Abstract
Endogenous cannabinoids (endocannabinoids) and neurotrophins, particularly brain-derived neurotrophic factor (BDNF), are potent synaptic modulators that are expressed throughout the forebrain and play critical roles in many behavioral processes. Although the effects of BDNF at excitatory synapses have been well characterized, the mechanisms of action of BDNF at inhibitory synapses are not well understood. Previously we have found that BDNF suppresses presynaptic GABA release in layer 2/3 of the neocortex via postsynaptic tropomyosin-related kinase receptor B (trkB) receptor-induced release of endocannabinoids. To examine the intracellular signaling pathways that underlie this effect, we used pharmacological approaches and whole cell patch-clamp techniques in layer 2/3 pyramidal neurons of somatosensory cortex in brain slices from juvenile Swiss CD1 mice. Our results indicated that phospholipase Cγ (PLCγ) is involved in the CB1 receptor-mediated synaptic effect of BDNF, because the BDNF effect was blocked in the presence of the broad-spectrum PLC inhibitors U-73122 and edelfosine, whereas the inactive analog U-73343 did not alter the suppressive effect of BDNF at inhibitory synapses. Endocannabinoid release can also be triggered by metabotropic glutamate receptor (mGluR)-mediated activation of PLCβ, and BDNF has been shown to enhance spontaneous glutamate release. An mGluR antagonist, E4CPG, however, did not block the BDNF effect. In addition, the effect of BDNF was independent of other signaling pathways downstream of trkB receptor activation, namely, mitogen-activated protein kinase and phosphoinositide 3-kinase pathways, as well as protein kinase C signaling.
Keywords: BDNF, cannabinoid, cerebral cortex, phospholipase C
brain-derived neurotrophic factor (BDNF) is as an important modulator of excitatory and inhibitory synaptic transmission. During the past 20 years, much has been learned about the mechanism of action of BDNF at excitatory synapses (reviewed in Carvalho et al. 2008; Gottmann et al. 2009). However, the current knowledge of the role and underlying mechanisms of BDNF in modulating inhibitory synaptic transmission is much less clear. The diverse effects of BDNF at inhibitory synapses depend on a variety of factors, including age of the animal, tissue preparation (slice vs. cell culture), and brain region and cell type being studied, as well as BDNF treatment time course. For example, BDNF has been found to suppress inhibitory postsynaptic currents (IPSCs) in cerebellar granule cells (Cheng and Yeh 2003, 2005) and to enhance postsynaptic GABA receptor responsiveness in Purkinje cells (Cheng and Yeh 2005). In cortical and hippocampal cell cultures, acute application of BDNF rapidly enhances miniature IPSC amplitude, followed by a prolonged suppression. This switch in the direction of effect was concurrent with protein kinase C (PKC)-mediated phosphorylation (Jovanovic et al. 2004). In terms of the locus of BDNF effect, Frerking et al. (1998) found that the suppressive effect of BDNF on IPSCs is expressed presynaptically in the hippocampal CA1 region, whereas several other studies indicated the involvement of postsynaptic tropomyosin-related kinase receptor B (trkB) receptors (Hewitt and Bains 2006; Tanaka et al. 1997).
We recently showed that at inhibitory synapses onto layer 2/3 cortical pyramidal neurons, acute application of BDNF rapidly suppresses GABAergic transmission via release of endocannabinoids from the postsynaptic pyramidal cell, which act in a retrograde manner to suppress presynaptic transmitter release (Lemtiri-Chlieh and Levine 2010). This effect of BDNF is initiated by postsynaptic trkB signaling, because the effect is blocked when postsynaptic trkB receptors are selectively inhibited by intracellular loading of a tyrosine kinase inhibitor or when endocannabinoid synthesis and release from the postsynaptic cell is prevented. The suppression of inhibitory transmission is expressed as a presynaptic decrease in GABA release probability, because it is associated with changes in the paired-pulse ratio, the coefficient of variation, and the frequency of miniature IPSCs, and the BDNF effect is also blocked by antagonists to the predominantly presynaptically expressed CB1 receptor (Lemtiri-Chlieh and Levine 2010). However, the signaling pathway linking BDNF-trkB activation to endocannabinoid mobilization is not known.
The trkB receptor is the major receptor for BDNF signaling in the brain and mediates most of the effects of BDNF on synaptic transmission and plasticity. Upon binding to trkB receptors, BDNF stimulates at least three major downstream intracellular signaling pathways via tyrosine phosphorylation, namely, Ras/mitogen-activated protein kinase (MAPK) pathway, phosphatidylinositol 3-kinases (PI3K)/Akt pathway, and phospholipase Cγ (PLCγ) pathway. Activation of PLCγ leads to cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 induces Ca2+ release from intracellular Ca2+ stores upon binding to its receptor and thus increases intracellular Ca2+ concentration (reviewed in Huang and Reichardt 2003). DAG leads to activation of PKC, which has been found to mediate some of the effects of BDNF at inhibitory synapses (Henneberger et al. 2002; Jovanovic et al. 2004). Activation of the Ras/MAPK and PI3K/Akt pathways are generally considered to be critical for neuronal survival and differentiation (reviewed in Huang and Reichardt 2003; Patapoutian and Reichardt 2001; Segal and Greenberg 1996), although activation of MAPK may also mediate some of the acute effects of BDNF on long-term potentiation and excitatory synaptic transmission (Jovanovic et al. 2000; Ying et al. 2002). The current study examines the potential intracellular signaling pathways that underlie BDNF-endocannabinoid interactions at inhibitory synapses onto layer 2/3 cortical pyramidal neurons.
MATERIALS AND METHODS
Animal handling and slice preparation.
All animal procedures were conducted using protocols approved by the University of Connecticut Health Center Animal Care Committee. Postnatal day 15–27 Swiss CD-1 mice (Charles River, Wilmington, MA) were anesthetized by 3.5% isoflurane inhalation, followed by decapitation. Whole brains were removed and immersed in ice-cold slicing solution containing (in mM) 110 choline chloride, 2.5 KCl, 1.25 NaH2PO4·H2O, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2·6H2O, 25 dextrose, 11.6 sodium ascorbate, and 3.1 sodium pyruvate, equilibrated with 95% O2-5% CO2 (pH 7.3, 310 ± 5 mosmol/kg). Transverse slices (350 μm) containing somatosensory cortex were cut with a Dosaka EM DTK-1000 vibratome (Kyoto, Japan) and transferred to an incubating chamber. Slices were then incubated for 30 min at 33–35°C in carboxygenated incubating solution containing (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 3.5 MgCl2·6H2O, 4 sodium lactate, 2 sodium pyruvate, 25 dextrose, and 0.4 ascorbic acid (pH 7.3, 310 ± 5 mosmol/kg) before being transferred to room temperature. Slices were then individually transferred to a recording chamber (room temperature) fixed to the stage of an Olympus BX51WI upright microscope fitted with a ×40 water-immersion objective lens (0.8 NA). The recording chamber was continuously perfused at 1.5–2 ml/min with carboxygenated artificial cerebrospinal fluid (aCSF) consisting of (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 2 MgCl2·6H2O, and 25 dextrose (pH 7.3, 305 ± 5 mosmol/kg).
Electrophysiology.
Whole cell recordings were obtained from layer 2/3 somatosensory cortex pyramidal neurons. Neurons were visually identified by their morphology and position under infrared differential interference contrast video microscopy. Patch electrodes (2–4 MΩ) were pulled from borosilicate glass capillaries using a Flaming/Brown P-97 micropipette puller (Sutter Instrument, Novato, CA). Pipette internal solution contained (in mM) 130 CsCl, 10 HEPES, 1 EGTA, 0.1 CaCl2, 1.5 MgCl2, 4 Na2-ATP, 0.3 Na-GTP, 10 di-tris-phosphocreatine, and 5 QX-314 (pH 7.3, 290 ± 5 mosmol/kg). A bipolar tungsten electrode (1 MΩ; WPI, Sarasota, FL) was positioned 100–150 μm lateral to the patched pyramidal neuron to elicit electrically evoked IPSCs (eIPSCs). Extracellular stimuli consisted of individual square-wave current pulses (170 μs, 4–30 μA) and were delivered every 15 s.
The chloride equilibrium potential (ECl) with the use of the above internal and external solutions was close to 0 mV; thus IPSCs were recorded as inward currents. Ionotropic glutamate receptor antagonists 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM) and 3-[(R)-2-carboxypiperazin-4-yl]-propyl-1-phosphonic acid (CPP; 3 μM) were used to isolate inhibitory activity. Cells were voltage clamped at −70 mV during recording. All electrical events were filtered at 2.9 kHz and digitized at >6 kHz using a HEKA EPC9 amplifier and ITC-16 digitizer (HEKA Elektronik, Darmstadt, Germany). Series resistance (Rs) was compensated up to 50% at 10- to 100-μs lag. Input resistance (Ri) was monitored with 10-mV (50 ms) hyperpolarizing voltage steps at the end of each sweep. Cells were rejected from analyses if Ri changed by >15% or fell below 50 MΩ during the course of an experiment.
Chemicals.
Unless otherwise stated, all drugs were obtained from Tocris Biosciences (Bristol, UK) and were delivered by bath perfusion. Drugs were first prepared as concentrated stock solution in solvents and stored at −20°C. The stock solutions of DNQX, U-73122, U-73343, GF-109203X, PD-98059, LY-294002, and (S)-3,5-dihydroxyphenylglycine (DHPG) were dissolved in 100% dimethyl sulfoxide (DMSO). Edelfosine (ET-18-OCH3) was dissolved in anhydrous ethanol. CPP, DNQX disodium, chelerythrine chloride (Sigma-Aldrich, St. Louis, MO), (RS)-α-ethyl-4-carboxyphenylglycine (E4CPG), and BDNF (PeproTech, Rocky Hill, NJ) were dissolved in 18-MΩ water. Drug stock solutions were diluted into aCSF on the day of recording to the final concentrations. The final concentration of DMSO did not exceed 0.1%, and the final ethanol concentration was 0.04%, which by themselves had no effect on synaptic transmission.
Data analysis.
Off-line analysis was carried out using Clampfit 10 (Molecular Devices, Sunnyvale, CA) and Prism 5 (GraphPad Software, La Jolla, CA). In individual examples, sweeps of evoked responses were averaged traces of five consecutive eIPSCs before and after 10 min of BDNF application. Group data are reported as means ± SE. Statistical comparisons were made using one-way ANOVA and Dunnett's multiple comparison test for post hoc comparison or paired Student's t-test. P < 0.05 was taken as a statistically significant effect.
RESULTS
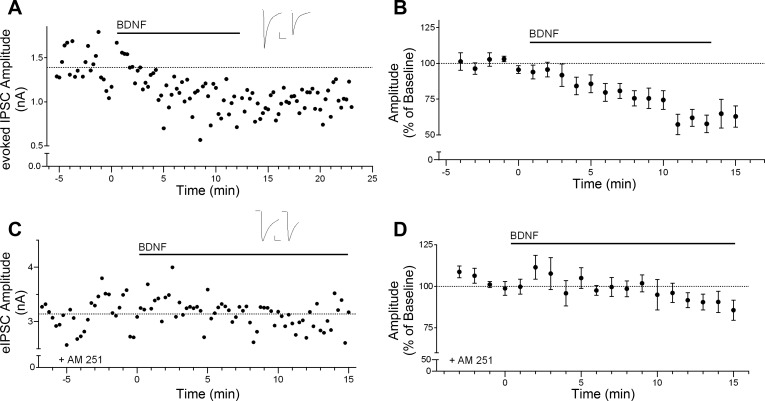
We first examined the effect of BDNF on inhibitory transmission in layer 2/3 pyramidal neurons. As shown in the individual example in Fig. 1A, bath application of 20 ng/ml (0.8 nM) BDNF rapidly reduced the peak amplitude of eIPSCs. This effect persisted during BDNF application and typically showed little or no recovery up to 15 min after washout of BDNF. Overall, peak eIPSC amplitude was significantly decreased to 75.6 ± 6.6% of baseline after 10 min of BDNF exposure, as shown in the group time course in Fig. 1B [F(7,11) = 5.698, P < 0.05, n = 8; baseline, 896.8 ± 181.7 pA; BDNF, 712.4 ± 163.5 pA]. Post hoc tests revealed a significant decrease after 4 min of BDNF treatment, which likely reflects penetration time of BDNF in the brain slice. In contrast, application of the vehicle solution had no significant effect on eIPSC amplitude (100.6 ± 3.0%, n = 3). We also confirmed that this effect of BDNF required activation of CB1 cannabinoid receptors (CB1R). As shown in Fig. 1, C and D, the BDNF effect on eIPSC amplitude was completely blocked in the presence of the CB1R antagonist AM251 (5 μM; 100.6 ± 6.5% of baseline, n = 5; AM251 baseline, 1,564.0 ± 482.3 pA; BDNF + AM251, 1,539.0 ± 437.9 pA).
Fig. 1.
Brain-derived neurotrophic factor (BDNF) rapidly suppresses inhibitory transmission via cannabinoid CB1 receptor signaling. A: example time course showing the effect of bath application of BDNF (20 ng/ml) on evoked inhibitory postsynaptic current (eIPSC) amplitude. Horizontal bar above the trace indicates BDNF application. Inset shows example sweeps before and after 10 min of BDNF application. Scale bars: 250 pA, 25 ms. B: group time course showing the effect of BDNF on normalized peak amplitude of eIPSCs (n = 8). C: example time course showing the effect of BDNF on eIPSC amplitude in the presence of the CB1 receptor antagonist AM251 (5 μM). Inset shows example sweeps before and after 10 min of BDNF application. Scale bars: 500 pA, 25 ms. D: group time course illustrating the lack of effect of BDNF on eIPSC amplitude in the presence of AM251 (n = 5).
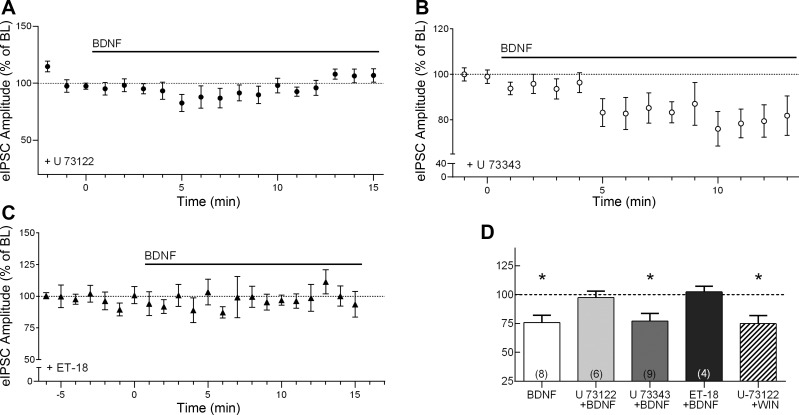
We hypothesized that the CB1R-mediated synaptic effect of BDNF may be dependent on PLCγ signaling. For example, PLCγ signaling has been implicated in the effect of BDNF at hippocampal and cerebellar inhibitory synapses (Cheng and Yeh 2005; Tanaka et al. 1997). In addition, the β-isoform of PLC (PLCβ), which is activated downstream of Gq protein-coupled receptors, is known to be involved in mobilizing endocannabinoids (Galante and Diana 2004; Hashimotodani et al. 2005; Varma et al. 2001). We therefore examined the effect of BDNF in the presence of U-73122 (2 μM), a selective membrane-permeable broad-spectrum PLC inhibitor (Gartner et al. 2006; Reyes-Harde and Stanton 1998). As shown in Fig. 2, A and D, U-73122 prevented the effect of BDNF (97.3 ± 5.7% of baseline, n = 6; baseline, 814.1 ± 148.6 pA; BDNF, 825.8 ± 191.4 pA). In contrast, the inactive analog U-73343 (5 μM) did not block the BDNF effect (Fig. 2, B and D). After 10 min of exposure, BDNF reduced eIPSC amplitude to 77.0 ± 6.6% of baseline in the presence of U-73343 (Fig. 2D, P < 0.05, n = 9), similar to the effect of BDNF alone. The latency to onset of the BDNF effect in the presence of U-73343 was also similar to that with BDNF alone.
Fig. 2.
BDNF suppression of IPSC amplitude requires phospholipase C (PLC) signaling. A: group time course of the effect of BDNF on peak eIPSC amplitude in the presence of U-73122, a broad-spectrum PLC inhibitor (n = 6). Horizontal bar above the trace indicates BDNF application. B: group time course showing the effect of BDNF on peak amplitude of eIPSCs in the presence of U-73343, an inactive analog of U-73122 (n = 9). C: group time course showing the effect of BDNF on peak amplitude of eIPSCs in the presence of the PLC inhibitor edelfosine (ET-18; n = 4). D: compiled group data for normalized eIPSC amplitude under various conditions. WIN, CB1 receptor agonist WIN 55,212-2. *P < 0.05 compared with baseline (BL).
To further confirm the involvement of PLC signaling, we used another selective PLC inhibitor, edelfosine (ET-18-OCH3, or ET-18; 5 μM). Similar to the result obtained with U-73122, the BDNF effect was completely abolished in the presence of ET-18 (Fig. 2, C and D, 102.3 ± 4.9% of baseline). Exposure to each of the PLC inhibitors alone did not have any significant effect on baseline eIPSC amplitude (U-73122, 118.6 ± 17% of baseline, n = 6; ET-18, 107.0 ± 17% of baseline, n = 4). The vehicles used to dissolve the PLC inhibitors also did not have any effect on eIPSC amplitudes (0.08% DMSO for U-73122 and U-73343: 101.4 ± 1.2% of baseline, n = 3; 0.04% anhydrous ethanol for ET-18: 94.7 ± 3.0% of baseline, n = 4). To verify that the effect of PLC inhibition is due to disruption of BDNF-trkB signaling in the postsynaptic cell, and not a disruption of presynaptic CB1R signaling, we tested the direct effect of a CB1R agonist, WIN 55,212-2 (WIN; 5 μM), in the presence of U-73122. As shown in Fig. 2D, exposure to WIN in the presence of the PLC inhibitor caused a significant suppression of eIPSC amplitude (74.8 ± 3.1% of baseline, n = 5, P < 0.05), which was similar to the effect of WIN alone (Lemtiri-Chlieh and Levine 2010). Taken together, these results indicate that the BDNF effect on cortical inhibitory transmission requires PLC signaling downstream of trkB activation.
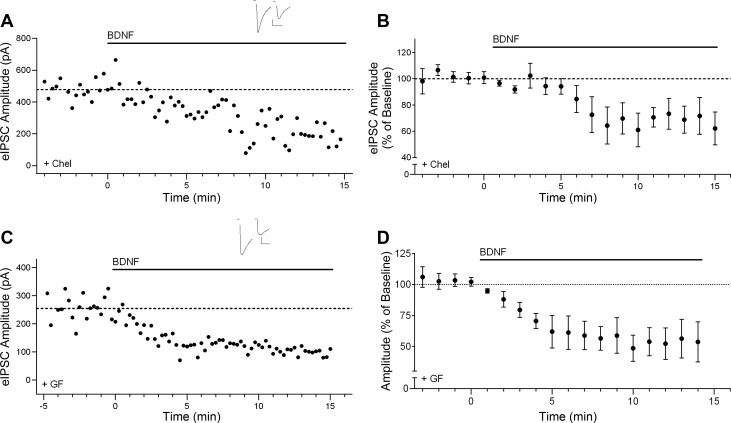
Activation of PLCγ leads to the generation of DAG and IP3. DAG is involved in several intracellular signaling pathways, including the DAG lipase (DGL)-dependent generation of the endocannabinoid 2-arachidonoyl glycerol (2-AG) (Stella et al. 1997). Previously, we have shown that the effect of BDNF is disrupted in the presence of the DGL inhibitor RHC-80267 (50 μM) (Lemtiri-Chlieh and Levine 2010), suggesting involvement of 2-AG. However, DAG also leads to the downstream activation of PKC, which has been found to mediate some of the effects of BDNF at inhibitory synapses (Henneberger et al. 2002; Jovanovic et al. 2004). Therefore, we also tested the possible involvement of PKC signaling. We used two different selective PKC inhibitors, namely, chelerythrine (Chel; 10 μM) and GF-109203X (GF; 1 μM). Neither drug blocked the BDNF effect (Chel, 63.9 ± 12.0% of baseline, P < 0.05, n = 5; GF, 64.6 ± 9.5% of baseline, P < 0.05, n = 5), as shown in individual examples and group data in Fig. 3. The latency and the magnitude of suppression by BDNF in the presence of either Chel or GF were not significantly different from the effect of BDNF alone.
Fig. 3.
Effect of BDNF on inhibitory transmission does not depend on protein kinase C (PKC) signaling. A: example time course for effect of BDNF on eIPSC amplitude in the presence of the selective PKC inhibitor chelerythrine (Chel; 10 μM). Inset shows example sweeps before and after 10 min of BDNF in the presence of Chel. Scale bars: 100 pA, 25 ms. B: group time course for the Chel experiments (n = 5). C: example time course of BDNF effect on eIPSC amplitude in the presence of the selective PKC inhibitor GF-109203X (GF; 1 μM). Inset shows example sweeps before and after 10 min of BDNF application in the presence of GF. Scale bars: 50 pA, 50 ms. D: group time course showing the effect of BDNF on normalized peak amplitude of eIPSCs in the presence of GF (n = 5).
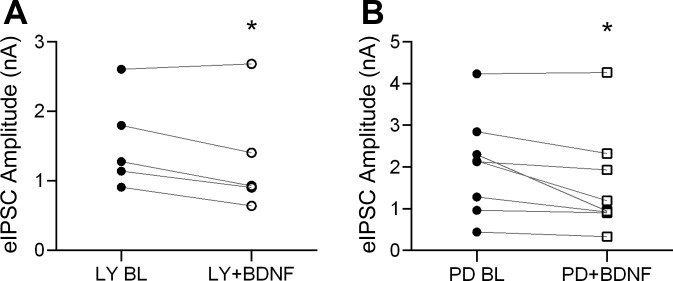
In addition to the above signaling cascades, PI3K and MAPK pathways are also activated by BDNF-trkB signaling. We therefore tested whether these pathways were involved in BDNF modulation of inhibitory transmission. We examined eIPSC amplitude in the presence of selective PI3K and MAPK inhibitors. As shown in Fig. 4A, the PI3K inhibitor LY-294002 (LY; 10 μM) did not block BDNF effect. By the end of 10-min bath application, BDNF significantly reduced eIPSC amplitude to 80.6 ± 5.8% of baseline (n = 5, P < 0.05). Similar results were seen with the MAPK inhibitor PD-98059 (PD; 10 μM), shown in Fig. 4B (74.6 ± 6.7% of baseline, n = 8, P < 0.05). Thus the PI3K and MAPK signaling pathways are not required for the BDNF effect at cortical inhibitory synapses.
Fig. 4.
BDNF effect on IPSC amplitude does not depend on phosphatidylinositol 3-kinase (PI3K) or mitogen-activated protein kinase (MAPK) signaling. A: group data showing eIPSC amplitude before (●) and after 10 min of BDNF exposure (○) in the presence of the PI3K inhibitor LY-294002 (LY; 10 μM; n = 5). B: group data showing eIPSC amplitude before (●) and after 10 min of BDNF exposure (○) in the presence of the MAPK inhibitor PD-98059 (PD; 10 μM; n = 5). Each point represents the average IPSC amplitude evoked during the last 2 min of BL or during minutes 9 and 10 of BDNF treatment (8 sweeps). *P < 0.05.
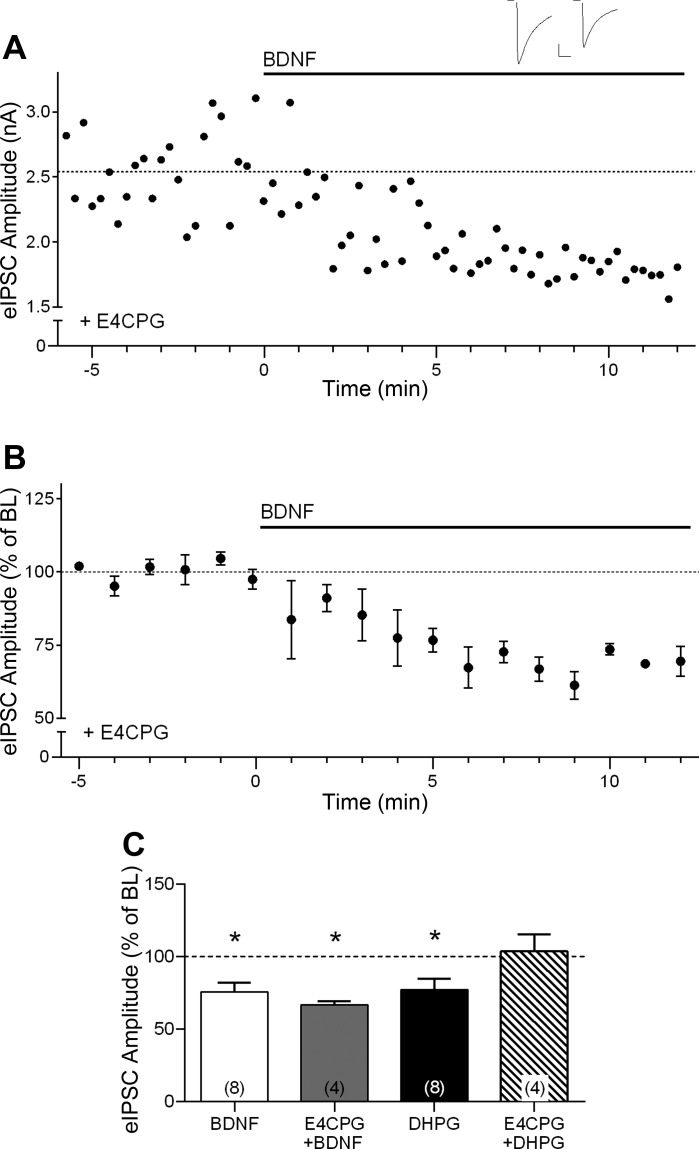
We also addressed whether the effect of BDNF could be mediated by glutamate, rather than a direct effect of BDNF-trkB signaling. BDNF can enhance presynaptic glutamate release (Numakawa et al. 2001, 2002; Zhang et al. 2013), and glutamate can induce endocannabinoid release via activation of metabotropic glutamate receptors (mGluRs) acting through PLCβ signaling (for reviews see Chevaleyre et al. 2006; Kano et al. 2009). Thus we examined the BDNF effect in the presence of 500 μM E4CPG, a group I/group II mGluR antagonist. Blocking mGluR signaling did not prevent the effect of BDNF or alter its time course (66.8 ± 2.5% of baseline at the end of 10-min BDNF application, n = 4, P < 0.05; Fig. 5).
Fig. 5.
BDNF suppression of IPSC amplitude does not require metabotropic glutamate receptor (mGluR) signaling. A: example time course showing BDNF effect on eIPSC amplitude in the presence of the group I/II mGluR inhibitor (RS)-α-ethyl-4-carboxyphenylglycine (E4CPG; 500 μM). Inset shows example sweeps before and after 10 min of BDNF application in the presence of E4CPG. Scale bars: 500 pA, 25 ms. B: group time course showing the effect of BDNF in the presence of E4CPG (n = 4). C: compiled group data for normalized eIPSC amplitude in the presence of BDNF alone (open bar; n = 8), BDNF in the presence of E4CPG (shaded bar; n = 4), the mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG) alone (solid bar; n = 8), and DHPG in the presence of E4CPG (hatched bar; n = 4). *P < 0.05 compared with BL.
As a positive control to ensure that E4CPG effectively blocked mGluR activation, we also examined the effect of DHPG, a selective group I mGluR agonist, on inhibitory transmission. We found that DHPG (50 μM) caused a suppression of eIPSC amplitude, similar to the effect of BDNF (77.2 ± 7.5% of baseline, P < 0.05, n = 8; Fig. 5C, solid bar). This effect of DHPG was completely prevented by E4CPG (103.6 ± 11.7% of baseline, n = 4; Fig. 5C, hatched bar). These results confirm the efficacy of the mGluR antagonist and indicate that the effect of BDNF on cortical inhibitory synaptic transmission is not dependent on glutamate-mediated mGluR activation. Because DHPG can also induce endocannabinoid release that is sensitive to DGL inhibition (Galante and Diana 2004; Gregg et al. 2012), we examined the interaction between BDNF and DHPG using the same concentrations as above. DHPG induced a stable suppression of eIPSC amplitude (73.5 ± 6.0% of baseline, n = 6), and subsequent addition of BDNF had no significant further effect on eIPSC amplitude (94.3 ± 3.7% of DHPG baseline, n = 6). Similarly, in the presence of BDNF, there was no effect of subsequent addition of DHPG (BDNF, 67.5 ± 5.3% of baseline; DHPG, 100.9 ± 8.1% of BDNF, n = 5). These results indicate that the effects of BDNF and DHPG occlude each other, suggesting possible saturation of endocannabinoid signaling.
DISCUSSION
Previously we have shown that the suppressive effect of BDNF on cortical layer 2/3 inhibitory synapses in somatosensory cortex is induced postsynaptically but expressed presynaptically. We have further shown that this effect requires endocannabinoid release from the postsynaptic cell and subsequent presynaptic CB1R activation (Lemtiri-Chlieh and Levine 2010). In the present studies, we investigated the signaling pathways that underlie the CB1R-mediated synaptic effect of BDNF at inhibitory synapses onto layer 2/3 pyramidal neurons in somatosensory cortex from juvenile mice. Our results showed that activation of PLCγ is necessary for this effect of BDNF, because it was blocked by two different PLC inhibitors, U-73122 and ET-18, while maintained in the presence of the inactive analog U-73343. In addition, PLC inhibition did not alter the direct suppressive effect of a cannabinoid agonist. Taken together, these results support a role for PLC signaling downstream of trkB activation. Furthermore, the CB1R-mediated synaptic effect of BDNF does not require PKC, MAPK, or PI3K signaling, because inhibition of these pathways did not prevent the effect of BDNF. Interestingly, this effect also does not require mGluR signaling, because the mGluR antagonist E4CPG did not block the BDNF effect on inhibitory transmission. Taken together with findings of our previous study (Lemtiri-Chlieh and Levine 2010), these results suggest that BDNF requires postsynaptic PLCγ signaling to induce endocannabinoid release, which leads to the CB1R-mediated synaptic effect of BDNF at inhibitory synapses.
We have previously shown that the effect of BDNF was blocked by the DGL inhibitor RHC-80267, suggesting involvement of 2-AG (Lemtiri-Chlieh and Levine 2010). Therefore, a potential signaling pathway underlying the BDNF-CB1R interaction could be trkB-induced activation of PLCγ, leading to generation of DAG and subsequent synthesis and release of 2-AG. This appears to be similar to the mechanism of mGluR-induced 2-AG release. However, it is important to note that elevated calcium is not necessary for mGluR-induced 2-AG production, although it can enhance it (Hashimotodani et al. 2005; Maejima et al. 2005), whereas elevated intracellular calcium appears to be necessary for the effect of BDNF at inhibitory cortical synapses (Lemtiri-Chlieh and Levine 2010). Therefore, different signaling mechanisms might be involved in mGluR-induced and BDNF-induced 2-AG release, even though they both require PLC signaling. On the other hand, it is possible that the other major endocannabinoid, anandamide (AEA), is also involved. It has been established that elevated intracellular calcium is needed for AEA synthesis (Cadas et al. 1996; Di Marzo et al. 1994). Moreover, an earlier study suggested an alternative pathway of AEA synthesis via PLC signaling (Liu et al. 2008). Further systematic studies are needed to identify the specific endocannabinoid(s) released by BDNF.
BDNF-induced endocannabinoid release may play a role in endocannabinoid-dependent depolarization-induced suppression of inhibition (DSI), a form of short-term synaptic plasticity at inhibitory synapses (Diana et al. 2002; Wilson and Nicoll 2001). During DSI, endocannabinoids are released from postsynaptic sites in response to depolarization-induced calcium influx and act retrogradely via presynaptic CB1Rs to suppress GABA release, similar to the effect of BDNF. It will therefore be interesting to explore whether BDNF contributes to endocannabinoid mobilization during DSI. In addition, BDNF-induced endocannabinoid release may play a role in the induction of long-term potentiation (LTP). Several studies have indicated an essential role for endogenous BDNF in the induction of LTP in hippocampus (Chen et al. 1999; Gartner and Staiger 2002; Kovalchuk et al. 2002) and cortex (Abidin et al. 2006; Aicardi et al. 2004; Inagaki et al. 2008; Lu et al. 2010). However, the underlying mechanisms remain largely unknown. A recent study from Lu et al. (2010) showed that BDNF facilitates LTP by suppressing GABAergic inhibition and enhancing pyramidal neuron excitability. Interestingly, that resembles the mechanism of endocannabinoid-dependent enhancement of plasticity. Moreover, high-frequency stimulation during LTP induction releases BDNF (Aicardi et al. 2004; Gartner and Staiger 2002). Endogenously released BDNF can act at both excitatory and inhibitory synapses, because BDNF can be released from the somatodendritic region as well as from axons and glial cells (for review see Edelmann et al. 2013). Thus it is possible that endogenously released BDNF may enhance LTP at least in part by inducing endocannabinoid release at inhibitory synapses.
In addition to suppressing inhibitory transmission, endocannabinoids have been shown to directly modulate excitatory synaptic transmission by suppressing glutamate release in many brain areas, including cortical layer 5 (Fortin and Levine 2007), amygdala (Kodirov et al. 2010), cerebellum (Kreitzer and Regehr 2001), and hippocampus (Hoffman et al. 2010). Interestingly, BDNF potentiates excitatory synaptic transmission in these same brain areas by enhancing glutamate release (Madara and Levine 2008; Schinder et al. 2000). Therefore, in addition to their synergistic action at inhibitory synapses, BDNF and endocannabinoids may have counteracting effects at excitatory synapses.
Several other lines of studies have also suggested mutual interactions between BDNF and endocannabinoid systems. In addition to the present findings, BDNF also has been shown to alter sensitivity to endocannabinoids via modulation of CB1Rs. For example, BDNF has been reported to increase CB1R expression in cerebellar granule neurons (Maison et al. 2009). On the other hand, BDNF can inhibit CB1R function in the striatum (De Chiara et al. 2010). Furthermore, endocannabinoid signaling has been implicated in the regulation of BDNF expression. For example, kainic acid-induced seizures induce BDNF expression in a CB1R-dependent manner (Marsicano et al. 2003), and BDNF has been reported to mediate CB1R-dependent protection against excitotoxicity (Khaspekov et al. 2004) It also has been found that the anxiogenic-like behavioral phenotype of CB1R knockout mice are related to decreased hippocampal BDNF level (Aso et al. 2008). Both BDNF and endocannabinoids are currently major targets for the development of novel therapeutics (Ashton and Moore 2011; Nagahara and Tuszynski 2011). Future studies are needed to further explore BDNF-endocannabinoid interactions and their role in regulating synaptic plasticity and neurological diseases.
In summary, the results above indicate that trkB-induced activation of PLCγ is required for the CB1R-mediated synaptic effect of BDNF at cortical inhibitory synapses, whereas MAPK, PI3K, and PKC signaling pathways are not involved. Our results also suggest that this effect is independent of mGluR-mediated signaling.
GRANTS
This work was supported by National Institute of Mental Health Grant MH-094896 (E. S. Levine).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.Z. and E.S.L. conception and design of research; L.Z. performed experiments; L.Z. analyzed data; L.Z. and E.S.L. interpreted results of experiments; L.Z. prepared figures; L.Z. drafted manuscript; L.Z. and E.S.L. approved final version of manuscript; E.S.L. edited and revised manuscript.
REFERENCES
- Abidin I, Kohler T, Weiler E, Zoidl G, Eysel UT, Lessmann V, Mittmann T. Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice. Eur J Neurosci 24: 3519–3531, 2006 [DOI] [PubMed] [Google Scholar]
- Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci USA 101: 15788–15792, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton CH, Moore PB. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr Scand 124: 250–261, 2011 [DOI] [PubMed] [Google Scholar]
- Aso E, Ozaita A, Valdizan EM, Ledent C, Pazos A, Maldonado R, Valverde O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J Neurochem 105: 565–572, 2008 [DOI] [PubMed] [Google Scholar]
- Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci 16: 3934–3942, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol 153, Suppl 1: S310–S324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci 19: 7983–7990, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. Brain-derived neurotrophic factor attenuates mouse cerebellar granule cell GABAA receptor-mediated responses via postsynaptic mechanisms. J Physiol 548: 711–721, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. PLCgamma signaling underlies BDNF potentiation of Purkinje cell responses to GABA. J Neurosci Res 79: 616–627, 2005 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 29: 37–76, 2006 [DOI] [PubMed] [Google Scholar]
- De Chiara V, Angelucci F, Rossi S, Musella A, Cavasinni F, Cantarella C, Mataluni G, Sacchetti L, Napolitano F, Castelli M, Caltagirone C, Bernardi G, Maccarrone M, Usiello A, Centonze D. Brain-derived neurotrophic factor controls cannabinoid CB1 receptor function in the striatum. J Neurosci 30: 8127–8137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372: 686–691, 1994 [DOI] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci 22: 200–208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann E, Lessmann V, Brigadski T. Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 2013 [DOI] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex 17: 163–174, 2007 [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol 80: 3383–3386, 1998 [DOI] [PubMed] [Google Scholar]
- Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J Neurosci 24: 4865–4874, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, Bonhoeffer T, Korte M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J Neurosci 26: 3496–3504, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci USA 99: 6386–6391, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res 199: 203–234, 2009 [DOI] [PubMed] [Google Scholar]
- Gregg LC, Jung KM, Spradley JM, Nyilas R, Suplita RL, 2nd, Zimmer A, Watanabe M, Mackie K, Katona I, Piomelli D, Hohmann AG. Activation of type 5 metabotropic glutamate receptors and diacylglycerol lipase-alpha initiates 2-arachidonoylglycerol formation and endocannabinoid-mediated analgesia. J Neurosci 32: 9457–9468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 45: 257–268, 2005 [DOI] [PubMed] [Google Scholar]
- Henneberger C, Juttner R, Rothe T, Grantyn R. Postsynaptic action of BDNF on GABAergic synaptic transmission in the superficial layers of the mouse superior colliculus. J Neurophysiol 88: 595–603, 2002 [DOI] [PubMed] [Google Scholar]
- Hewitt SA, Bains JS. Brain-derived neurotrophic factor silences GABA synapses onto hypothalamic neuroendocrine cells through a postsynaptic dynamin-mediated mechanism. J Neurophysiol 95: 2193–2198, 2006 [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci 30: 545–555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72: 609–642, 2003 [DOI] [PubMed] [Google Scholar]
- Inagaki T, Begum T, Reza F, Horibe S, Inaba M, Yoshimura Y, Komatsu Y. Brain-derived neurotrophic factor-mediated retrograde signaling required for the induction of long-term potentiation at inhibitory synapses of visual cortical pyramidal neurons. Neurosci Res 61: 192–200, 2008 [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci 3: 323–329, 2000 [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABAA receptor phosphorylation, activity, and cell-surface stability. J Neurosci 24: 522–530, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89: 309–380, 2009 [DOI] [PubMed] [Google Scholar]
- Khaspekov LG, Brenz Verca MS, Frumkina LE, Hermann H, Marsicano G, Lutz B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur J Neurosci 19: 1691–1698, 2004 [DOI] [PubMed] [Google Scholar]
- Kodirov SA, Jasiewicz J, Amirmahani P, Psyrakis D, Bonni K, Wehrmeister M, Lutz B. Endogenous cannabinoids trigger the depolarization-induced suppression of excitation in the lateral amygdala. Learn Mem 17: 43–49, 2010 [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science 295: 1729–1734, 2002 [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717–727, 2001 [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J Neurophysiol 104: 1923–1932, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, Razdan RK, Kunos G. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 54: 1–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron 67: 821–833, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurophysiol 100: 3175–3184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci 25: 6826–6835, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison P, Walker DJ, Walsh FS, Williams G, Doherty P. BDNF regulates neuronal sensitivity to endocannabinoids. Neurosci Lett 467: 90–94, 2009 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302: 84–88, 2003 [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10: 209–219, 2011 [DOI] [PubMed] [Google Scholar]
- Numakawa T, Matsumoto T, Adachi N, Yokomaku D, Kojima M, Takei N, Hatanaka H. Brain-derived neurotrophic factor triggers a rapid glutamate release through increase of intracellular Ca2+ and Na+ in cultured cerebellar neurons. J Neurosci Res 66: 96–108, 2001 [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yamagishi S, Adachi N, Matsumoto T, Yokomaku D, Yamada M, Hatanaka H. Brain-derived neurotrophic factor-induced potentiation of Ca2+ oscillations in developing cortical neurons. J Biol Chem 277: 6520–6529, 2002 [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11: 272–280, 2001 [DOI] [PubMed] [Google Scholar]
- Reyes-Harde M, Stanton PK. Postsynaptic phospholipase C activity is required for the induction of homosynaptic long-term depression in rat hippocampus. Neurosci Lett 252: 155–158, 1998 [DOI] [PubMed] [Google Scholar]
- Schinder AF, Berninger B, Poo M. Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron 25: 151–163, 2000 [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19: 463–489, 1996 [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388: 773–778, 1997 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci 17: 2959–2966, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci 21: RC188, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588–592, 2001 [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci 22: 1532–1540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Fan J, Ren Y, Zhou W, Yin G. The release of glutamate from cortical neurons regulated by BDNF via the TrkB/Src/PLC-gamma1 pathway. J Cell Biochem 114: 144–151, 2013 [DOI] [PubMed] [Google Scholar]