated in the spinal circuitry and projected to different motor pools. We also observed how the epidurally evoked potentials are modulated by sensory input projecting to a spinal cord that has not received any input from the brain for more than 2 yr. These observations provide the groundwork for understanding how the spinal circuitry of an injured spinal cord can respond to a range of pharmacological agonists and inhibitors of excitatory and inhibitory receptors known to be important in facilitating standing and stepping after a complete SCI in adult rats (Musienko et al. 2011).
We hypothesize that chronically implanted electrode arrays over the lumbosacral spinal cord in individuals with complete motor paralysis of the lower limbs can be used to assess functionally spinal circuitry linked to specific motor pools. To test this hypothesis, we have asked the following questions: 1) Are the modulatory features of epidurally evoked potentials spatially unique when the stimulus is induced at different anatomical points along the lumbosacral spinal cord relative to the anatomical location of the sensory-motor pathways being modulated? 2) To what degree can such spatially unique pathways be selectively activated by different stimulation configurations? 3) How is the composition of evoked muscle potentials affected by location and extension of the spinal-cord stimulation? 4) Are the potentials uniquely modulated when the subject is in a supine or standing position?
METHODS
Participants.
Three individuals with SCI participated in this study (Table 1). Participants provided written, informed consent for the experimental procedures, which were approved by the University of Louisville (Louisville, KY) and the University of California, Los Angeles (CA) Institutional Review Boards.
Table 1.
Characteristics of participants
| Motor Score |
Sensory Score |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant | Sex | Age at Implant | Years Since Injury | LOI | AIS | Right Arm | Left Arm | Right Light Touch | Left Light Touch |
| Right Leg | Left Leg | Right Pin Prick | Left Pin Prick | ||||||
| B07 | Male | 23 | 3.4 | T2 | B | 25 | 25 | 50 | 46 |
| 0 | 0 | 38 | 33 | ||||||
| A45 | Male | 24 | 2.2 | T4 | A | 25 | 25 | 24 | 22 |
| 0 | 0 | 22 | 22 | ||||||
| B13 | Male | 32 | 4.2 | C7 | B | 25 | 20 | 33 | 33 |
| 0 | 0 | 25 | 26 | ||||||
The motor score is based on the examination of 10 key muscles on each side. For each movement, force is measured and assigned a coefficient, from 0 (absence of muscle contraction) to 5, when contraction creates a movement in all of the joint amplitudes against a complete resistance. Light-touch and pin-prick sensitivity was assessed on a 0–2 scale at each of 28 dermatomes (0, absent; 1, impaired; 2, intact) on each side. The presented scores are given as a total out of 50 for each (left and right) side. LOI, neurological level of injury (most caudal segment with normal motor and sensory function, as per the International Standards for the Classification of Spinal Cord Injury); AIS, American Spinal Injury Association Impairment Scale.
Epidural electrical spinal stimulation.
A stimulation unit (RestoreAdvanced; Medtronic, Minneapolis, MN), in combination with a 16-electrode array (5-6-5; Medtronic), implanted at the T11–L1 vertebral levels over the spinal-cord segments L1–S2, as described previously (Harkema et al. 2011), was used to deliver electrical stimulation to the lumbosacral enlargement of the spinal cord (Fig. 1). The electrode array was positioned over the midline of the exposed dura. The neurosurgeon performed the initial placement based on anatomical landmarks and fluoroscopy. The array's location was also adjusted using electrophysiology during the surgery. Bilateral-evoked potentials from leg muscles were collected and evaluated in detail by spatial, temporal, and amplitude characteristics to optimize the location and symmetry of the electrode array placement. Once the electrode's position was optimized and confirmed, the array was sutured in place.
Fig. 1.
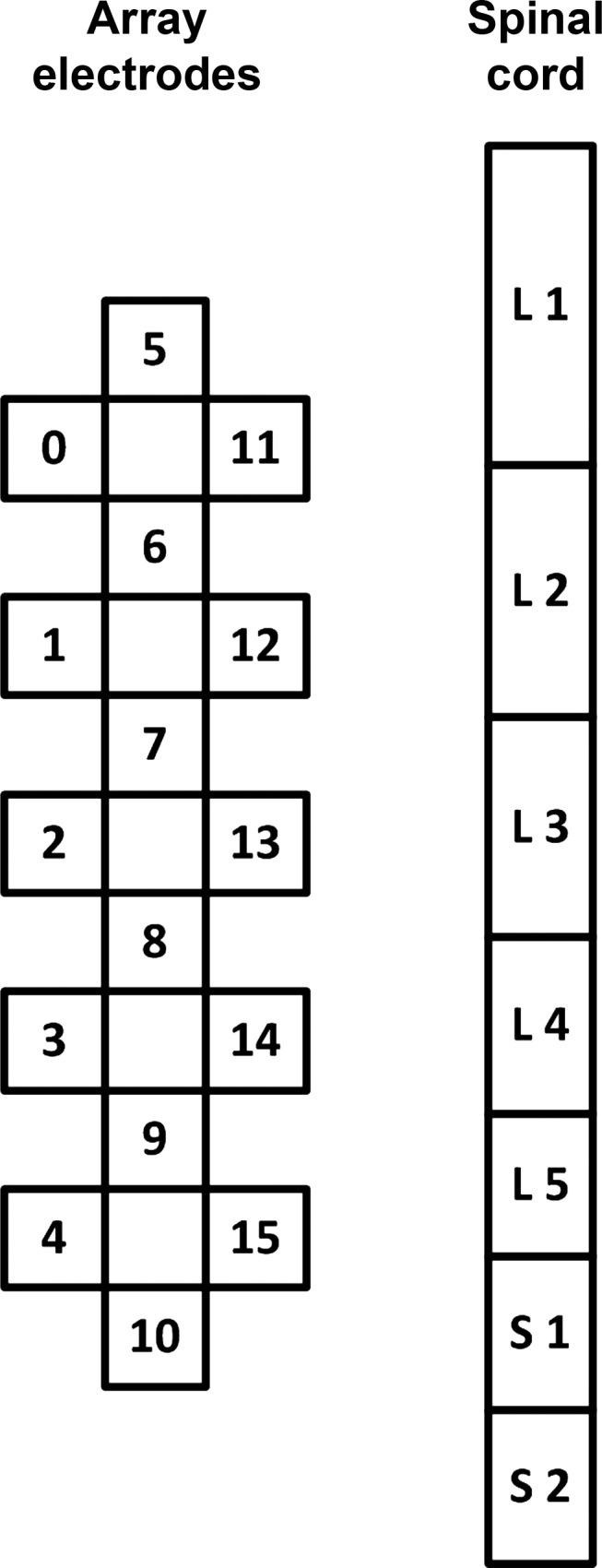
Schematic presentation of the 16-electrode array (left) and corresponding spinal-cord segments L1–S2 (right). The drawing is an estimation and might not be representative for each participant.
The evoked potentials to epidural spinal stimulation were investigated 2–3 wk after the surgery. The experiments were performed with the individual relaxed in a supine position. In participant A45, the data were also recorded during standing with a body weight load (BWL) of 60%. The Body Weight Support System (Innoventor, St. Louis, MO), with a harness, in combination with manual assistance, was used to provide body-weight support during standing. The stimulation current had a rectangular, biphasic pulse waveform with a pulse duration of 210 μs. With the use of 0.5-V increments, the stimulation intensity was increased from 0.5 to 10 V, or the maximum tolerable intensity, whichever was less. In some cases, the stimulation caused a tightness in the abdominal area at higher intensities; therefore, maximum intensity was kept below the level that would cause difficulty breathing. At each intensity, a minimum of five stimuli was delivered at a frequency of 2 Hz. Recruitment curves were collected at three localized bipolar (5−//6+, 8−//7+, 10−//9+) and two wide-field (0−/5−/11−//4+/10+/15+, 4−/10−/15−//0+/5+/11+) stimulation configurations (Fig. 1).
EMG recording and data collection.
Surface EMG signals were recorded bilaterally using bipolar surface electrodes (Motion Lab Systems, Baton Rouge, LA) that were placed longitudinally on the soleus (SOL), medial gastrocnemius (MG), tibialis anterior (TA), vastus lateralis (VL), rectus femoris, medial hamstrings (MH), and gluteus maximus (GL) muscles with a fixed interelectrode distance of 17 mm. EMG signals from the iliopsoas muscles (IL) were recorded with fine-wire electrodes (MA-416; Motion Lab Systems). In addition, two surface electrodes, placed symmetrically lateral to the electrode array incision site over the paraspinal muscles, were used to record the stimulation artifact, which was later used to define the onset of the stimulus. Reference electrodes were placed bilaterally over the distal part of the tibia bone. The EMG signals were differentially amplified using the MA300 EMG System (Motion Lab Systems) with a band-pass filter of 10 Hz–2 kHz (−3 dB). Finally, the EMG data were digitized at a sampling rate of 2,000 Hz. The power density of the signal was tested to ensure that there was negligible signal power beyond 500 Hz.
Data analysis.
The digitized MG, TA, VL, MH, GL, and IL EMG time series were full-wave rectified after subtraction of the mean background EMG. The latency of the response was defined as the time between the stimulus and the moment when the EMG activity reached levels higher than the mean baseline EMG, plus three times its SD. The magnitude of the evoked potentials was calculated by measuring the area under the curve across each component. Based on findings from previous studies (Andersen et al. 2003; Gerasimenko et al. 2006; Lavrov et al. 2006), three components of the evoked potentials, which are attributed to involvement of different pathways, were analyzed: early response (ER) and medium response (MR), as well as the long latency response (LLR). The onset of ER was determined based on the latency of the earliest response of a given muscle on each stimulation configuration across all stimulation intensities. The duration of ER was determined from the ensemble-averaged waveform displayed on a computer monitor; in case it was impossible to distinguish the ER and MR visually, the onset of MR was defined as 10 ms after the onset of ER, on the basis of reported latencies and durations (Gerasimenko et al. 2006; Lavrov et al. 2006). For the MR duration, a constant interval of 30 ms was used. In addition, the LLR was processed in a 60- to 250-ms poststimulation interval during wide-field stimulation configurations during supine and standing with a BWL of 60% in participant A45 (Andersen et al. 2003).
The evoked potential magnitude for each muscle was reported as a ratio, with respect to the maximum value across all stimulation configurations. The proportion of activation of different extensors or flexors attributable to each stimulation area was then calculated for each participant.
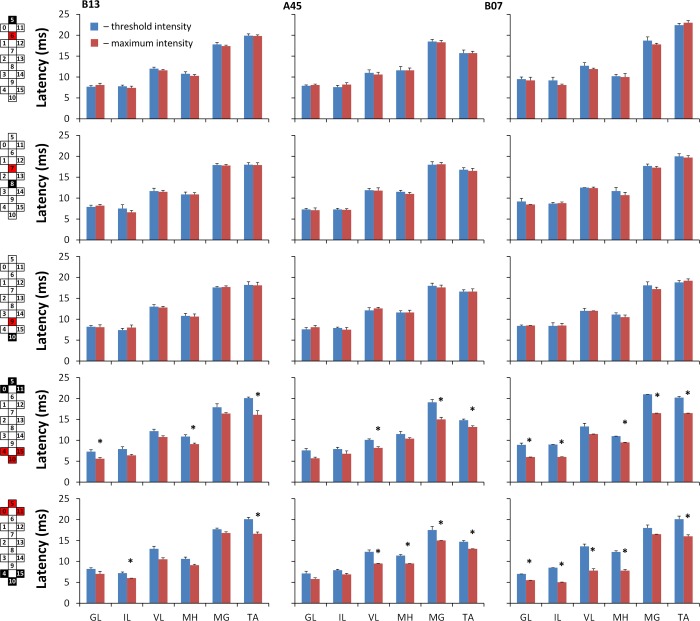
The latencies of the evoked potentials for each muscle in different configurations for each individual were analyzed using repeated-measures ANOVA. Where appropriate, F-statistics were corrected for violations of the sphericity assumption using the Greenhouse-Geisser procedure. Simple t-test comparisons with Bonferroni correction were made to decompose significant effects involving more than two means (α = 0.05). The two-way interactions for each participant and muscle are depicted (see Fig. 6). Results of the pooled data are presented as mean values ± SD.
Fig. 6.
The average latencies of the evoked potentials during localized (5−//6+, 8−//7+, 10−//9+) and wide-field (0−/5−/11−//4+/10+/15+, 4−/10−/15−//0+/5+/11+) stimulation configurations at threshold and maximum intensities. The average of 5 responses is presented for each stimulation intensity. Cathode (active) and anode (reference) electrodes are shown in black and red, respectively. Asterisks indicate statistically significant differences between the values at the threshold and maximum stimulation intensity (*P < 0.05).
RESULTS
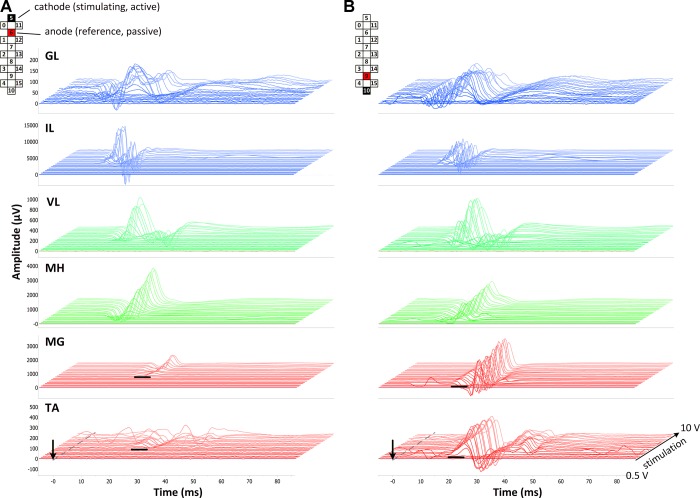
The stimulation resulted in visible single twitches in multiple muscles at higher stimulation intensities. Hip flexors, knee flexors, and plantar flexors dominated the force, so a bilateral hip/knee flexion and plantar flexion were observed. On the EMG, however, the threshold for evoked potentials occurred consistently at lower stimulation intensities compared with when twitches became visually apparent. The properties of the evoked potentials reflected the relative rostrocaudal position of the respective motor pools. Figure 2 demonstrates the averaged evoked potentials (n = 5) obtained during localized rostral (Fig. 2A) and caudal (Fig. 2B) stimulation at different intensities in participant B13. The magnitude of the potentials in most muscles was dependent on the location of the stimulation site. The potentials in proximal muscles, such as IL or VL, occurred with larger magnitude at the rostral configuration (Fig. 2A), whereas the responses in distal muscles, such as MG or TA, occurred at lower stimulation intensities and had the largest magnitude using the caudal configuration (Fig. 2B).
Fig. 2.
Evoked potentials in participant B13 during localized rostral (5−//6+; A) and caudal (10−//9+; B) stimulation configurations in a supine position. The average of 5 nonrectified responses is shown for each stimulation intensity from 0.5 to 10 V, with increments of 0.5 V. GL, gluteus maximus; IL, iliopsoas; VL, vastus lateralis; MH, medial hamstrings; MG, medial gastrocnemius; TA, tibialis anterior muscles. Arrows indicate the onset of the stimulus. Bold, black lines indicate the threshold of MG and TA response. Cathode (active) and anode (reference) electrodes are shown in black and red, respectively.
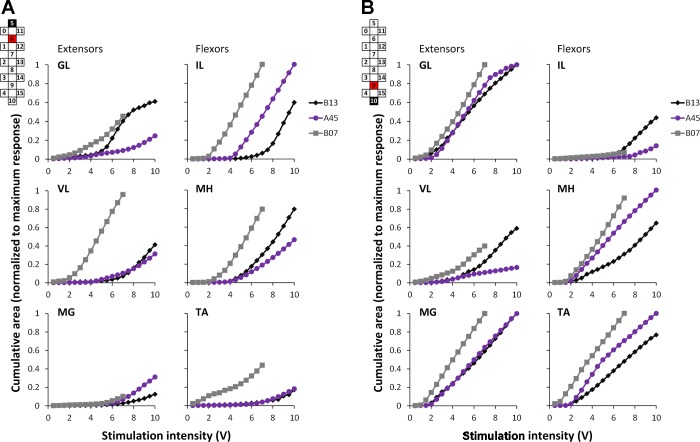
Figure 3 provides some perspective on the level of specificity in selecting different combinations of motor pools using our electrode array. This can be accomplished by stimulating with the anode and cathode closely spaced, as well as with the anode and cathode at the maximum distance. There was a relationship between the magnitude of evoked potentials during localized and wide-field stimulation configurations for each participant. Furthermore, there were several common characteristics in the stimulus-response relationships in different stimulation configurations across all three participants. First, the magnitude of the responses was dependent on the following variables: 1) stimulation delivered via rostral electrodes of the array primarily activated proximally located IL; 2) stimulation delivered via a medium portion of the array activated primarily knee muscles—VL and MH; and 3) stimulation delivered via caudal electrodes of the array activated predominantly distally located MG and TA, as well as GL. Second, thresholds of the muscle activation were dependent on the stimulation configuration: during wide-field stimulation, the thresholds were lower and occurred at the intensities as low as 1 V. Third, the pattern of the recruitment curves, in many cases, was different during wide-field stimulation compared with the localized one; i.e., the initial increment of the response's magnitude was followed by its decay with a subsequent secondary increment of the magnitude. The latter occurred at higher stimulation intensities, and the response's magnitude during that was often two to three times larger than during the first increment. During localized stimulation, the magnitude of the response increased in a more linear fashion and either reached a plateau or had a tendency to decrease at higher stimulation intensities. Finally, when the stimulation location was “nonspecific” for a given muscle, a substantial increment in the response magnitudes remained so at maximum stimulation intensities (for instance, see IL during localized caudal stimulation in participant B13 and MG and TA during localized rostral stimulation in all participants). In addition to the common characteristics, there were distinguishing differences in the evoked potentials among participants (Fig. 3). For instance, in participant B13, the amplitude of GL was prominent in the localized rostral stimulation configuration, whereas in participants A45 and B07, higher activation of GL occurred in localized caudal configurations. Another example was VL potentials across localized vs. wide-field stimulation configurations in different participants. In subject B13, the greatest magnitude of VL potentials occurred in the localized middle configuration, whereas in participant B07, the largest VL potentials were evoked in wide-field stimulation configurations.
Fig. 3.
Area of the evoked potentials [sum of early and medium responses (ER and MR, respectively)] in extensors and flexors recorded in a supine position during localized (5−//6+, 8−//7+, 10−//9+) and wide-field (0−/5−/11−//4+/10+/15+, 4−/10−/15−//0+/5+/11+) stimulation configurations normalized to the maximum response of each muscle in all configurations. Cathode (active) and anode (reference) electrodes are shown in black and red, respectively.
On the other hand, a striking example of similarities among subjects was evident in the cumulative level of activation in different muscles during localized rostral and caudal stimulation configurations, with increasing intensities of stimulation in three participants (Fig. 4). Predominant activation of IL during rostral stimulation and GL, MG, and TA during caudal stimulation can be seen clearly across all individuals.
Fig. 4.
Cumulative area of the evoked potentials (sum of ER and MR) in extensors and flexors recorded in a supine position during localized rostral (5−//6+; A) and caudal (10−//9+; B) stimulation configurations, normalized to the maximum response of each muscle. Cathode (active) and anode (reference) electrodes are shown in black and red, respectively.
Figure 5 shows the shape and latency of evoked potentials in MG compared with the TA in participant B07 during localized (A) and wide-field (B) caudal stimulations. During wide-field stimulation, the latency of the responses changed as a function of stimulation intensity. In the MG, the onset of the response shifted without a noticeable change in the ER magnitude, whereas in the TA, the shift was accompanied by an increase of the ER (Fig. 5B; 5–10 V). Figure 5C depicts recruitment curves of ER and MR during the localized and wide-field stimulations. During wide-field stimulation, there was an initial increment of MR preceding the ER, with a subsequent decrement in its magnitude at 4.0 V, followed by the second magnitude increment. The stimulus-response relationships of ER and MR were different in MG and TA, with a predominant occurrence of MR in MG and ER in TA. The latencies of the evoked potentials for each muscle in different configurations for each individual did not differ during localized stimulation, whereas during wide-field stimulation, the latency was significantly shorter in most muscles at the maximum stimulation intensity (Fig. 6).
Fig. 5.
Evoked potentials in participant B07 during localized (10−//9+; A) and wide-field (4−/10−/15−//0+/5+/11+; B) caudal stimulation configurations. The average of 5 nonrectified responses is shown for each stimulation intensity. Orange, dashed lines show the epochs of ER and MR, determined based on the latency of the earliest response of a given muscle at all stimulation intensities. Red, dotted lines indicate the changes of the onset of the response with an increment of the stimulation intensity. C: area of the ER and MR during localized (left) and wide-field (right) stimulation configurations in the supine position. Cathode (active) and anode (reference) electrodes are shown in black and red, respectively.
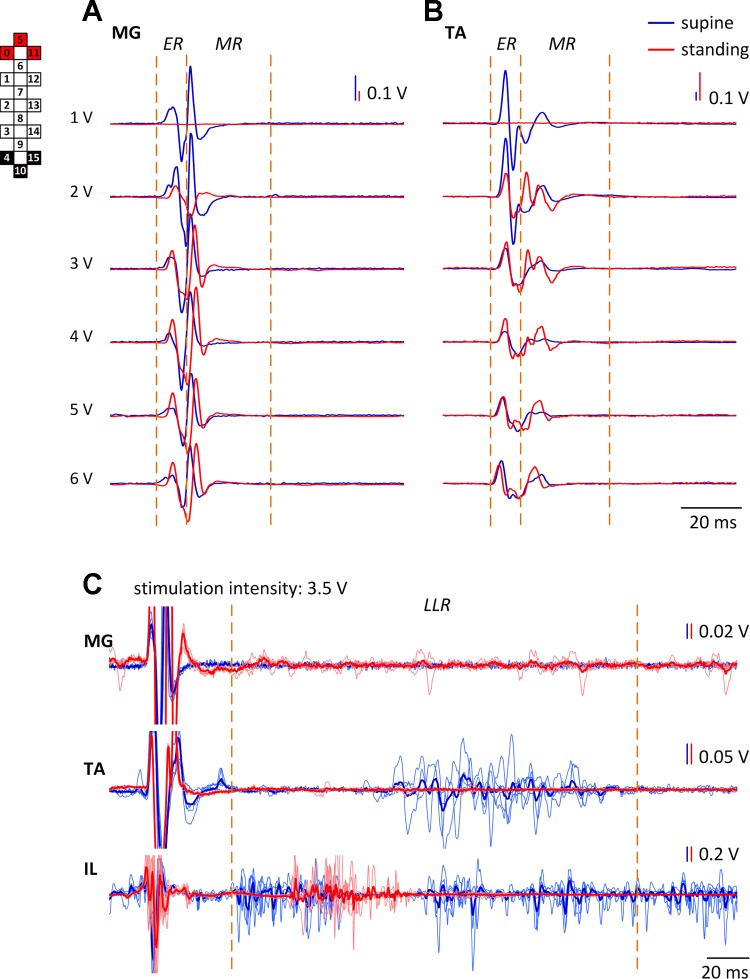
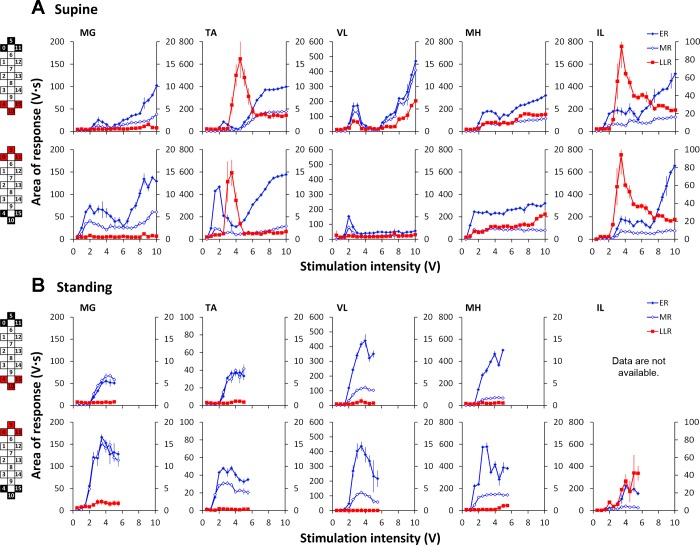
In the supine position, the changes of the ER and MR components of the evoked responses during wide-field stimulation differed substantially from those recorded during standing (BWL of 60% in participant A45; Figs. 7 and 8). The thresholds of the responses were considerably lower in the supine compared with the standing position (Figs. 7, A and B, and 8). The magnitude of the ER and MR was larger in MG during standing (Fig. 7A) and in TA during supine (Fig. 7B). The MR amplitudes were higher during standing (Figs. 7B and 8B). A dramatic difference between the two positions occurred in the LLR (Figs. 7C and 8). In the supine position, the LLR was prominent only in flexors (TA, IL, MH), whereas that was suppressed in extensors (except VL during rostral stimulation; Figs. 7C and 8). The most pronounced occurrence of the LLR was present during the mild-to-medium stimulation intensities, and its manifestation seemed to be reciprocal with the ER and MR development (Fig. 8; TA and IL). During standing, the LLR was suppressed in flexors—completely in TA and MH and substantially in IL, whereas the irregular and asynchronous long latency activity was present in the extensors (MG; Fig. 7C).
Fig. 7.
Evoked potentials (ER and MR) in participant A45 during wide-field caudal (4−/10−/15−//0+/5+/11+) stimulation configuration in MG (A) and TA (B) in the supine and standing [body weight load (BWL) = 60%] positions. The average of 5 nonrectified responses is shown for each stimulation intensity. Orange, dashed lines show the epochs of the ER and MR. C: the long latency responses (LLR) in MG, TA, and IL muscles are presented for both positions at the stimulation intensity of 3.5 V. The thin lines indicate individual trials, whereas the bold lines indicate the average of 5 trials recorded in the supine (blue) and standing (red) positions. Orange, dashed lines show the epoch the LLR. Cathode (active) and anode (reference) electrodes are shown in black and red, respectively.
Fig. 8.
Area of the ER, MR, and LLR in participant A45 during wide-field rostral (0−/5−/11−//4+/10+/15+; top) and caudal (4−/10−/15−//0+/5+/11+; bottom) stimulation configurations in the supine (A) and standing (B; BWL = 60%) positions. Left axis corresponds to the ER and MR values; right axis corresponds to the LLR values. Note that the recruitment curve during standing was not recorded at the higher stimulation intensities, due to discomfort experienced by the participant. Cathode (active) and anode (reference) electrodes are shown in black and red, respectively.
DISCUSSION
We demonstrated that epidural electrical stimulation of rostral and caudal areas of lumbar spinal cord results in a selective topographical recruitment of proximal and distal leg muscles, as revealed by both magnitude and thresholds of the evoked potentials. We also found that the threshold, structure, and latency of the responses changed with the stimulation intensity at different electrode configurations, suggesting an activation of different components, length, and complexity of the transmitting pathways. Furthermore, our findings demonstrate an example of the difference in the structure of the responses evoked during standing compared with those in a supine position, suggesting a weight-bearing-induced reorganization of the spinal circuitries.
Spatiotemporal patterns of motor-pool activation along the rostrocaudal axis of the spinal cord.
Many myotomal maps have been published on the approximate rostrocaudal location of motoneuron pools innervating different muscles in the human spinal cord, derived from various sources, including autopsy, clinical, neuroimaging, and electrophysiologic studies [see Wilbourn and Aminoff (1998) for a review]. Nevertheless, some variation in the topographical root innervation of muscles remains debatable, and all myotomal charts can usually be considered only as “approximate guides,” given that anomalous innervation occurs frequently (Ivanenko et al. 2006; Phillips and Park 1991; Stewart 1992). Also, motoneuron density might exhibit intersegment, intermuscle, and intersubject variability (Arber 2012; Ivanenko et al. 2006). Moreover, most muscles are innervated by and project sensory information extensively to excitatory and inhibitory neurons associated with multiple motor pools among several spinal segments.
In our study, we compared the proportion of activation of extensors and flexors attributable to each stimulation area, instead of evaluation of the absolute values of each muscle-recruitment curve in different segments. We assumed that the optimal site and configuration of stimulation of particular muscles can be derived from their relationships at each configuration. Our data are generally consistent with the anatomy and myotomal maps of the spinal cord and lumbosacral roots (Altman and Bayer 2001; Ivanenko et al. 2006; Kendall et al. 1993; Sharrard 1955, 1964): ES of rostral and caudal areas of lumbar spinal cord resulted in a predominant activation of proximal and distal muscles' motoneuron pools, respectively (except GL). Figures 3 and 4 demonstrate remarkable consistency in the pattern of different muscle recruitment during localized rostral and caudal stimulations across all three participants. Importantly, however, the order of the proximal-to-distal muscles' recruitment cannot be described as a simple linear relationship between the rostral and caudal stimulation sites. Such “nonlocation-specific” responses occurred during higher stimulation intensities delivered through wide-field configurations and were characterized by high magnitude of the evoked responses in both proximal and distal muscles. This phenomenon, to some degree, might be expected, given that most muscles are innervated by several spinal segments, and the network of motoneurons appears to be widely spaced over extensive regions of the spinal cord (Arber 2012; Ivanenko et al. 2006). Another contributing factor is that current flows through the well-conducting cerebrospinal fluid surrounding the spinal cord and in the vertebral canal (Holsheimer 1998; Ladenbauer et al. 2010; Minassian et al. 2007a). Thus spinal roots from multiple adjacent segments can be stimulated with even relatively narrowly placed electrodes at a localized stimulation configuration. But, in spite of this broad area of excitation, it appears that spatiotemporal patterns of spinal-cord activations can have surprisingly specific consequences on motor behavior and therefore, have important clinical implications (Gad et al. 2013).
Whereas previous studies provide a basis from which it is possible to predict the properties of evoked potentials when stimulating with different parameters and at different sites along the spinal cord, none of these data were derived from individuals with chronic, complete motor paralysis (>2 yr postinjury), and none were obtained for obvious ethical reasons via chronically implanted epidural electrodes in uninjured subjects. Here, we show the feasibility of the method and provide the beginning of a comprehensive database that can be used to compare primordial spinal circuitry properties across individuals with different injuries, as well as for repeated measures over time within the same individual. The spatiotemporal maps of the neural networks that lead to different motor pools along the rostrocaudal axis of the human spinal cord may be used to control and monitor selectively the output state and plasticity of the neural network in the course of recovery of motor functions in patients with various motor disorders. The present data are consistent with the conclusion that the selective recruitment of the specific motoneuron pools can be titrated during localized stimulation of the spinal cord, particularly at lower stimulation intensities (Gad et al. 2013).
ES can activate both afferent and efferent pathways.
Previous studies in animals have described different components of the evoked potentials—ER and MR—which were attributed, at least in part, to the preferential involvement of efferent (motoneurons, anterior roots) and afferent (posterior roots, groups Ia and II) structures, respectively (Gerasimenko et al. 2006; Lavrov et al. 2006). We have applied a similar approach to our data. An analysis of ER and MR recruitment curves during wide-field stimulation configurations has revealed that in many cases, their relationship was reciprocal: the increment of ER was often accompanied by a substantial decrement of MR (Figs. 5C and 8). With increasing intensity, the stimulus-response relationship in many muscles shares some characteristics with the H-reflex and M-wave recruitment curve; that is, it was characterized by two peaks of the response magnitude increment, separated by a period of depression, with an early, low-threshold, small, and short-lasting peak and a late, higher threshold, and large peak, followed by a plateau. These findings suggest that the lower stimulation intensities result in initial recruitment of the lower threshold afferent structures, whereas with a progression of the intensity, more of efferent volleys are involved, causing an occlusion effect of the afferent pathways and leading to an activation of motoneurons and/or anterior roots. This notion is supported by a significant decrement of the responses' latency at maximum intensities of the stimulation (Figs. 5 and 6) and concurs with prior reports obtained in experiments with transcutaneous spinal-cord stimulation (Minassian et al. 2007a). We suggest that localized stimulation produces complete recruitment of targeted afferent structures, allowing more focused motoneuron activation, whereas wide-field stimulation activates afferent and intersegmental efferent structures, which contribute to both MR and ER and can be distinguished clearly from each other at maximum stimulation intensities.
Previous electrophysiological (Hunter and Ashby 1994; Maertens de Noordhout et al. 1988; Minassian et al. 2004; Murg et al. 2000) and computational (Ladenbauer et al. 2010; Rattay et al. 2000) studies have reported that the structures, stimulated directly and electrically by lumbar spinal-cord stimulation, are predominantly afferent fibers of the posterior roots. It has been demonstrated that the segmental effects of spinal-cord stimulation resulted from the simultaneous orthodromic and antidromic activation of the central projections of primary afferents (Hunter and Ashby 1994). It has been also suggested that the volleys elicit segmental muscle responses in the lower limbs and coactivate lumbar interneuronal circuits via synaptic projections (Jilge et al. 2004; Minassian et al. 2004, 2007a). The degree to which different components of the spinal networks are activated, however, is, to a large extent, a function of the site and stimulation parameters. Also, it is important to recognize that the present experiments are designed to characterize the responsiveness of the spinal networks associated with a given motor pool at relatively high voltages. This contrasts with a stimulation strategy, in which we prioritize an “enabling” stimulation scenario, which includes intensities of stimulation near or below motor thresholds. This stimulation strategy allows the spinal circuits to process both peripheral sensory and supraspinal inputs, for instance, to facilitate the control of movement (Harkema et al. 2011). In this case, the excitability of interneuronal circuits can be modulated more extensively in the absence or rarely occurring direct activation of motor pools (Gad et al. 2013).
Also, it is important to recall that most, if not all, descending pathways are nonfunctional in individuals with complete motor paralysis, and therefore, the impact of stimulation via the dorsal columns in mediating evoked responses would conceivably differ from that of the uninjured spinal cord. Although of scientific interest, it is not realistic to investigate the structures being activated via implanted epidural electrode array in individuals with an intact spinal cord. The difference among the threshold, composition, latency, and stimulus-response relationship of the evoked potentials during localized and wide-field stimulation configurations is consistent with the interpretation that spinal-cord electrical stimulation activates the intraspinal structures (Danner et al. 2011; Partanen et al. 2000). The difference in the responses evoked in the supine and standing positions in the present study also supports this point. We acknowledge a probability of the direct and indirect involvement of the intraspinal structures during spinal stimulation. However, the amount of contribution of each mechanism during ES can be different than during other methods, for instance, during transcutaneous stimulation. This difference, in turn, may be critical for the therapeutic and functional efficacy of those approaches.
Neuromodulatory effects during standing.
It is plausible that changes in the geometry of the thoraco-lumbar spine and the relative position of the electrode array can influence the flow of current (Ranger et al. 2008) and thus can contribute to the observed difference in the neuromodulation of the evoked potentials during the supine and standing positions. Further studies during movements and in different positions are required to investigate this in detail. At the same time, it is well known that functional load through weight-bearing activities plays a significant role in modulating spinal motoneuron excitability during human posture and movement (Harkema et al. 1997; Knikou et al. 2009; Kozlovskaya et al. 2007; Nakazawa et al. 2004). Proprioceptive, cutaneous-tactile, and nociceptive-afferent inputs are known to converge onto spinal interneurons in the reflex pathway (Schomburg 1990; Zehr and Stein 1999). As such, spatial and temporal summation of activity in different afferent projections can be attributed to the effects observed during standing. However, few studies have investigated the capacity to gate afferent input via spinal circuitry among different motoneuron pools in people with SCI (Dietz 2010; Dy et al. 2010; Knikou et al. 2007). Positive augmentative interaction between the epidural spinal stimulation and sensory activity during stepping on a treadmill has been demonstrated previously in cats with SCI (Gerasimenko et al. 2003, 2008; Guevremont et al. 2006), as well as in individuals with incomplete SCI [American Spinal Injury Association Impairment Scale (AIS) C] (Herman et al. 2002). In the present study, we demonstrated in individuals with motor complete paralysis (AIS A and B) that the relationship among the different components of evoked responses is different at the same stimulation configurations during supine and standing positions.
The difference in the magnitude of the earlier latency components (ER and MR) with their larger magnitude in extensors during standing and in flexors during the supine position suggests higher excitability of extensors and inhibition of flexors during weight bearing. It may seem contradictory to the previous findings on the inhibitory effects of the peripheral sensory inputs on the extensors' motor pool, specifically, on the SOL H-reflex, in nondisabled individuals (Brooke et al. 1997; Koceja et al. 1995; Stein 1995). This could be explained, however, by the difference in the amount of inhibitory control of spinal circuits between the individuals without and with SCI, as well as by the larger contribution of the spinal interneuronal network during epidural spinal stimulation. As it has been shown previously, the spinal lesion deprives the relevant pathways of a tonic excitatory drive and/or removes tonic-inhibitory control of a spinal circuit (Pierrot-Deseilligny and Burke 2005; Taylor et al. 1984). On the other hand, as opposed to the SOL H-reflex, which is a response from a single muscle, the potentials evoked by the spinal ES engage the lumbar-cord networks, including the interneuronal circuits. As such, the excitatory status of the extensors and flexors during spinal stimulation reflects the intraspinal, intersegmental, and multisensory interaction differently than during the H-reflex test. This notion can be supported by the findings on the LLR modulatory effects during supine and standing.
It has been suggested previously that the LLR reflect activation of polysynaptic circuitry associated with the flexor reflex afferent system and may underlie the voluntary standing and stepping motor activity (Dimitrijevic et al. 1998; Gerasimenko et al. 2006; Musienko et al. 2011). For instance, during locomotion, the withdrawal pattern is highly dependent on the present motor task, indicating that proprioceptive input and spinal motor systems functionally modulate the withdrawal pattern (Andersen et al. 2003; Crenna and Frigo 1984; Duysens et al. 1990; Zehr and Stein 1999). In the present study, the balance between the magnitude of the LLR in extensors and flexors was shifted toward flexors during the supine position and toward extensors during standing. A prominent flexor activation in rest is most likely associated with propriospinal connectivity between the hip flexor and ankle flexor motoneuron pools (Grillner 1985). Smaller flexor activation, relative to extensors during standing, has been observed previously in nondisabled participants (Andersen et al. 2003; Rossi and Decchi 1994) and is associated with maintaining the equilibrium (Paquet et al. 1996). It has been shown that the plantar flexors are more excitable for Ia afferent inputs than dorsi-flexors, whereas the reverse is true for corticospinal inputs (Pierrot-Deseilligny and Burke 2005). Compared with the supine position, the more prominent LLR in extensors most likely reflects changes in motoneuron excitability based on the multisensorial convergence on spinal-reflex pathways (Schomburg 1990). Therefore, the characteristics of the evoked potentials cannot be attributed only to a certain excitation level of the motor pools inside of the spinal circuits but eventually, rely on the interplay of multiple peripheral sensory resources and related interneurons.
It has been suggested recently that individuals with SCI gradually lose some intrinsic properties of spinal circuitry, as they progress from the acute to the chronic state, supporting that the loss of sensory and motor input leads to reorganization of the underlying spinal networks (Ashby et al. 1974; Calancie et al. 1993; Hiersemenzel et al. 2000; Skinner et al. 1996). However, our findings showing that the state of excitability of lumbar-cord networks can be modulated effectively during such a functional task, such as standing, look promising in light of the functional rehabilitation of individuals that can occur at the chronic stages after the injury (Harkema et al. 2012).
Our data provide evidence that in the absence of supraspinal-descending drive, the human lumbosacral spinal circuitries can gate afferent input during weight bearing. As shown in animals after complete spinal transection, as well as in human subjects with complete motor paralysis, this state-dependent modulation of potentials demonstrates the important role of the spinal interneuronal networks in processing complex sensory input in the regulation of the motor output generated during standing and locomotion.
Conclusion.
Stimulation of rostral and caudal areas of lumbar spinal cord via epidurally placed electrodes results in a relatively selective activation of proximal and distal motoneuron pools. ES can activate both afferent and efferent pathways. The neural pathways that can mediate motor-evoked potentials to all muscles of the lower limbs are highly dependent on the stimulation parameters. Depending on these parameters and sensory environment, the pathways mediating these responses can have monosynaptic and polysynaptic and orthodromic and antidromic components. Stimulation, limited to a more localized set of electrodes within an electrode array, allows more selective activation of proximal and distal and flexor and extensor motor pools. Wide-field stimulation results in a more generalized pattern of activation between proximal and distal muscles. Our findings provide a better understanding of the mechanisms by which the general levels of excitability of specific neuronal networks within the spinal circuitry are modulated with varying stimulation parameters. As these neural mechanisms become more apparent, ES techniques applied to the injured spinal cord will become increasingly more valuable as an electrophysiological and clinical assessment tool.
GRANTS
Support for this work was provided by the National Institute of Biomedical Imaging and Bioengineering (grant 1R01EB007615-01A1); National Institute of General Medical Science (grant 8P30GM103507); Russian Foundation for Basic Research (grant 13-04-01091); Online Funding Inquiry-M (grants 13-04-12030 and 13-04-12023); Christopher and Dana Reeve Foundation; Kessler Foundation; University of Louisville Foundation; Frazier Rehab Institute and University of Louisville Hospital, Kentucky One Health; Jewish Hospital and St. Mary's Foundation; and Leona M. and Harry B. Helmsley Charitable Trust.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
AUTHOR CONTRIBUTIONS
Author contributions: C.A., S.J.H., V.R.E., and Y.P.G. conception and design of research; C.A. and S.J.H. performed experiments; D.G.S. analyzed data; D.G.S., C.A., S.J.H., V.R.E., and Y.P.G. interpreted results of experiments; D.G.S. prepared figures; D.G.S. and Y.P.G. drafted manuscript; C.A., S.J.H., V.R.E., and Y.P.G. edited and revised manuscript; D.G.S., C.A., S.J.H., V.R.E., and Y.P.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the research volunteers for their valuable contributions to this study. We gratefully acknowledge Jonathan Hodes for the surgical implantations; Joel Burdick and Zhao Liu for their contribution to software development for data analysis; Valery Smith, Martha Meng, Andrea Willhite, Christie Ferreira, Carie Tolfo, and Michael Tolfo for their contribution to the data collection and analysis; Rebekah Morton and Matthew Green for their support of the research volunteers; and Enrico Rejc for valuable discussions.
REFERENCES
- Altman J, Bayer SA. Development of the Human Spinal Cord: An Interpretation Based on Experimental Studies in Animals. Oxford; New York: Oxford University Press, 2001 [Google Scholar]
- Andersen OK, Sonnenborg F, Matjacic Z, Arendt-Nielsen L. Foot-sole reflex receptive fields for human withdrawal reflexes in symmetrical standing position. Exp Brain Res 152: 434–443, 2003 [DOI] [PubMed] [Google Scholar]
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron 74: 975–989, 2012 [DOI] [PubMed] [Google Scholar]
- Ashby P, Verrier M, Lightfoot E. Segmental reflex pathways in spinal shock and spinal spasticity in man. J Neurol Neurosurg Psychiatry 37: 1352–1360, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke JD, McIlroy WE, Miklic M, Staines WR, Misiaszek JE, Peritore G, Angerilli P. Modulation of H reflexes in human tibialis anterior muscle with passive movement. Brain Res 766: 236–239, 1997 [DOI] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol 89: 177–186, 1993 [DOI] [PubMed] [Google Scholar]
- Crenna P, Frigo C. Evidence of phase-dependent nociceptive reflexes during locomotion in man. Exp Neurol 85: 336–345, 1984 [DOI] [PubMed] [Google Scholar]
- Danner SM, Hofstoetter US, Ladenbauer J, Rattay F, Minassian K. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif Organs 35: 257–262, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V. Behavior of spinal neurons deprived of supraspinal input. Nat Rev Neurol 6: 167–174, 2010 [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci 860: 360–376, 1998 [DOI] [PubMed] [Google Scholar]
- Duysens J, Trippel M, Horstmann GA, Dietz V. Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res 82: 351–358, 1990 [DOI] [PubMed] [Google Scholar]
- Dy CJ, Gerasimenko YP, Edgerton VR, Dyhre-Poulsen P, Courtine G, Harkema SJ. Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J Neurophysiol 103: 2808–2820, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Choe J, Nandra MS, Zhong H, Roy RR, Tai YC, Edgerton VR. Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats. J Neuroeng Rehabil 10: 2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Roy RR, Edgerton VR. Epidural stimulation: comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp Neurol 209: 417–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci Behav Physiol 33: 247–254, 2003 [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods 157: 253–263, 2006 [DOI] [PubMed] [Google Scholar]
- Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science 228: 143–149, 1985 [DOI] [PubMed] [Google Scholar]
- Guevremont L, Renzi CG, Norton JA, Kowalczewski J, Saigal R, Mushahwar VK. Locomotor-related networks in the lumbosacral enlargement of the adult spinal cat: activation through intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng 14: 266–272, 2006 [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol 77: 797–811, 1997 [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil 93: 1508–1517, 2012 [DOI] [PubMed] [Google Scholar]
- Herman R, He J, D'Luzansky S, Willis W, Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 40: 65–68, 2002 [DOI] [PubMed] [Google Scholar]
- Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology 54: 1574–1582, 2000 [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord 36: 531–540, 1998 [DOI] [PubMed] [Google Scholar]
- Hunter JP, Ashby P. Segmental effects of epidural spinal cord stimulation in humans. J Physiol 474: 407–419, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Spinal cord maps of spatiotemporal α-motoneuron activation in humans walking at different speeds. J Neurophysiol 95: 602–618, 2006 [DOI] [PubMed] [Google Scholar]
- Jilge B, Minassian K, Rattay F, Pinter MM, Gerstenbrand F, Binder H, Dimitrijevic MR. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res 154: 308–326, 2004 [DOI] [PubMed] [Google Scholar]
- Kendall FP, McCreary EK, Provance PG. Muscles: Testing and Function. Baltimore: Williams & Wilkins, 1993 [Google Scholar]
- Knikou M, Angeli CA, Ferreira CK, Harkema SJ. Soleus H-reflex gain, threshold, and amplitude as function of body posture and load in spinal cord intact and injured subjects. Int J Neurosci 119: 2056–2073, 2009 [DOI] [PubMed] [Google Scholar]
- Knikou M, Kay E, Schmit BD. Parallel facilitatory reflex pathways from the foot and hip to flexors and extensors in the injured human spinal cord. Exp Neurol 206: 146–158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koceja DM, Markus CA, Trimble MH. Postural modulation of the soleus H reflex in young and old subjects. Electroencephalogr Clin Neurophysiol 97: 387–393, 1995 [DOI] [PubMed] [Google Scholar]
- Kozlovskaya IB, Sayenko IV, Sayenko DG, Miller TF, Khusnutdinova DR, Melnik KA. Role of support afferentation in control of the tonic muscle activity. Acta Astronautica 60: 285–294, 2007 [Google Scholar]
- Ladenbauer J, Minassian K, Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng 18: 637–645, 2010 [DOI] [PubMed] [Google Scholar]
- Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol 96: 1699–1710, 2006 [DOI] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Rothwell JC, Thompson PD, Day BL, Marsden CD. Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psychiatry 51: 174–181, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, Dimitrijevic MR. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42: 401–416, 2004 [DOI] [PubMed] [Google Scholar]
- Minassian K, Persy I, Rattay F, Dimitrijevic MR, Hofer C, Kern H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 35: 327–336, 2007a [DOI] [PubMed] [Google Scholar]
- Minassian K, Persy I, Rattay F, Pinter MM, Kern H, Dimitrijevic MR. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci 26: 275–295, 2007b [DOI] [PubMed] [Google Scholar]
- Murg M, Binder H, Dimitrijevic MR. Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. muscle twitches—a functional method to define the site of stimulation. Spinal Cord 38: 394–402, 2000 [DOI] [PubMed] [Google Scholar]
- Musienko P, van den Brand R, Marzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR, Courtine G. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J Neurosci 31: 9264–9278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Miyoshi T, Sekiguchi H, Nozaki D, Akai M, Yano H. Effects of loading and unloading of lower limb joints on the soleus H-reflex in standing humans. Clin Neurophysiol 115: 1296–1304, 2004 [DOI] [PubMed] [Google Scholar]
- Paquet N, Tam F, Hui-Chan CW. Functional modulation of the human flexion and crossed extension reflexes by body position. Neurosci Lett 209: 215–217, 1996 [DOI] [PubMed] [Google Scholar]
- Partanen J, Merikanto J, Kokki H, Kilpelainen R, Koistinen A. Antidromic corticospinal tract potential of the brain. Clin Neurophysiol 111: 489–495, 2000 [DOI] [PubMed] [Google Scholar]
- Phillips LH, Park TS. Electrophysiologic mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve 14: 1213–1218, 1991 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. Cambridge: Cambridge University Press, 2005 [Google Scholar]
- Ranger MR, Irwin GJ, Bunbury KM, Peutrell JM. Changing body position alters the location of the spinal cord within the vertebral canal: a magnetic resonance imaging study. Br J Anaesth 101: 804–809, 2008 [DOI] [PubMed] [Google Scholar]
- Rattay F, Minassian K, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord 38: 473–489, 2000 [DOI] [PubMed] [Google Scholar]
- Rossi A, Decchi B. Flexibility of lower limb reflex responses to painful cutaneous stimulation in standing humans: evidence of load-dependent modulation. J Physiol 481: 521–532, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg ED. Spinal sensorimotor systems and their supraspinal control. Neurosci Res 7: 265–340, 1990 [DOI] [PubMed] [Google Scholar]
- Sharrard WJ. The distribution of the permanent paralysis in the lower limb in poliomyelitis; a clinical and pathological study. J Bone Joint Surg Br 37-B: 540–558, 1955 [DOI] [PubMed] [Google Scholar]
- Sharrard WJ. The segmental innervation of the lower limb muscles in man. Ann R Coll Surg Engl 35: 106–122, 1964 [PMC free article] [PubMed] [Google Scholar]
- Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. Brain Res 729: 127–131, 1996 [PubMed] [Google Scholar]
- Stein RB. Presynaptic inhibition in humans. Prog Neurobiol 47: 533–544, 1995 [DOI] [PubMed] [Google Scholar]
- Stewart JD. Electrophysiological mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve 15: 965–966, 1992 [PubMed] [Google Scholar]
- Taylor S, Ashby P, Verrier M. Neurophysiological changes following traumatic spinal lesions in man. J Neurol Neurosurg Psychiatry 47: 1102–1108, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbourn AJ, Aminoff MJ. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. American Association of Electrodiagnostic Medicine. Muscle Nerve 21: 1612–1631, 1998 [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999 [DOI] [PubMed] [Google Scholar]