Abstract
The hippocampus has been shown to undergo significant changes in rodent models of neuropathic pain; however, the role of the hippocampus in human chronic pain and its contribution to pain chronification have remained unexplored. Here we examine hippocampal processing during a simple visual attention task. We used functional MRI to identify intrinsic and extrinsic hippocampal functional connectivity (synchronous neural activity), comparing subacute back pain (SBP, back pain 1–4 mo) and chronic back pain (CBP, back pain >10 yr) patients to control (CON) subjects. Both groups showed more extensive hippocampal connectivity than CON subjects. We then examined the evolution of hippocampal connectivity longitudinally in SBP patients who recovered (SBPr, back pain decreased >20% in 1 yr) and those with persistent pain (SBPp). We found that SBPp and SBPr subjects have distinct changes in hippocampal-cortical connectivity over 1 yr; specifically, SBPp subjects showed large decreases in hippocampal connectivity with medial prefrontal cortex (HG-mPFC). Furthermore, in SBP patients the strength of HG-mPFC reflected variations in back pain over the year. These relationships were replicated when examined in a different task performed by SBP patients (rating fluctuations of back pain), indicating that functional connectivity of the hippocampus changes robustly in subacute pain and the nature of these changes depends on whether or not patients recover from SBP. The observed reorganization of processing within the hippocampus and between the hippocampus and the cortex seems to contribute to the transition from subacute to chronic pain and may also underlie learning and emotional abnormalities associated with chronic pain.
Keywords: hippocampus, transition to chronic pain, functional connectivity, back pain, medial prefrontal cortex
the hippocampus is known to undergo robust molecular and synaptic changes in animals with chronic neuropathic pain (Mutso et al. 2012; Ren et al. 2011). However, the nature of this modulation in humans has not been investigated, and the involvement of the hippocampus in pain chronification remains unclear. A myriad of behavioral changes observed in chronic pain patients, including elevated anxiety and depression (Gore et al. 2012), problems in emotional decision making (Apkarian et al. 2004; Gupta et al. 2009), spatial working memory deficits (Abrahams et al. 1999; Dick and Rashiq 2007; Kim et al. 2012), and difficulty in classical conditioning tasks (Flor et al. 2002), suggest abnormal hippocampal physiology in chronic pain. Indeed, some hippocampal functional changes have recently been identified in migraine pain (Maleki et al. 2013), but investigations of hippocampal functional properties in the transition to chronic pain are completely absent.
We previously proposed that chronic pain can be redefined as a state of continual learning coupled with an inability to extinguish aversive associations (Apkarian 2008; Apkarian et al. 2009, 2011). Recent work has identified particular brain reorganization that seems to be causally involved in pain chronification (Baliki et al. 2012). In particular, nucleus accumbens (NAc) and medial prefrontal cortex (mPFC) connectivity was found to predict the transition to chronic back pain, indicating that this motivation-valuation circuitry is potentially mediating such an aversive teaching signal. Since the hippocampus is an integral component of this mesocorticolimbic circuitry, especially involved in aversive learning (Keleta and Martinez 2012), and is functionally connected to the NAc (O'Donnell and Grace 1995) and mPFC (Jay et al. 1992), we further hypothesize that hippocampal physiology plays a role early in the transition from subacute (SBP; at least 1 mo of persistent pain) to chronic (CBP; at least 1 yr of persistent pain) back pain and to the evolution of pain during this period.
In this study we investigate hippocampal processing within itself and in relation to the rest of the brain, interrogated cross-sectionally for SBP and CBP and longitudinally in SBP subgroups whose pain resolves (SBPr) or persists (SBPp) over a 1-yr observation period. Given that the properties of the brain at rest can be captured in simple visual attentional tasks (Fox et al. 2005; Greicius et al. 2003) and the first study to demonstrate disruption of healthy brain networks in CBP was based on a visual attention task (Baliki et al. 2008), here we take the same approach and examine hippocampal functional connectivity (synchronous neural activity between regions) during the same visual tasks as in Baliki et al. (2008). This allows us to identify hippocampal functional properties independent of task specifics. Our results indicate that there are specific hippocampal functional network changes that differentiate subacute patients who recover from those who develop chronic pain, and a number of these anticipate changes in pain intensity over time.
METHODS
Subjects.
Data presented here are part of an ongoing longitudinal brain imaging study of SBP and the transition to CBP. All participants were right-handed, all patients were diagnosed by a clinician for back pain and had pain intensity greater than 40/100 on the visual analog scale (VAS 0, no pain; 100, “worst pain imaginable”), and subacute patients had pain duration of 4–16 wk at initial interview. Subjects were excluded if they reported any other chronic painful conditions, systemic disease, history of head injury, psychiatric diseases, or more than mild depression [score > 19, according to Beck's Depression Inventory (BDI)]. For this study, data were available for 15 healthy control (CON) subjects (6 women, 9 men; age: 38.5 ± 8.0 yr, mean ± SD), 17 sex- and age-matched CBP patients (7 women, 10 men; age: 41.24 ± 7.3 yr), and 32 matched SBP patients (16 women, 16 men; age: 40.8 ± 11 yr), all of whom had been previously examined by Baliki et al. (2012).
SBP and CON subjects were scanned at four visits over the course of a year, while CBP patients were scanned only once. SBP patients were scanned as soon as possible after injury (duration from injury at visit 1: 12.9 ± 4.9 wk, mean ± SD; range: 5–26 wk), and SBP and CON subjects were followed over the subsequent year (visit 2: 20.2 ± 4.8 wk, range 11–36 wk; visit 3: 42.8 ± 8.0 wk, range: 32–69 wk; visit 4: 66 ± 5.1 wk, range: 59–77 wk; mean ± SD wk from injury; see Baliki et al. 2012 for details on scan times). Some SBP patients exhibited recovery from pain (SBPr, decrease in pain of at least 20% between visits 1 and 4; n = 17), while for others pain was persistent (SBPp; n = 15). One subject from each of these groups lacked visit 2 data and was excluded from longitudinal analyses. All subjects gave full informed consent to procedures approved by the Northwestern University Institutional Review Board. The medication used, demographics, pain, and mood characteristics collected at each visit closely follow data shown in Fig. 1, S9, and Table S.2A in Baliki et al. (2012), although it should be noted that the number of subjects is slightly different in the present study.
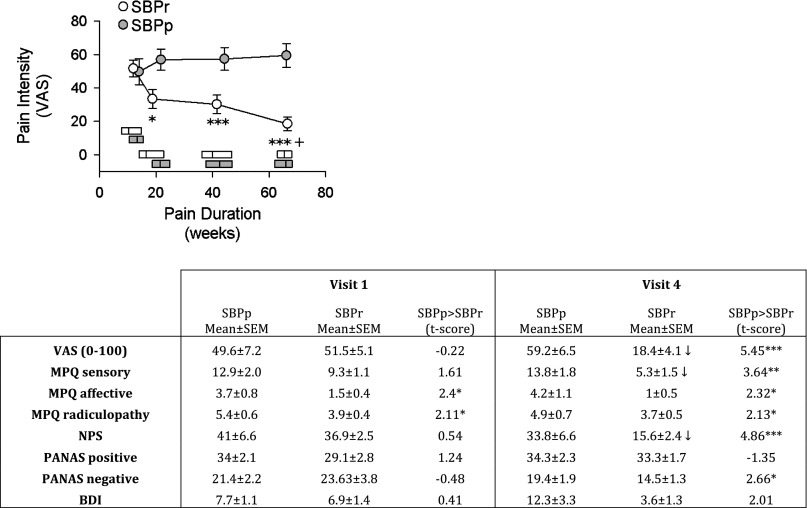
Fig. 1.
Pain characteristics in subacute back pain (SBP) patients, as a function of grouping and time. Top: graph shows that SBP patients whose pain resolves (SBPr, n = 16) report decreases in pain on a visual analog scale (VAS) over the course of the study and ultimately differ significantly from SBP patients whose pain persists (SBPp, n = 14). Horizontal bars represent median and interquartile range of pain durations for each group. rm-ANOVA showed a significant group × time effect (P < 10−5). Post hoc (Tukey) tests: +P <0.05, within-group comparison to visit 1; *P < 0.05, ***P < 0.001, comparison between groups at corresponding times. Bottom: table shows pain and mood parameter differences for SBPp and SBPr. Significant decreases between visit 1 (at entry into the study) and visit 4 (1 yr after entry into study) (paired t-test, P < 0.01) are displayed as ↓. MPQ, McGill Pain Questionnaire; NPS, Neuropathic pain scale; BDI, Beck's Depression Inventory. PANAS, Positive Affect Negative Affect Scale. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired t-test.
Pain and mood parameters.
At all visits, SBP and CBP patients completed the short form of the McGill Pain Questionnaire (MPQ). The main component of the MPQ consists of 12 sensory and 4 affective descriptors, which were used to compute separate sensory and affective scores. Radiculopathy scores were quantified from pain locations that patients had shaded on the MPQ form (Chanda et al. 2011). Patients also completed the Positive Affect Negative Affect Score (PANAS), Pain Detect, and BDI. All questionnaires were given 1 h prior to brain scanning.
Scanning parameters.
High-resolution T1-anatomical brain images were acquired with a Siemens 3.0-T whole body system with a standard radio frequency head coil with the following parameters: voxel size = 1 × 1 × 1 mm, TR = 2,500 ms, TE = 3.36 ms, flip angle = 90°, in-plane matrix size = 256 × 256, slices = 160, and field of view = 256 mm. fMRI images were acquired on the same day and scanner with the following parameters: multislice T2*-weighted echo-planar images with TR = 2.5 s, TE = 30 ms, flip angle = 90°, number of volumes = 244, slice thickness = 3 mm, in-plane resolution = 64 × 64. The 36 slices covered the whole brain from the cerebellum to the vertex.
Gray matter segmentation.
Left and right hippocampus were segmented from each subject at visit 1 with FMRIB's integrated registration and segmentation tool (FIRST), which uses Bayesian location and shape parameters to identify subcortical structures (Patenaude et al. 2011) and measure volume. All completed segmentations were linearly aligned to a reference space. Appropriate transformation parameters were determined by least-squares fitting of native whole brains to a standard MNI152 T1 brain (Jenkinson et al. 2002) with a subcortical weighting function. A conjunction was taken of individual subject segmentations, yielding two hippocampal masks, one for left and another for right hippocampus. In addition, cortical gray matter was segmented with FAST in each subject to produce gray matter masks (Zhang et al. 2001). Cerebellum and brain stem were removed to avoid signal confounded by large vertical intensity gradients. All masks were transformed to each subject's functional space for data analysis.
fMRI data acquisition and preprocessing.
During one fMRI scan subjects used a finger span device to perform a standardized visual task. They were asked to use the finger device to match the motion of a horizontal bar projected on a screen inside the scanner as it moved up and down according to a preprogrammed stimulus sequence. All subjects underwent an initial training phase before scanning to learn the use of the finger span device and ensure adequate task performance (defined as r > 0.8 for correlation of stimulus time course with subject feedback). During imaging sessions, stimulus onset coincided with fMRI acquisition onset and frame rate was synchronized with fMRI TEs.
An additional fMRI scan was performed with identical acquisition parameters and duration. During this second scan pain patients were instructed to use their finger span device to continuously rate their spontaneous pain on a visual analog scale. Patients were provided with visual feedback in the form of the same horizontal bar used during the standard visual task. During the spontaneous pain rating scan the horizontal bar did not move according to an automated stimulus sequence but rather reflected the magnitude of the subject's finger span device ratings in real time (Baliki et al. 2006). Healthy control subjects did not receive this scan.
Preprocessing of each subject's time series fMRI data was performed with an in-house processing pipeline that included brain extraction, slice timing correction, motion correction, spatial smoothing using a Gaussian kernel (fwhm = 5 mm), nonlinear high-pass temporal filtering (100 s), intensity normalization, and global signal correction. Motion correction was implemented by using MCFLIRT (Jenkinson et al. 2002) to identify six rotation and translation parameters and realign volumes with linear registration. These six parameters were additionally regressed out of the BOLD signal. The first four volumes were removed to allow for signal stabilization. FSL v. 4.1.8 was used during preprocessing and for further data analysis.
fMRI spatial transformations.
fMRI data were spatially normalized to a 2-mm MNI152 T1 brain template with a two-step registration. First, each fMRI volume was registered with a 7 degrees of freedom affine transformation to the corresponding T1 brain. Transformation parameters were also computed by registering all T1 brains to the MNI152 template with 12 degrees of freedom. Combining the two transformations by matrix multiplication yielded transformation parameters normalizing fMRI data to standard space, and this process was applied to both standard visual and spontaneous pain rating fMRI data. Inverse matrices were computed to transform standard space templates and regions of interest (ROIs) into subject functional spaces. Transformations performed using these parameters were implemented with linear interpolation.
Task performance.
To ensure that task-related effects did not confound results, visual task performance was examined between groups and across all four time points. Task performance was quantified by the correlation coefficient of stimulus time course and subject feedback and normalized by z-Fisher transformation. SBPp (n = 14), SBPr (n = 14), and all CON subjects were modeled as separate treatment levels in an rm-ANCOVA of task performance across the four visits. Several SBPp and SBPr patients were excluded because of incomplete task feedback data, while CBP patients were not included in this analysis because of a lack of data at later visits of the study. To examine CBP with respect to the other groups, a second ANCOVA analysis was performed that included CBP in addition to the same SBPr, SBPp, and CON subjects but only looked at task performance at visit 1, where CBP data were available. Both analyses controlled for sex and age confounds.
Analysis of functional connectivity extent.
In-house developed software (Apkarian Brain Linkage Map, ABLM) was used to examine the extent of intrinsic and extrinsic hippocampal functional connectivity, based on a procedure we have described in the past (Eguiluz et al. 2005) and related to procedures used by other groups for computing “degree count” (Achard et al. 2006; Buckner et al. 2009; Salvador et al. 2005; Sporns 2013). Given a source region and target region, we compute the number of functional connections between source region voxels and target region voxels. A map is generated in which source region voxels are assigned a value corresponding to the number of target region voxels with which they share a suprathreshold connection. Two voxels were considered functionally connected if their BOLD signals correlated with r > 0.3. Bartlett's theory identifies 84 degrees of freedom in the BOLD time series after accounting for hemodynamic smoothing (Jenkins and Watts 1968), suggesting that voxels correlated with r > 0.3 could be considered functionally connected at a false positive rate α = 0.001. This conservative threshold was used for strong control on the false positive rate.
Two complementary analyses were performed. First, the extent of connectivity of the hippocampus to itself was measured (intrinsic connectivity) by using each subject's hippocampal segmentations as both ROI and target region. Second, the extent of connectivity of hippocampus with other gray matter regions of the brain was examined (extrinsic connectivity). For this second analysis the same ROI was used as for the first, and each subject's total gray matter mask less hippocampi was used to define the target region. Both analyses produce a map of the hippocampus, but the result of the first analysis produces a map indicating which hippocampal voxels are connected to most other hippocampal voxels, whereas the second analysis indicates which hippocampal voxels are most connected to extrahippocampal gray matter voxels.
Analyses were performed in native space without any spatial manipulations. Subsequently, counts of connectivity extent were projected to a standard MNI152 T1 space. Voxels outside the group hippocampal conjunction mask were excluded to further account for hippocampal shape differences across subjects. The subject mean was computed for each group for illustrative purposes. To identify voxelwise differences in connectivity extent between various subject groups, bootstrap testing at P < 0.05 was used with 5,000 resamples (Kennedy 1995; Nichols and Holmes 2002) while controlling for age and sex and correcting for multiple comparisons at the α = 0.05 level with threshold-free cluster enhancement (TFCE) (Smith and Nichols 2009).
Longitudinal analysis was also performed for SBP subjects. Gray matter and hippocampal masks, as well as spatial transformation parameters, were generated in the same manner as for visit 1. Extent of both intrinsic and extrinsic connectivity was computed in subject space before transformation to standard space. Nonparametric rm-ANOVA was performed separately for SBPp and SBPr with one factor at four levels, one for each visit. Once again, bootstrap testing at P < 0.05 with 5,000 resamples and TFCE α = 0.05 level was used to identify any significant changes, but this time without statistical control for sex and age. Unlike the cross-sectional analysis, repeated-measures analysis provides an inherent experimental control for within-subject variability.
Region of interest analysis.
Analysis of hippocampal connectivity extent suffers from limitations in specificity and sensitivity. Although it can reveal connectivity differences between subject groups, it is not clear what nonhippocampal regions might drive such connectivity differences. Moreover, connectivity extent is computed based on a relatively high connectivity threshold (r = 0.3) to mitigate the contribution of spurious correlations to the total count of connectivity extent. This may hide more subtle but nevertheless true time effects or differences between patient groups, particularly SBPp and SBPr.
Analysis of hippocampal connectivity extent identifies a particular region of the hippocampus that shows differences in patient groups compared with CON subjects. This region was selected as an ROI to resolve uncertainties inherent to connectivity extent analysis and to identify differences between SBPp and SBPr. It was projected into native subject space, and a seed-based connectivity analysis in the visual task data was performed for each subject (see Fig. 3). The hippocampal BOLD and its first derivative were used with FMRIB's Improved Linear Model (FILM) (Woolrich et al. 2001) to determine hippocampal functional connectivity with gray matter regions throughout the rest of the brain at visit 1. This analysis was also performed at visit 4 for CON and SBP subjects. Moreover, hippocampal functional connectivity was also computed in data obtained during the spontaneous pain-rating task in all SBP patients at visits 1 and 4 for validation purposes. Maps of parameter estimates (COPEs) and associated uncertainty (varcopes) were projected back into standard space, where parameter estimates were normalized by smoothing with a Gaussian kernel (fwhm = 4 mm). This process yielded maps of hippocampal functional connectivity in standard space for each subject at visits 1 and 4.
Fig. 3.
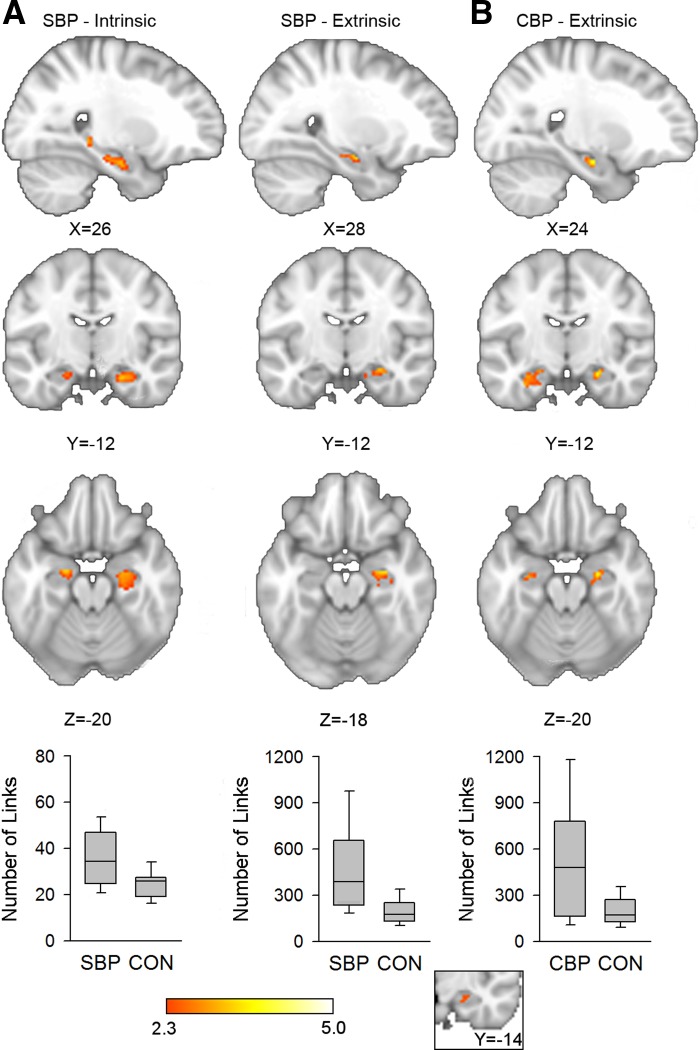
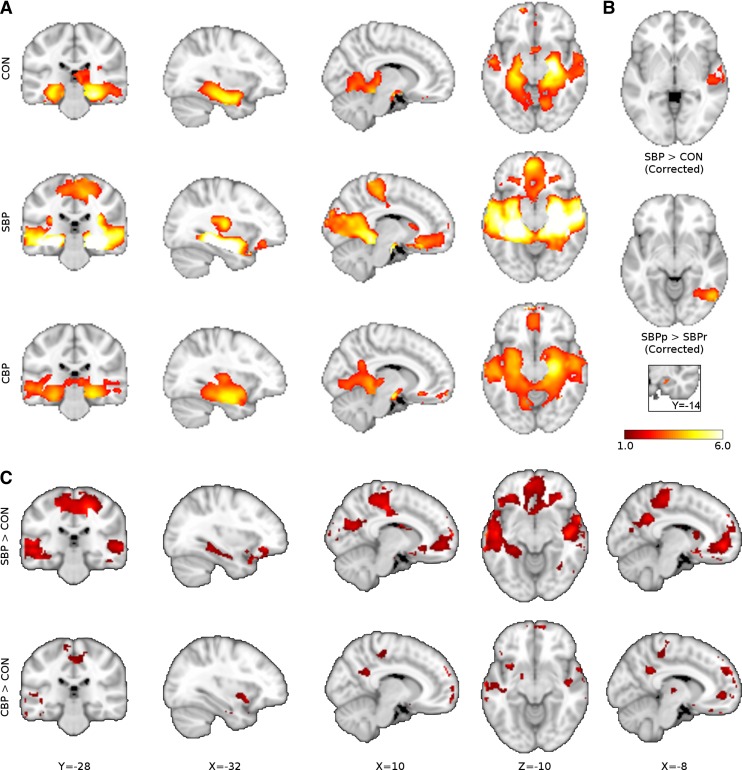
SBP and CBP patients show greater hippocampal connectivity than CON subjects. A: SBP patients (n = 32) show greater intrinsic (left) and extrinsic (right) hippocampal connectivity than CON subjects (n = 15) bilaterally and in the right hippocampus, respectively [bootstrap test, z > 2.3, threshold-free cluster enhancement (TFCE) cluster P < 0.05, corrected for age and sex]. Mean value of significant voxels was computed in each subject post hoc, and the connectivity across each group is shown as a graph below corresponding slices (post hoc Mann-Whitney U-test: intrinsic connectivity P < 0.001, extrinsic connectivity P = 0.007). B: CBP patients (n = 17) show greater extrinsic hippocampal connectivity than CON subjects bilaterally (bootstrap test, z > 2.3, TFCE P < 0.05 for multiple comparisons, data controlled for age and sex; post hoc U-test P = 0.007). Inset illustrates the conjunction of the 3 parametric maps shown in A and B. Bar graphs indicate median, quartile, and range of number of links.
First, to inform results from analyses of connectivity extent, maps of mean hippocampal connectivity during the visual task were generated for CON, SBP, and CBP groups at visit 1. Parametric contrasts were then performed for SBP vs. CON, CBP vs. CON, and SBPp vs. SBPr. FMRIB's Local Analysis of Mixed Effects (FLAME) (Beckmann et al. 2003) was used to perform contrasts and control for age and sex. Control for multiple comparisons was enforced by cluster thresholding within the framework of Gaussian random field theory (voxel z > 2.3, cluster P < 0.05; see Fig. 3A) (Poline et al. 1997). Additionally, to obtain a more accurate representation of nonspecific and global connectivity extent differences between groups, we also performed contrasts without cluster correction and at a reduced voxelwise threshold (z > 1). Because negative correlations in the BOLD signal do not lend themselves to clear neurobiological interpretation (Fox et al. 2009), we used postthreshold masking on regions that on average show negative functional connectivity to the hippocampus across subjects to exclude these regions from the contrasts.
Second, longitudinal analysis was performed to identify how persisting and recovering SBP patients differ in their development of hippocampal connectivity. We limited this analysis to patients who had visual task data available at all four time points of the study (SBPr n =16, SBPp n = 14). This requires an rm-ANCOVA and examination of group × time interactions while controlling for confounds like sex and age, but performing such an analysis voxelwise is computationally prohibitive. To reduce the computational complexity of higher-level analysis we engaged hippocampal connectivity differences between SBPp and SBPr patients, using a two-tiered approach. First, within-group contrasts were performed of visual task data from visit 1 vs. visit 4 to define relevant ROIs. FLAME with cluster correction for multiple comparisons was used (voxel z > 2.3, cluster P < 0.05). To attain our ultimate objective, a second multifactor analysis was performed in these resulting clusters. Mean hippocampal connectivity was computed for each ROI in each subject at each scan. Post hoc rm-ANCOVA was used to identify group and time interactions while controlling for sex and age. Fifteen CON subjects were also examined for differences between visits 1 and 4 in the same manner, and rm-ANOVAs were performed to determine whether CON subjects showed a time effect for regions changing in time in SBPp or SBPr.
ROI task dependence.
To provide further reassurance that task-related differences between groups did not influence our findings, the hippocampal seed was examined in the visual task specifically for task effects. The normalized mean time series for ROI voxels was obtained for each subject in native space. The correlation of the stimulus time course with the average time series across subjects was computed to quantify task dependence across the entire subject sample. Two analyses were then needed to ensure that there were no differences between groups at visit 1 or in time. To identify differences between groups at visit 1, the subject ROI time series were individually correlated with the stimulus time course; correlation coefficients were z-Fisher transformed, and an ANCOVA was used to identify any latent differences between subject groups at visit 1 (CON n = 15, SBPr n = 14, SBPp n = 16, CBP n = 17). Second, rm-ANCOVA was used to identify any group-related effects in time (CON n = 15, SBPr n = 14, SBPp n = 16). Sex and age were modeled as confounding covariates.
ROI analysis of additional potential confounds: head motion, depression, and total hippocampal volume effects.
We specifically examined hippocampal functional connectivity for effects of depression, total hippocampal volume, or head motion-related effects. The mean intrinsic and extrinsic hippocampal connectivity within the hippocampal seed was used to quantify connectivity extent across all subjects (n = 64). Additionally, several ROIs showed distinctive connectivity to the hippocampal seed for SBPp and SBPr, and their mean functional connectivity to the hippocampus was examined. These latter ROIs were only examined in CON and SBP subjects who were analyzed longitudinally (n = 45). Motion during the spontaneous pain task has been examined elsewhere and was not found to differ significantly between SBPp and SBPr at either visit 1 or visit 4 (Baliki et al. 2012). Here we additionally examine motion in terms of mean absolute head displacement during the standard visual task. Depression is quantified by BDI, and volume of the hippocampus was computed during segmentation by FIRST. Motion was available in all patients, while BDI was not available at visit 1 in 1 SBP and 1 CBP patient (n = 62) and was only available at visit 4 in 15 SBP and 12 CON subjects (n = 27). ANOVA was used to identify between-group differences in motion (visit 1 groups: CON, SBPr, SBPp, CBP; visit 4 groups: CON, SBPr, SBPp). A regression analysis was used to determine whether any of the functional parameters (COPEs) showed a relationship with either motion or depression at visits 1 and 4 and whether motion, depression, or hippocampal volume affected connectivity extent at visit 1 after controlling for sex and age.
Connectivity and change in pain.
Change in pain was defined as (VAS1 − VAS4)/VAS1, where VAS1 and VAS4 are the VAS scores of a patient at visits 1 and 4, respectively. One subject experienced a transient remission of pain and reported zero pain at visit 1. This subject was excluded from analyses of pain because of an inability to define a useful measure of percent change in pain.
RESULTS
Task performance.
Task performance did not differ between groups (CON n = 15, SBP n = 32, CBP n =17) at visit 1 [ANCOVA: F(3,51) = 1.478, P = 0.230], nor were any significant time (visits 1–4, over 1-yr period) [rm-ANCOVA: F(3,108) = 0.081, P = 0.97], group [F(2,36) = 1.08, P = 0.35], or group × time [F(6,108) = 0.50, P = 0.81] interactions identified longitudinally.
Pain characteristics.
By experimental design, pain duration was not different between SBPp and SBPr groups. There was no difference in pain intensity between SBPp and SBPr groups at visit 1 as measured by VAS (SBPr VAS = 50 ± 7, SBPp VAS = 51 ± 5, t-test t = 0.21, mean ± SE). However, this measure diverged within 3 mo between the two groups, and by 1 yr (visit 4) pain intensity remained unchanged from baseline in SBPp patients but decreased to around 20 in SBPr patients (Fig. 1). Other pain-related characteristics showed a similar pattern, where most questionnaire outcomes related to pain, anxiety, or depression remained unchanged over the year in SBPp patients and most of these values decreased in SBPr patients (table in Fig. 1).
Extent of hippocampal connectivity.
Extent of functional connectivity was examined in CON (n = 15), SBP (n = 32), and CBP (n =17) subjects at visit 1 (Fig. 2, Table 1). Relative to CON subjects, SBP subjects showed greater extrinsic left hippocampal connectivity and greater intrinsic hippocampal connectivity bilaterally while CBP patients showed increased extrinsic connectivity bilaterally (bootstrap test; voxelwise t > 2.3, TFCE at α = 0.05; Fig. 3, A and B, Table 2). A contrast of SBPp (n = 15) and SBPr (n = 17) subjects showed no significant differences in either extrinsic or intrinsic connectivity extent. Moreover, neither SBPp (n = 14) nor SBPr (n = 16) subjects showed any changes in either intrinsic or extrinsic connectivity extent over time (visits 1–4, over 1-yr period).
Fig. 2.
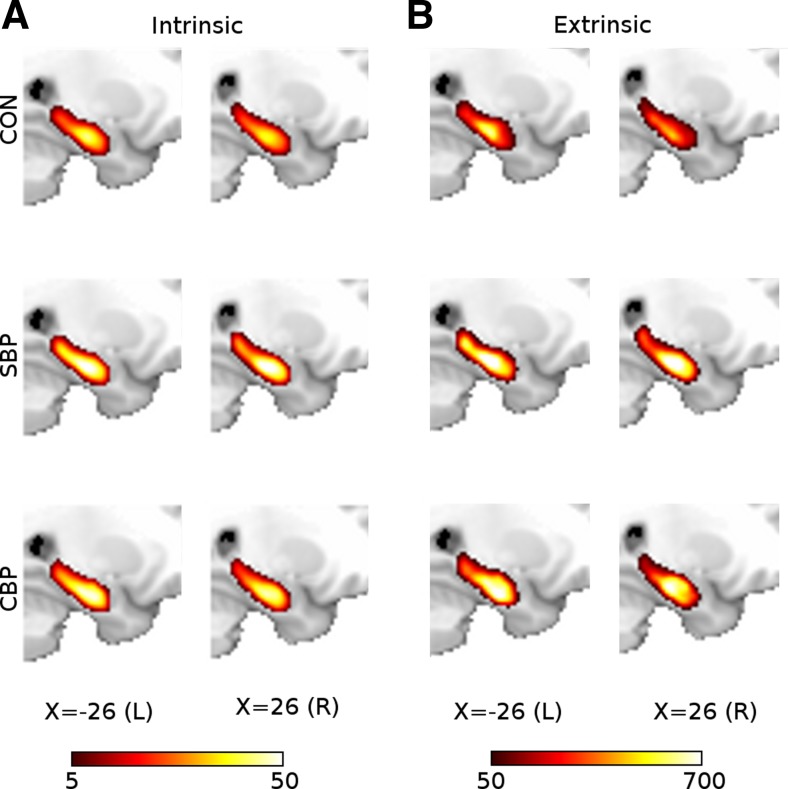
Mean intrinsic and extrinsic hippocampal connectivity in control (CON, n = 15), SBP (n = 32), and chronic back pain (CBP, n = 17) at visit 1 during a standard visual task. A: intrinsic hippocampal functional connectivity was computed for each voxel by identifying the number of other hippocampal voxels to which it was functionally connected. Extent of connectivity averaged across each group is illustrated with a heat map overlaid on a standard brain. B: extrinsic connectivity was computed by counting the number of nonhippocampal voxels to which hippocampal voxels were connected. Two voxels were considered functionally connected if their time series showed a correlation of r > 0.3. Hippocampal voxels colored yellow/white are functionally connected to more target region voxels than voxels in red/orange. Color bars indicate link count.
Table 1.
Most highly connected hippocampal voxels at visit 1 (illustrated in Fig. 2)
| Group and Scope | Hemisphere | x | y | z | Median Links (r > 0.3) | Link IQ Range |
|---|---|---|---|---|---|---|
| CON Extrinsic | L | −24 | −20 | −20 | 282 | [158, 575] |
| CON Extrinsic | R | 26 | −18 | −22 | 265 | [83, 336] |
| SBP Extrinsic | L | −26 | −20 | −20 | 379 | [280, 572] |
| SBP Extrinsic | R | 24 | −18 | −20 | 428 | [263, 601] |
| CBP Extrinsic | L | −24 | −16 | −24 | 358 | [50, 562] |
| CBP Extrinsic | R | 26 | −20 | −20 | 243 | [57, 761] |
| CON Intrinsic | L | −24 | −18 | −20 | 33 | [18, 39] |
| CON Intrinsic | R | 24 | −16 | −20 | 37 | [23, 49] |
| SBP Intrinsic | L | −26 | −18 | −20 | 44 | [26, 54] |
| SBP Intrinsic | R | 24 | −16 | −20 | 44 | [38, 56] |
| CBP Intrinsic | L | −24 | −14 | −22 | 39 | [21, 48] |
| CBP Intrinsic | R | 22 | −12 | −20 | 45 | [25, 52] |
SBP, subacute back pain; CBP, chronic back pain; CON, control; L, left; R, right.
Table 2.
Locations with greatest connectivity increase relative to control subjects at visit 1 (illustrated in Fig. 3)
| Group and Scope | Hemisphere | x | y | z | t Statistic |
|---|---|---|---|---|---|
| SBP Extrinsic | R | 30 | −10 | −18 | 3.50 |
| CBP Extrinsic | L | −32 | −14 | −26 | 3.31 |
| CBP Extrinsic | R | 24 | −10 | −18 | 3.67 |
| SBP Intrinsic | L | −20 | −8 | −20 | 3.67 |
| SBP Intrinsic | R | 24 | −12 | −22 | 3.27 |
Seed-based connectivity analysis.
We performed seed-based connectivity analyses of the hippocampus to unravel the brain regions underlying the observed overall connectivity changes. A pain-relevant seed was defined from visit 1 whole hippocampus connectivity analyses by taking the conjunction of results from SBP and CBP contrasts with CON. This included regions for which both extrinsic and intrinsic connectivity extent differed at visit 1 in SBP compared with CON (Fig. 3A) and regions that in CBP showed only extrinsic connectivity abnormalities compared with CON (Fig. 3B). This produced a ROI comprised of 30 voxels (60 mm3) in the right hippocampus (Fig. 3 inset, Fig. 4), hereon “hippocampal seed.” A whole brain connectivity analysis of the right hippocampal seed was performed for CON, SBP, and CBP at visit 1 (Fig. 5A, Table 3). To identify specific brain areas influencing hippocampal connectivity extent we contrasted the functional connectivity of each patient group with CON subjects and observed significantly higher connectivity between hippocampus and right middle temporal lobe in SBP, and no specific brain region showed greater connectivity in CBP relative to CON. Contrasts of persisting and recovering SBP show that recovering patients have greater connectivity to right fusiform gyrus (GF; mixed-effects analysis controlling for age and sex, z > 2.3, cluster P < 0.05; Fig. 5B, Table 3). Although the results obtained identify some specific targets, they do not fully explain the source of hippocampal connectivity extent differences. Therefore, we examined the more general connectivity profile by removing cluster thresholding and examining voxelwise differences at a low threshold (based on 1 standard deviation of COPE). Figure 5C shows widespread increased connectivity in SBP and in CBP. Therefore, increased extrinsic hippocampal connectivity extent in patients relative to CON subjects is due primarily to weak but widespread differences.
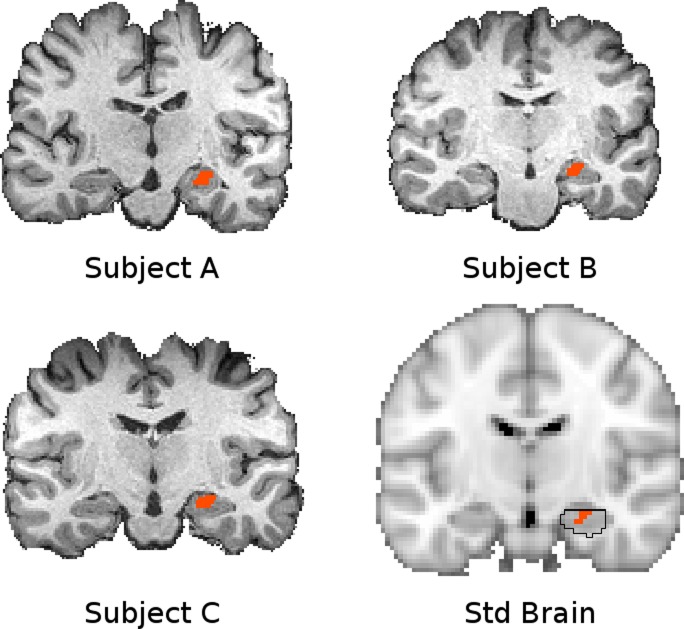
Fig. 4.
The hippocampal seed shown in standard space (bottom right, orange, Y = −14) and native space for 3 subjects. The region of interest (ROI) was defined in standard space according to a region that showed greater connectivity extent in pain patients compared with CON subjects. This region is circumscribed by the hippocampus defined in the Harvard-Oxford subcortical atlas (bottom right, black outline), which identifies the likelihood of any particular voxel belonging to a particular subcortical structure (black outline indicates P > 0.5 likelihood for hippocampus). This ROI was transformed into native space for seed-based analysis in each subject. The fidelity of this transformation is indicated with T1 volumes from 3 representative subjects with the corresponding native space seed overlaid. Connectivity maps were transformed back into standard space subsequently for higher-level analysis.
Fig. 5.
Hippocampal-cortical connectivity differences between pain patients and CON subjects. A: whole brain functional connectivity of hippocampal seed (inset) in CON (n = 15), SBP (n = 32), and CBP (n = 17) at visit 1 during a standard visual task (mixed-effects analysis controlling for age and sex, voxel z > 2.3, cluster P < 0.05). B: hippocampal functional connectivity is distinct at visit 1 between groups. Middle temporal lobe connectivity to parahippocampus is greater in SBP than CON subjects, while SBPr (n = 17) show greater fusiform gyrus connectivity to parahippocampus than SBPp (n = 15; voxel z > 2.3, cluster P < 0.05). C: contrasts of CBP and SBP with CON performed at a low threshold (z > 1.0) without cluster correction reveal that the increases in cortical connectivity to the hippocampal seed are weak but widespread. Color bar illustrates z statistic of group mean hippocampal connectivity and group contrasts.
Table 3.
| Region | Hemisphere | x | y | z | z Score |
|---|---|---|---|---|---|
| CON visit 1 | |||||
| Thalamus | R | 10 | −30 | 0 | 3.42 |
| NAc | R | 2 | 8 | −10 | 3.05 |
| SII | R | 48 | −10 | 18 | 3.08 |
| Hypothalamus | R | 8 | 0 | −14 | 6.37 |
| Hippocampus | R | 24 | −12 | −18 | 7.98 |
| MTG | R | 60 | 2 | −20 | 4.75 |
| Lingual gyrus | R | 14 | −54 | 0 | 3.78 |
| Hippocampus | L | −22 | −8 | −20 | 6.03 |
| MTG | L | −56 | −8 | −18 | 4.43 |
| Lingual gyrus | L | −12 | −58 | 6 | 3.49 |
| mPFC | 2 | 40 | −20 | 3.72 | |
| SBP visit 1 | |||||
| Caudate | R | 6 | 20 | 6 | 3.48 |
| Hippocampus | R | 24 | −12 | −18 | 12.1 |
| OFC | R | 30 | 34 | −12 | 3.44 |
| MTG | R | 60 | −14 | −10 | 6.52 |
| STG | R | 60 | −8 | −2 | 6.26 |
| pIns | R | 34 | −18 | 12 | 5.48 |
| Postcentral gyrus | R | 16 | −34 | 70 | 4.29 |
| Postcentral gyrus | R | 16 | −30 | 54 | 4.10 |
| Lingual gyrus | R | 22 | −56 | −2 | 5.06 |
| Cuneus | R | 8 | −74 | 22 | 4.40 |
| V1 | R | 2 | −94 | 12 | 4.61 |
| NAc | L | −14 | 18 | −6 | 2.86 |
| NAc | L | −4 | 6 | −6 | 3.11 |
| Caudate | L | −8 | 14 | 10 | 2.92 |
| Hippocampus | L | −34 | −12 | −22 | 8.47 |
| OFC | L | −38 | 38 | −20 | 3.81 |
| MTG | L | −52 | −14 | −18 | 6.15 |
| pIns | L | −36 | −16 | 8 | 4.81 |
| Postcentral gyrus | L | −10 | −32 | 56 | 3.65 |
| Lingual gyrus | L | −18 | −52 | 2 | 5.55 |
| Precuneus | L | −8 | −58 | 20 | 5.02 |
| mPFC | −4 | 52 | −14 | 5.10 | |
| Subcallosal area | −2 | 20 | −20 | 4.60 | |
| CBP visit 1 | |||||
| Thalamus | L | −4 | −22 | 2 | 3.52 |
| Thalamus | L | −4 | −10 | 4 | 3.53 |
| Hippocampus | L | −24 | −16 | −20 | 5.19 |
| MTG | L | −54 | −8 | −22 | 4.16 |
| pIns | L | −32 | −16 | 6 | 3.31 |
| PCC | L | −4 | −50 | 6 | 4.21 |
| Lingual gyrus | L | −12 | −62 | −8 | 3.39 |
| mPFC | 0 | 30 | −20 | 4.28 | |
| Visit 1 SBP > CON | |||||
| MTL | R | 66 | −12 | −2 | 3.30 |
| Visit 1 SBPr > SBPp | |||||
| GF | R | 46 | −64 | −10 | 4.32 |
| SBPr visit 1 > visit 4 | |||||
| GF | R | 34 | −60 | −8 | −3.79 |
| MFG | L | −30 | 6 | 56 | 3.26 |
| SBPp visit 1 > visit 4 | |||||
| CG | L | −4 | −50 | −10 | −3.82 |
| mPFC | R | 10 | 62 | −4 | −3.33 |
| Lpc | R | 12 | −32 | 64 | −3.27 |
| CON visit 1 > visit 4 | |||||
| ACC | L | −8 | 30 | 24 | 3.60 |
SBPp, SBP with persistent pain; SBPr, SBP with resolution of pain; NAc, nucleus accumbens; SII, secondary somatosensory cortex; MTG, middle temporal gyrus; mPFC, medial prefrontal cortex; OFC, orbital frontal cortex; STG, superior temporal gyrus; pIns, posterior insula; V1, primary visual cortex; PCC, posterior cingulate cortex; MTL, Medial temporal lobe; GF, fusiform gyrus; MFG, middle frontal gyrus; CG, cingulate gyrus; Lpc, paracentral lobule; ACC, anterior cingulate cortex.
Longitudinal comparison of seed-based connectivity.
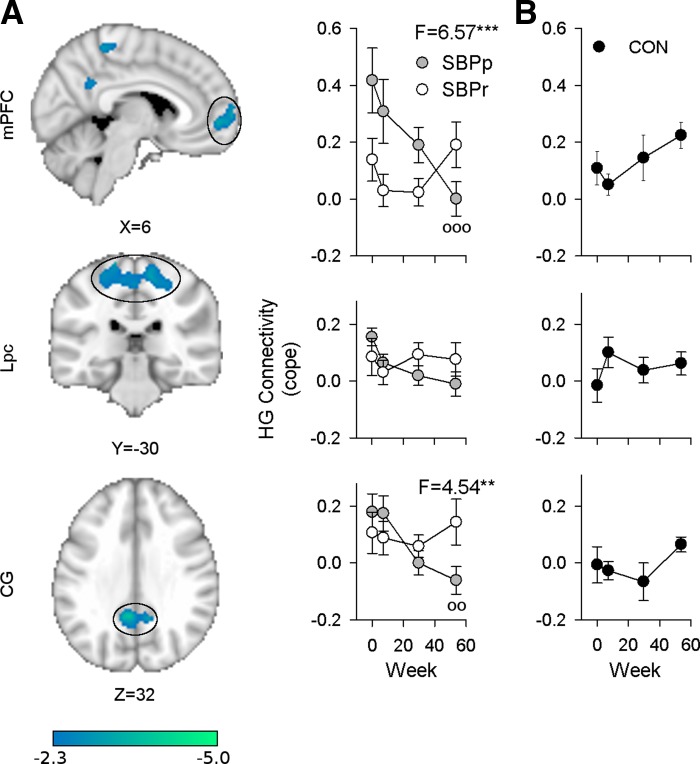
Because chronic and subacute pain appear to produce higher than normal hippocampal connectivity compared with pain-free CON subjects, and some subacute patients experience a reduction in their pain with time (SBPr) while for others pain persists (SBPp), we hypothesized that hippocampal connectivity would evolve differently between these two subgroups. In a whole brain contrast between visit 1 and visit 4 1 yr later, SBPr patients showed a reduction in hippocampal connectivity to right GF and an increase in connectivity to left middle frontal gyrus (MFG; n = 16, mixed effects, paired t-test, z > 2.3, cluster α = 0.05, Table 3). When these two brain regions were examined (ROI analyses) there was indeed a significant difference between SBPr and SBPp in the temporal evolution of hippocampal connectivity [SBPp n = 14, right GF: rm-ANCOVA, group × time interaction F(3,75) = 9.00, P = 4 × 10−5; left MFG: group × time interaction F(3,75) = 3.85, P = 0.0128; corrected for age and sex] and GF connectivity was significantly higher at visits 1 and 2 in SBPr than SBPp (Tukey's test, P = 0.0008, P = 0.030, respectively), reiterating the results found in full brain voxelwise contrasts at visit 1 (Fig. 5B).
Significant differences between visits 1 and 4 in SBPp patients were also identified: hippocampal connectivity to mPFC, paracentral lobule (Lpc), and cingulate gyrus (CG) all decreased significantly in time [mixed effects, paired t-test, z > 2.3, cluster α = 0.05; Fig. 6A, Table 3]. Hippocampal-mPFC and hippocampal-CG connectivity (HG-mPFC and HG-CG, respectively) showed a significantly different evolution in SBPp compared with SBPr as well [rm-ANCOVA, group × time interaction: mPFC, F(3,75) = 6.57, P = 0.00053; CG, F(3,75) = 4.54, P = 0.0056; corrected for age and sex; Fig. 6A]. Regions showing differences between SBPp and SBPr showed no time effect when examined in CON [rm-ANOVA: mPFC, F(3,42) = 2.24, P = 0.10; CG, F(3,42) = 1.9, P = 0.15; GF, F(3,42) = 1.1, P = 0.36; MFG, F(3,42) = 1.8, P = 0.16; Fig. 6B]. These results indicate that hippocampal connectivity to distinct brain regions is changing in opposite directions in SBPp and SBPr.
Fig. 6.
Longitudinally, SBPp and SBPr subjects show different changes in connectivity over 1 yr. A: right hippocampal gyrus (HG) in SBPp subjects (n = 14) shows significant changes in connectivity to medial prefrontal cortex (mPFC), paracentral lobule (Lpc), and cingulate gyrus (CG) over 1 yr (mixed effects, paired t-test visit 1 vs. visit 4, z > 2.3, cluster P < 0.05). Post hoc analysis controlling for age and sex showed that time evolution differs between SBPp and SBPr (n = 16) for mPFC and CG [rm-ANCOVA, group × time interactions: mPFC, F(3,75) = 6.57, P < 0.00053; CG, F(3,75) = 4.54, P = 0.0056]. Graphs are aligned to corresponding ROI. B: healthy CON subjects (n = 15) show no significant changes in HG to mPFC, Lpc, and GF over 1 yr [rm-ANOVA, F(3,42) = 2.24, P = 0.10 and F(3,42) = 1.1, P = 0.36, respectively]. **P < 0.01, ***P < 0.001, group × time interactions after controlling for sex and age. Mean ± SE shown for each visit.
BOLD task dependence of hippocampal seed.
Although task performance was consistent across groups, this does not exclude the possibility of differential task effects on hippocampal BOLD signal, so the relationship between the standard visual task and hippocampal BOLD was also examined to confirm that no task-related confound was underlying the differences between groups in time. The hippocampal BOLD averaged across all subjects at visit 1 showed a significant inverse correlation to the task (n = 64, r2 = 0.18, P < 10−12). There was some variability in this correlation across subjects, but it was generally negative [−0.08 ± 0.01, mean Fisher-z(r) ± SE] and showed no difference between groups at visit 1 [ANCOVA: F (3,53) = 1.6, P = 0.19]. The magnitude of this correlation decreased in time in CON, SBPp, and SBPr [CON n = 17, SBPp n = 14, SBPr n = 16; rm-ANCOVA time effect: F(3,114) = 2.86, P = 0.04], but hippocampal task dependence changes did not differ between groups [group × time effect: F(6,114) = 1.24, P = 0.29; group effect: F(2,38) = 0.72, P = 0.49], unlike hippocampal connectivity changes. Thus the possibility that task effects are responsible for the differences we are seeing between patient groups can be ruled out both behaviorally and neurophysiologically. Task performance was not found to differ between SBPp and SBPr over the course of the study, and hippocampal BOLD signal dependence on task was not different between groups or visits, so task effects are constant across all conditions.
Connectivity of hippocampus in relation to additional potential confounds: head motion, depression, and hippocampal volume.
There were no differences between groups in motion at either visit 1 [F(3,60) = 0.88130, P = 0.456] or visit 4 [F(2,44) = 2.583, P = 0.087]. There was also no significant correlation between motion and visit 1 connectivity extent (right intrinsic HG: ρ = −0.098, P = 0.446, df = 62; right extrinsic HG: ρ = −0.02, P = 0.871, df = 62), visit 1 hippocampal functional connectivity (HG-mPFC: ρ = 0.060, P = 0.70; HG-CG: ρ = −0.115, P = 0.461; HG-GF: ρ = −0.098, P = 0.532; HG-MFG: ρ = 0.181, P = 0.254; df = 45), or visit 4 functional connectivity (HG-mPFC: ρ = 0.120, P = 0.444; HG-CG: ρ = −0.257, P = 0.095; HG-GF: ρ = 0.275, P = 0.076; HG-MFG: ρ = 0.166, P = 0.287; n = 45).
Depression as measured by BDI did not show any significant correlation with connectivity extent (right intrinsic HG: ρ = 0.230, P = 0.076, n = 62; right extrinsic HG: ρ = 0.205, P = 0.116, df = 62), visit 1 functional connectivity (HG-mPFC: ρ = 0.179, P = 0.257; HG-CG: ρ = 0.137, P = 0.387; HG-GF: ρ = 0.069, P = 0.663; HG-MFG: ρ = −0.049, P = 0.757; n = 44), or visit 4 functional connectivity (HG-mPFC: ρ = −0.257, P = 0.214; HG-CG: ρ = −0.258, P = 0.213; HG-GF: ρ = 0.288, P = 0.162; HG-MFG: ρ = −0.223, P = 0.284; n = 27). Neither did hippocampal volume correlate with connectivity extent (right intrinsic HG: ρ = −0.041, P = 0.821; right extrinsic HG: ρ = 0.207, P = 0.233). Nevertheless, the relationship between BDI and connectivity extent approaches significance, so we verified that SBP and CBP showed significantly different hippocampal connectivity after controlling for BDI, age, and sex with ANCOVA. No effects were significant in the ANCOVA of CON and SBP intrinsic connectivity, but extrinsic connectivity remained significantly greater in patients than CON subjects [ANCOVA CBP and CON group effect: F = 7.487, P = 0.011; SBP and CON group effect: F(1,40) = 4.278, P = 0.046]. Thus these potential confounds show no identifiable effects on intrinsic or extrinsic connectivity of the hippocampus.
Hippocampal connectivity and pain.
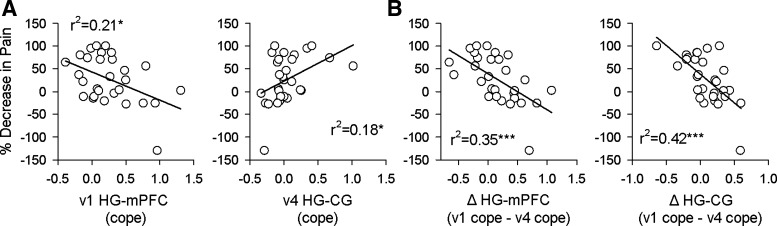
mPFC connectivity has been implicated in the future experience of pain. Strong mPFC-NAc connectivity during the spontaneous pain-rating task in our subjects was previously reported to be predictive of maintenance of pain (Baliki et al. 2012). Similarly, after painful thermal stimulation over several consecutive days hippocampal-ventromedial PFC (vmPFC) connectivity preceding painful stimulation correlates with the intensity of induced pain in healthy control subjects (Riedl et al. 2011). These findings suggest that HG-mPFC connectivity might correlate with future pain in our subjects. Indeed, a comparison of the percent decrease in a patient's pain ratings over the course of the study showed a significant negative correlation with visit 1 HG-mPFC functional connectivity (n = 29, P = 0.013, r2 = 0.21; Fig. 7A). Notably, this connectivity was independent of current pain (HG-mPFC visit 1 vs. visit 1 VAS: r2 = 0.004, P = 0.73). On the other hand, HG-CG connectivity only at visit 4 showed a positive relationship with how much back pain had decreased over the preceding year (r2 = 0.18, P = 0.02) but was borderline related to current pain (HG-CG visit 4 vs. visit 4 VAS: r2 = 0.13, P = 0.052). When similar relationships were examined for MFG and GF no significant behavioral correlates were found. In SBP, we observe that the greater the loss of functional connectivity between HG-mPFC or between HG-CG over the course of the study, the less the change in pain (n = 29, ΔmPFC, ΔVAS r2 = 0.35, P = 0.0007; ΔCG, ΔVAS r2 = 0.42, P = 0.00014; Fig. 7B). Overall, hippocampal connectivity to mPFC and to CG, and their reorganization in time, reflect the variation in pain in SBP over a 1-yr period, which in turn underlies the distinction between SBPp and SBPr.
Fig. 7.
HG connectivity reflects past and future pain in SBP (n = 29). A: HG-mPFC at visit 1 (v1) is significantly anticorrelated with decrease in pain by visit 4 (v4) 1 yr later (r2 = 0.21, P = 0.013, significant after correcting for multiple comparisons), while HG-CG at visit 4 correlates significantly with the amount that pain has decreased since visit 1 (r2 = 0.18, P = 0.02, uncorrected, not significant after correcting for multiple comparisons). B: decrease in connectivity shows a stronger anticorrelation to decrease in pain for both mPFC and CG. The more HG-mPFC and HG-CG connectivity drops over the course of 1 yr, the more persistent the pain at visit 4 (mPFC r2 = 0.35, P = 0.0007, CG r2 = 0.42, P = 0.00014), supporting the notion of an early hippocampal contribution to pain chronification. *P < 0.05, ***P < 0.001.
Hippocampal connectivity and pain across tasks.
The relationships between back pain and hippocampal connectivity with mPFC and CG provide the most direct relationship between the hippocampal-cortical connectivity and chronification of back pain. To test the extent to which these results are task independent and to validate this outcome in an alternative condition, we examined HG-mPFC and HG-CG functional connections in a second task, in which SBP patients rated fluctuations of back pain intensity. We related outcomes to back pain as well as to corresponding hippocampal functional connections identified during the visual rating task.
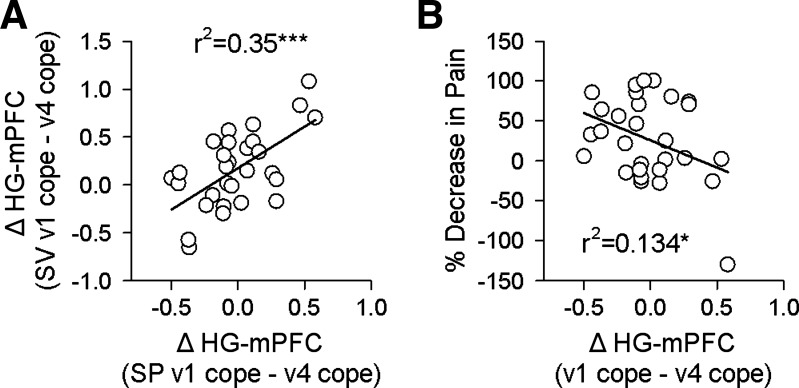
The connectivity of the hippocampal seed to the rest of the brain was computed at visits 1 and 4, and mean COPEs corresponding to the mPFC ROI and CG ROI were extracted from each subject at both visits. Functional connectivity of HG-mPFC at visit 1 and visit 4 correlated between the two tasks (P = 0.01, r2 = 0.21 and P = 0.013, r2 = 0.20, respectively), and HG-CG functional connectivity correlated across tasks as well at visit 1 but not at visit 4 (P = 0.0029, r2 = 0.41 and P = 0.66, r2 = 0.12, respectively). Decrease in connectivity from visit 1 to visit 4 was also highly consistent across tasks for HG-mPFC (P = 0.001, r2 =0.35; Fig. 8A) but not for HG-CG (P = 0.115, r2 = 0.09). For the pain-rating task, HG-mPFC connectivity at visit 1 showed a weak correlation with future pain (P = 0.10), while decrease in HG-mPFC connectivity between visits 1 and 4 showed a significant anticorrelation (P = 0.05, r2 = 0.134, n = 29; Fig. 8B). Overall, the HG-mPFC functional connectivity properties generalize better across tasks than the HG-CG connectivity.
Fig. 8.
Decrease in HG-mPFC connectivity during a spontaneous pain-rating (SP) task (n = 29) shows the same relationship with pain identified in the standard visual (SV) task. A: decreases in HG-mPFC connectivity from visit 1 to visit 4 are significantly correlated between both the SV and SP tasks in SBP patients (r2 = 0.35, P = 0.001). B: in the SP task in SBP patients this connectivity decrease also shows a significant anticorrelation with decrease in pain (r2 = 0.134, P = 0.05). *P < 0.05, ***P < 0.001.
DISCUSSION
In this study we use hippocampal activity during a simple visual tracking task to gain insight into information exchange within the hippocampus, between it and the rest of the brain cross-sectionally, and during the development of chronic pain. Our results reveal weak but widespread increases in hippocampal connectivity in pain patients compared with control subjects, demonstrating a disruption in the normal network of the hippocampus that may impact its standard functions in learning, memory, and emotional regulation. Longitudinally we find distinct localized decreases in hippocampal functional connectivity in persisting SBP. The longitudinal changes in HG-mPFC functional connectivity reflect pain intensity changes and show consistency across tasks. These differences help identify the evolution of hippocampal function during pain persistence and remission.
Intrinsic and extrinsic hippocampal connectivity was increased in SBP in the anterior region of the hippocampus. Increased intrinsic connectivity in this area has been suggested to play a role in mild stages of memory loss in early Alzheimer's disease (Das et al. 2013). Our identification of a similar region of abnormal intrinsic connectivity in pain patients, who often suffer from problems in emotional decision making (Apkarian et al. 2004) and working memory (Dick and Rashiq 2007; Kim et al. 2012; Ling et al. 2007), may indicate a similar physiological mechanism for these deficits, particularly at the subacute stage of pain. Moreover, decreased hippocampal synaptic plasticity and neurogenesis are observed in animal models of chronic pain (Mutso et al. 2012; Terada et al. 2008), contributing to decreased synaptic connectivity. Thus Alzheimer's disease and the early stages of pain may share concomitantly increased connectivity extent and decreased plasticity contributing to memory deficits.
A similar region of anterior hippocampus has recently been identified during a memory task paired with acute pain stimulation. This region showed reduced extrinsic connectivity, which contributed to pain-specific interference with an early stage of memory formation (Forkmann et al. 2013), likely reflecting a reduction in memory encoding-related activity during painful stimulation. The heightened extrinsic connectivity we find in chronic pain during a nonmemory task complements this study and suggests that ongoing pain-related activity might chronically compete with non-pain-related memory processing in the hippocampus. Moreover, heightened connectivity may indicate a significant increase in pain-related memory formation and consolidation in these patients, consistent with our finding that greater HG-mPFC connectivity at visit 1 leads to more pain retained over time. This corresponds to our hypothesis that chronic pain is a state of continual learning coupled with an inability to extinguish aversive associations (Apkarian 2008; Apkarian et al. 2009, 2011; Mutso et al. 2012).
While the hippocampus plays an important role in memory consolidation (Abrahams et al. 1999; Morris et al. 1986; Zola et al. 2000), the specific anterior region we identify is also involved in regulating emotional and motivating behaviors (Sahay and Hen 2007), in particular interacting with the amygdala during fear conditioning in both human subjects (Alvarez et al. 2008) and animal models (LeDoux 2000). Additionally, ventral hippocampus of rodents, corresponding to this anterior hippocampal region in humans, is suggested to modulate other areas of the mesolimbic system, particularly mPFC, amygdala, and NAc (Fanselow and Dong 2010). Projections from this area are necessary for mPFC (Ferino et al. 1987; Parent et al. 2010), effectively gating NAc activity through postsynaptic depolarization (O'Donnell and Grace 1995), and are important in selecting emotional spatial information (Phillips and LeDoux 1992). This hippocampal involvement supports our hypothesis, suggesting that the increased anterior hippocampal connectivity we see in pain patients may play a role in aversive learning and contribute to heightened anxiety observed in patients (Gore et al. 2012). To study the longitudinal development of hippocampal connectivity in SBP, we examine the conjunction of our cross-sectional analysis of SBP and CBP versus CON; we identify a ROI whose functionally connected regions cause the differences between pain groups and CON subjects.
The identified hippocampal ROI shows distinctive longitudinal connectivity changes between SBPp and SBPr patients. While several regions differ between groups, the strongest evidence of hippocampal contributions to chronic pain transition is the behavioral correlates reflecting temporally displaced events. Specifically, HG-mPFC and HG-CG connectivity manifest retrospective- and prospective-like memory encoding: the former anticipates pain outcome, while the latter appears to reflect the developmental course of pain, suggesting an active role of the hippocampus in encoding diverse pain-related memories. The HG-mPFC is of particular interest since abnormalities in this circuit appear to be retained across tasks in SBP and have bearing on the progression of pain without showing any relationship with current ongoing pain. Moreover, this connectivity was reproduced in the same spontaneous pain data previously used to show the predictive abilities of NAc-mPFC connectivity (Baliki et al. 2012), inviting direct comparison between the two findings and enabling development of a more generalized circuit model for pain chronification.
Both HG and mPFC are implicated in working memory (Stern et al. 2001), and recent animal studies have directly linked altered HG-mPFC functional connectivity in chronic pain to spatial working memory deficits (Cardoso-Cruz et al. 2013). Additionally, HG-mPFC bidirectional interactions are necessary for making choices between different response strategies (Goto and Grace 2008), suggesting that HG-mPFC interactions are essential for mediating behaviorally relevant spiking patterns to utilize memory in driving goal-directed behavior (Goto and Grace 2008; Squire 1992). If interpreted in light of these results, our findings suggest that in addition to memory deficits, the decrease in HG-mPFC activity longitudinally in SBPp may also contribute to difficulty in emotional decision making observed in chronic pain (Apkarian et al. 2004). These cognitive deficits, and underlying HG-mPFC connectivity decreases, may themselves be catalysts for pain chronicity (Apkarian 2008; Apkarian et al. 2009).
The connectivity strength of HG-mPFC in SBP correlates with the persistence of pain over time, confirming the involvement of this circuitry in the transition to chronic pain. As HG-mPFC connectivity is predominantly comparable in magnitude between SBPr and CON at visit 1, our findings point toward an early, transient HG-mPFC contribution in the transition to chronicity. Previously, mPFC-NAc connectivity was shown to predict this transition to chronic pain (Baliki et al. 2012), and the mPFC was found to then represent spontaneous pain in chronicity (Baliki et al. 2006). Our findings complement the observation of hippocampal gating of mPFC-NAc connectivity (O'Donnell and Grace 1995) and hippocampal modulation of mPFC, amygdala, and NAc function (Baliki et al. 2012; Fanselow and Dong 2010). Taken together, these observations and our discovery of an early HG-mPFC contribution to pain chronification bolster the findings of Baliki et al. (2012) and extend the understanding of how brain reorganization, particularly the hippocampus, is involved in pain chronification. Specifically, we propose that the hippocampus may play an early role in facilitating the hyperactive NAc-mPFC connectivity causally involved in pain chronification.
While chronic pain patients often suffer from depression (Gore et al. 2012), which is known to be strongly associated with decreased hippocampal volume (Cole et al. 2011) and leads to altered hippocampal connectivity in depressed patients, our results indicate that neither depression nor hippocampal volume correlates with levels of hippocampal connectivity extent in pain patients. Therefore, while depression is often seen comorbid with chronic back pain, depression levels do not directly contribute to the evolution of hippocampal connectivity observed in chronic pain. Additionally, no other behavioral measures or medication usage are found to correlate with hippocampal connectivity extent. While undiscovered potential confounds may remain, including the possibility that the visual task was more difficult for patients or postrecovery lifestyle changes could influence brain connectivity, our results strongly indicate that distinct hippocampal connectivity in persistent pain is unaffected by other behavioral or emotional factors.
Our findings provide new details bearing on default mode network (DMN) disruptions in chronic pain. Task-free fMRI and diffusion tensor imaging have identified the hippocampus as a component of the DMN, a network of brain regions including mPFC and CG that are more activated during rest (Fox et al. 2005; Greicius et al. 2003, 2009) and are disrupted in chronic back pain (Baliki et al. 2011). In particular, recent evidence has demonstrated increased connectivity between DMN and non-DMN regions in chronic back pain (Birnbaum et al. 2004; Loggia et al. 2013; Tagliazucchi et al. 2010; Troparevsky et al. 2009). Our results are consistent with these findings, showing that hippocampal BOLD is anticorrelated with the standard visual task, that functional connectivity is abnormal during a task at visit 1, that extrinsic connectivity of the hippocampus in both CBP and SBP is increased, and finally that hippocampal connectivity to regions associated with the DMN is disrupted as pain transitions to chronicity.
In summary, our results offer strong evidence that hippocampal connectivity extent is distinct between pain patients and control subjects, and that hippocampal connectivity shows unique longitudinal changes over time between patients who transition from subacute to chronic pain and those who recover. In particular, the changing HG-mPFC connectivity over time in SBPp, which reflects back pain intensity changes over time, suggests a significant role of this circuitry in the early transition to chronic pain and the learning and emotional deficits seen in chronic pain patients.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant NS-035115.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.A.M., B.P., L.H., M.N.B., S.T., K.M.H., T.J.S., and A.V.A. performed experiments; A.A.M., B.P., and L.H. analyzed data; A.A.M., B.P., L.H., M.N.B., T.J.S., and A.V.A. interpreted results of experiments; A.A.M. and B.P. prepared figures; A.A.M., B.P., and A.V.A. drafted manuscript; A.A.M., B.P., M.N.B., T.J.S., and A.V.A. edited and revised manuscript; A.A.M., B.P., L.H., M.N.B., S.T., K.M.H., T.J.S., and A.V.A. approved final version of manuscript; M.N.B., T.J.S., and A.V.A. conception and design of research.
ACKNOWLEDGMENTS
The authors thank all subjects who volunteered time and effort for this study. We also thank all Apkarian lab members who read and commented on earlier versions of the manuscript. Dr. T. Parrish and the staff at Northwestern University Center for Brain Imaging were helpful in the initial design of brain scans and in data collection.
REFERENCES
- Abrahams S, Morris RG, Polkey CE, Jarosz JM, Cox TC, Graves M, Pickering A. Hippocampal involvement in spatial and working memory: a structural MRI analysis of patients with unilateral mesial temporal lobe sclerosis. Brain Cogn 41: 39–65, 1999 [DOI] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci 26: 63–72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci 28: 6211–6219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol 18: 464–468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol 87: 81–97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152: S49–S64, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain 108: 129–136, 2004 [DOI] [PubMed] [Google Scholar]
- Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci 31: 13981–13990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 26: 12165–12173, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 28: 1398–1403, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15: 1117–1119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage 20: 1052–1063, 2003 [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science 306: 882–884, 2004 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 29: 1860–1873, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Cruz H, Lima D, Galhardo V. Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity. J Neurosci 33: 2465–2480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda ML, Alvin MD, Schnitzer TJ, Apkarian AV. Pain characteristic differences between subacute and chronic back pain. J Pain 12: 792–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord 134: 483–487, 2011 [DOI] [PubMed] [Google Scholar]
- Das SR, Pluta J, Mancuso L, Kliot D, Orozco S, Dickerson BC, Yushkevich PA, Wolk DA. Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus 23: 1–6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg 104: 1223–1229, 2007 [DOI] [PubMed] [Google Scholar]
- Eguiluz VM, Chialvo DR, Cecchi GA, Baliki M, Apkarian AV. Scale-free brain functional networks. Phys Rev Lett 94: 018102, 2005 [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon's horn to the medial prefrontal cortex in the rat. Exp Brain Res 65: 421–426, 1987 [DOI] [PubMed] [Google Scholar]
- Flor H, Knost B, Birbaumer N. The role of operant conditioning in chronic pain: an experimental investigation. Pain 95: 111–118, 2002 [DOI] [PubMed] [Google Scholar]
- Forkmann K, Wiech K, Ritter C, Sommer T, Rose M, Bingel U. Pain-specific modulation of hippocampal activity and functional connectivity during visual encoding. J Neurosci 33: 2571–2581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101: 3270–3283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 37: E668–E677, 2012 [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex 18: 1407–1414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19: 72–78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia 47: 1686–1693, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. Contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci 4: 1285–1295, 1992 [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Watts DG. Spectral Analysis and Its Applications. San Francisco, CA: Holden-Day, 1968 [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002 [DOI] [PubMed] [Google Scholar]
- Keleta YB, Martinez JL. Brain circuits of methamphetamine place reinforcement learning: the role of the hippocampus-VTA loop. Brain Behav 2: 128–141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PE. Randomization tests in econometrics. J Business Econ Stat 13: 85–94, 1995 [Google Scholar]
- Kim SH, Kim SK, Nam EJ, Han SW, Lee SJ. Spatial versus verbal memory impairments in patients with fibromyalgia. Rheumatol Int 32: 1135–1142, 2012 [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184, 2000 [DOI] [PubMed] [Google Scholar]
- Ling J, Campbell C, Heffernan TM, Greenough CG. Short-term prospective memory deficits in chronic back pain patients. Psychosom Med 69: 144–148, 2007 [DOI] [PubMed] [Google Scholar]
- Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain 154: 24–33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct Funct 218: 903–912, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Hagan JJ, Rawlins JN. Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q J Exp Psychol B 38: 365–395, 1986 [PubMed] [Google Scholar]
- Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci 32: 5747–5756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15: 3622–3639, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro. Cereb Cortex 20: 393–403, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56: 907–922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285, 1992 [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5: 83–96, 1997 [DOI] [PubMed] [Google Scholar]
- Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents. Neuropsychopharmacology 36: 979–992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl V, Valet M, Woller A, Sorg C, Vogel D, Sprenger T, Boecker H, Wohlschlager AM, Tolle TR. Repeated pain induces adaptations of intrinsic brain activity to reflect past and predict future pain. Neuroimage 57: 206–213, 2011 [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci 10: 1110–1115, 2007 [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex 15: 1332–1342, 2005 [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44: 83–98, 2009 [DOI] [PubMed] [Google Scholar]
- Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol 23: 162–171, 2013 [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195–231, 1992 [DOI] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus 11: 337–346, 2001 [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett 485: 26–31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M, Kuzumaki N, Hareyama N, Imai S, Niikura K, Narita M, Yamazaki M, Suzuki T. Suppression of enriched environment-induced neurogenesis in a rodent model of neuropathic pain. Neurosci Lett 440: 314–318, 2008 [DOI] [PubMed] [Google Scholar]
- Troparevsky MC, Zhao K, Xiao D, Zhang Z, Eguiluz AG. Tuning the electronic coupling and magnetic moment of a metal nanoparticle dimer in the nonlinear dielectric-response regime. Nano Lett 9: 4452–4455, 2009 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14: 1370–1386, 2001 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20: 45–57, 2001 [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci 20: 451–463, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]