Abstract
Individuals with spinal cord injury (SCI) above the T6 spinal segment suffer from orthostatic intolerance. How cerebral blood flow (CBF) responds to orthostatic challenges in SCI is poorly understood. Furthermore, it is unclear how interventions meant to improve orthostatic tolerance in SCI influence CBF. This study aimed to examine 1) the acute regional CBF responses to rapid changes in blood pressure (BP) during orthostatic stress in individuals with SCI and able-bodied (AB) individuals; and 2) the effect of midodrine (alpha1-agonist) on orthostatic tolerance and CBF regulation in SCI. Ten individuals with SCI >T6, and 10 age- and sex-matched AB controls had beat-by-beat BP and middle and posterior cerebral artery blood velocity (MCAv, PCAv, respectively) recorded during a progressive tilt-test to quantify the acute CBF response and orthostatic tolerance. Dynamic MCAv and PCAv to BP relationships were evaluated continuously in the time domain and frequency domain (via transfer function analysis). The SCI group was tested again after administration of 10 mg midodrine to elevate BP. Coherence (i.e., linearity) was elevated in SCI between BP-MCAv and BP-PCAv by 35% and 22%, respectively, compared with AB, whereas SCI BP-PCAv gain (i.e., magnitudinal relationship) was reduced 30% compared with AB (all P < 0.05). The acute (i.e., 0–30 s after tilt) MCAv and PCAv responses were similar between groups. In individuals with SCI, midodrine led to improved PCAv responses 30–60 s following tilt (10 ± 3% vs. 4 ± 2% decline; P < 0.05), and a 59% improvement in orthostatic tolerance (P < 0.01). The vertebrobasilar region may be particularly susceptible to hypoperfusion in SCI, leading to increased orthostatic intolerance.
Keywords: cerebral autoregulation, orthostatic tolerance, alpha-1 agonist
spinal cord injury (sci) is a devastating chronic condition that results in not only motor and sensory deficits, but also autonomic dysfunction (22). As a consequence of decentralization of sympathetic fibers, those with SCI suffer from low resting blood pressure (BP) and episodes of severe hypotension when moving to an upright position (orthostatic hypotension) (8). Orthostatic hypotension has been shown to be an important independent risk factor for the development of stroke in able-bodied (AB) individuals (10), and stroke is two to three times more likely in those with SCI (51).
A fundamental property of cerebral vessels is their capacity to locally regulate cerebral blood flow (CBF). Effective cerebral autoregulation (CA) to buffer changes in BP requires an integrated response from myogenic, neurogenic, metabolic, and systemic factors (46). Brief disruptions in CBF caused by impaired BP control during orthostatic hypotension may cause irreversible neuronal cell death (2, 11). Consequently, poor BP control in individuals with SCI makes appropriate regulation of CBF crucial for preventing stroke.
A number of studies have examined static CA [i.e., the ability to maintain CBF during a range of steady-state (0 Hz) BPs] in those with SCI, and there is consistent evidence that static CA is preserved in this population [reviewed in (33)]. In contrast, the dynamic relationship between BP and CBF has been examined only in two studies (37, 49). Both static and dynamic CA are believed to be regulated by a combination of myogenic, metabolic, and neurogenic control mechanisms [for review see (32)]; however, static and dynamic CA may rely on relatively different influences from various regulatory factors (9). For example, it has been suggested that neural control more heavily influences dynamic CA as opposed to static CA (9), whereas endothelial (i.e., nitric oxide) dependence has been shown not to influence dynamic or static CA (52). The two studies on dynamic CA in individuals with SCI reported only spontaneous analyses of cerebral pressure-flow relationships (37, 49), which may not reflect how the cerebrovasculature responds to rapid perturbations in BP (30, 40, 41). In addition, the relationship between CBF and BP in a hemodynamically stable, closed-loop situation is unlikely to simulate the drastic reductions in cerebral perfusion pressure that occur during orthostatic challenges (40). In both these studies (37, 49), only metrics from the middle cerebral artery (MCA) were reported as opposed to arteries of the vertebrobasilar system; arteries that may be more related to orthostatic tolerance as it perfuses the medulla oblongata, which contains associated autonomic control centers and discrete regions responsible for consciousness (39). Furthermore, several studies have recently shown that the internal carotid/MCA region is differentially sensitive to orthostatic challenges compared with the vertebral/posterior cerebral artery (PCA) region (3, 38, 47, 48).
Midodrine, an alpha-1 adrenoreceptor agonist, is used as a first line of defense to improve orthostatic tolerance in those with acute SCI (23). Although a 10-mg dose of midodrine has been shown to mitigate orthostatic hypotension in SCI, its influence on CBF is unclear (44, 45). One study reported no change in supine CBF velocity of the MCA (MCAv) after midodrine administration in individuals with SCI; however, reductions in MCAv during an orthostatic challenge were attenuated (45). In a follow-up study from that research group, MCAv was increased at baseline, and was not changed during an orthostatic challenge (44). Both of these studies, however, examined the relationship between MCAv in a homeostatic/steady-state situation (i.e., 0 Hz), and these findings are likely to be clarified and enhanced by an evaluation of the dynamic relationship between BP and CBF both at rest and during orthostatic stress. Furthermore, these studies used only self-reported symptoms of presyncope, and did not report any statistical analysis between trials (i.e., midodrine vs. no midodrine) (44, 45).
This study had four main objectives. The first was to compare static and dynamic regional CBF responses to orthostasis in SCI and AB individuals. The second was to evaluate how midodrine influences the static and dynamic regional CBF responses to orthostasis in SCI. The third objective was to evaluate orthostatic tolerance in individuals with SCI before and after midodrine. The final objective was to evaluate the relationship between dynamic systemic BP regulation and the cerebral pressure-flow relationship in individuals with SCI. We hypothesized that 1) static and dynamic CA in those with SCI would be preserved in both the MCA and PCA; 2) midodrine would not influence static CA but would partially normalize dynamic cerebral pressure-flow metrics to values reported in AB; 3) orthostatic tolerance would be improved after midodrine administration; and 4) dynamic cerebral pressure-flow relationships would be uncoupled from cardiac baroreflex function in individuals with SCI.
METHODS
Ten individuals with SCI participated in this study (C4-T5; American Spinal Cord Injury Association impairment scale A and B; Table 1). Eight participants were <1 yr postinjury; two were >1 yr postinjury (Table 1). All participants were referred for autonomic testing due to clinical observations. The control group consisted of 10 age- and sex-matched AB individuals. All testing took place at GF Strong Rehabilitation Centre, Vancouver, BC, Canada. Participant characteristics are presented in Tables 1 and 2. All participants were instructed to abstain from exercise and alcohol for 24 h before testing. No caffeine was permitted the day of testing. Additionally, participants abstained from all other medications on the day of testing, and had a small meal (e.g., a small yogurt) approximately 1 h before testing. Those who were smokers or had any history of cardiovascular disease were excluded from participation. All participants provided written informed consent in accord with the Clinical Research Ethics Board at the University of British Columbia, which approved this study.
Table 1.
Individual demographic information for participants with spinal cord injury
| Participant | SCI level | DOI | AIS grade | Age, yr | Stature, cm | Mass, kg | Education* | Sex |
|---|---|---|---|---|---|---|---|---|
| 1 | T5 | 11 | A | 27 | 158.0 | 58.0 | 5 | F |
| 2 | T5 | 324 | A | 43 | 165.5 | 66.0 | 4 | F |
| 3 | C4 | 6.5 | A | 47 | 175.5 | 79.0 | 2 | M |
| 4 | C5 | 144 | A | 36 | 180.5 | 70.0 | 5 | M |
| 5 | C5 | 7 | A | 17 | 175.0 | 54.0 | 0 | M |
| 6 | C5 | 7 | B | 42 | 175.0 | 71.0 | 0 | M |
| 7 | C5 | 5 | A | 19 | 189.0 | 70.5 | 1 | M |
| 8 | C5 | 10 | B | 28 | 178.0 | 94.0 | 0 | M |
| 9 | C6 | 8 | A | 22 | 162.0 | 45.5 | 1 | F |
| 10 | C7 | 11 | A | 26 | 177.0 | 74.0 | 0 | M |
DOI, duration of injury, in weeks; AIS, American Spinal Injury Association impairment scale.
Postsecondary years.
Table 2.
Selected cardiovascular variables of study participants
| Variable | AB | SCI | P |
|---|---|---|---|
| Age, yr | 31 ± 11 | 29 ± 10 | 0.44 |
| Mass, kg | 71 ± 15 | 68 ± 14 | 0.57 |
| BMI, kg·m−2 | 24.5 ± 3.5 | 22.6 ± 3.7 | 0.12 |
| Education, years postsecondary | 1.8 ± 2.1 | 2.3 ± 1.6 | 0.38 |
| No. of women participants | 3 | 3 | N/A |
| TBI | 0 | 1 | N/A |
AB, able-bodied controls, n = 10; SCI, high-level spinal cord injury, n = 10; BMI, body mass index, TBI; traumatic brain injury; N/A, not applicable.
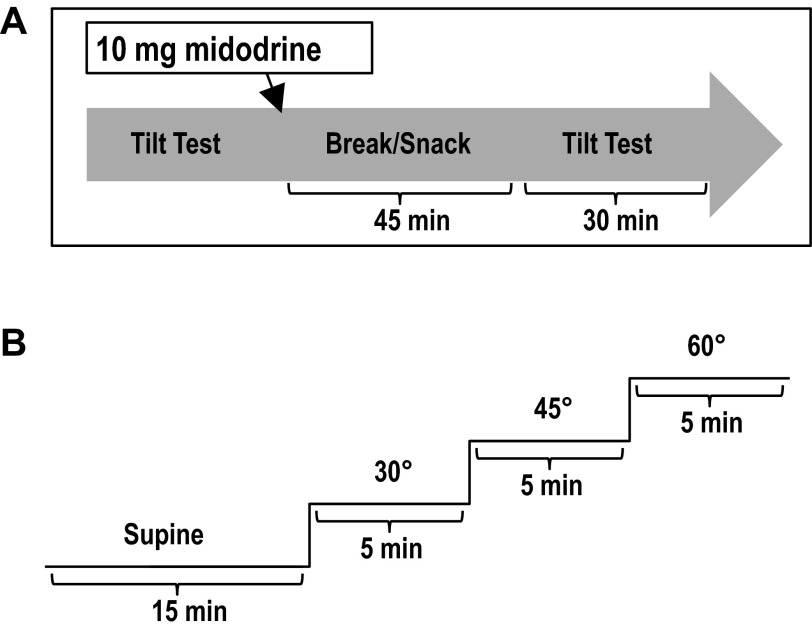
Participants were tested over 2 days. Each testing day was separated by at least 48 h and took place between 10:00 a.m. and 12:00 pm. The testing days were identical except for the administration of 10 mg midodrine on one of the days. The order of days (i.e., whether baseline or midodrine trial went first) was randomized. On the midodrine day, a 10-mg oral dose was administered. Data from the SCI-with-midodrine group are referred to as SCImido, whereas data from the SCI-without-midodrine group are referred to as SCI. Midodrine is converted to the pharmacologically active metabolite desglymidodrine, which has a half-life of approximately 3 h (15). The setup for postmidodrine testing was initiated precisely 45 min after midodrine administration (Fig. 1A) to conduct physiological assessments at the time of peak response, which is approximately 1 h after ingestion (50). A 10-mg dose was chosen because it has been shown to elicit the greatest improvements in orthostatic hypotension and symptoms of orthostatic intolerance, with no additional side effects than a 5-mg dose (50). Participants rested quietly in the supine position for 15 min prior to initiation of testing.
Fig. 1.
Illustration of study procedures. A: order of testing; B: tilt-testing protocol.
Protocol
Participants were transferred to the tilt table and rested supine for 15 min while baseline hemodynamic data were recorded (Fig. 1B). Participants were progressively tilted from supine to 30°, 45°, and 60°. Five minutes of continuous data were collected at each tilt level, and participants were instructed to keep their eyes open throughout the test. Transition between tilt levels was achieved in less than 5 s. Participants were asked about the presence of presyncopal symptoms (i.e., dizziness, light-headedness, nausea) at the beginning, middle, and end of each stage. Participants were asked to rate their symptoms between 1 and 10 (with 5 being slightly dizzy and 10 being about to lose consciousness or vomit), and were also instructed to notify the testing team if at any point their rank of symptoms became greater than 7/10. The stage and time (in seconds) at which each participant withdrew or was withdrawn from tilt were recorded. Orthostatic tolerance index (OTi) was calculated by the formula [OTi] = [Final tilt degree (°)] × [time (s) the last stage was tolerated].
Data Acquisition
For each participant, brachial BP was measured (BpTRU-BPM-100; Coquitlam, VSM Medical, Vancouver, BC, Canada) on the right arm at least two times at each stage of tilt. Beat-by-beat BP via finger photoplethysmography (Finometer PRO; Finapres Medicine Systems, Amsterdam, The Netherlands), heart rate (electrocardiogram ML 132; ADInstruments, Colorado Springs, CO), end-tidal carbon dioxide partial pressure (PetCO2) (17515 CO2 Analyzer Gold Edition; VacuMed, Ventura, CA), left MCAv and right PCA blood flow velocity (PCAv) (Doppler-Box, Compumedics DWL, Singen, Germany) was measured. Using two 2-MHz probes mounted bilaterally on the temporal bones using a fitted head strap, the P1 segment of the PCA was insonated at depths between 60–70 mm; the MCA was insonated at 45–55 mm. Arteries were confirmed using ipsilateral common carotid artery compression, ensuring an increase in PCA velocity and decrease in MCA velocity. All data were collected at 1,000 Hz using an analog-to-digital converter (PowerLab/16SP ML 795; ADInstruments) interfaced with data acquisition software (LabChart 7; ADInstruments) on a laptop computer. Finger photoplethysmograph signal was corrected to the brachial level.
Data Analysis
Following 2 min of acclimation to the new tilt stage, 3 min of steady-state BP, heart rate, and PetCO2 were recorded. Systolic and diastolic BP (DBP), and peak MCAv/PCAv and minimum MCAv/PCAv were then extracted to generate mean steady-state values for the given stage. From these values, mean arterial pressure (MAP) as (2 ∗ DBP + systolic BP)/3 and mean MCAv/PCAv as (2 ∗ MCAv/PCAv minimum + MCAv/PCAv maximum)/3 were calculated. This also allowed for the calculation of cerebrovascular conductance (CVC) as mean MCAv/PCAv/MAP. To estimate myocardial work, rate pressure product was calculated as (heart rate ∗ systolic blood pressure).
Time-domain pressure-flow relationships.
For evaluation of time-domain dynamic pressure-flow relationships during a clinically relevant orthostatic stimulus, acute changes in BP and CBF velocity (CBFv) for 15 s prior and 60 s after tilt were evaluated. The BP, MCAv, and PCAv responses occurring after each tilt stage were averaged for each participant. Not every tilt resulted in large decreases in BP in all participants. As such, to be included in the average response the tilt had to result in a decrease in MAP >15 mmHg over the first 30 s. All signals were visually inspected for artifacts or noise, and corrected by linear interpolation. CBFv signals were filtered by a low-pass filter with a cutoff frequency of 10 Hz (LabChart 7). All hemodynamic variables were sampled on a heart beat-by-beat basis (as detected by the electrocardiogram), whereas PetCO2 was sampled on a breath-by-breath basis (as detected by the peak of the first derivative of the PetCO2 waveform). All signals were then transferred to Excel software (Microsoft, Redmond, WA) with a custom-designed cubic spline interpolation package that allowed for resampling at 5 Hz. Mean CBFv and CVC were calculated according to the above formulas. After resampling, a mean response from the various orthostatic trials was generated for 60 s after tilt for each participant. Average and peak changes in CBFv and conductance of the MCA and PCA were recorded over the first 60 s of tilt. The 60 s was separated into two 30-s-long bins. The 30-s period immediately after tilt was also divided into six 5-s-long averages for analysis. It was not possible to insonate the PCA in one SCI participant. As such, steady-state and perturbed dynamic pressure-flow metrics for the PCA are limited to nine individuals.
Transfer function analysis.
Using transfer function analysis, the dynamic relationships between spontaneous oscillations in BP and MCAv/PCAv were evaluated. Beat-by-beat R-R interval, MAP, and CBFv signals were resampled at 4 Hz and divided into five successive windows that overlapped by 50% and then passed through a Hanning window and were fast Fourier transformed (49). Briefly, the transfer function H(f) between the signals was calculated as H(f) = Sxy(f)/Sxx(f), where Sxy(f) denotes the cross-spectrum between the two signals, and Sxx(f) is the autospectrum of the input signal (i.e., BP). The relationship between BP and CBFv with regard to amplitude and time were denoted as transfer function gain and phase shifts in the low (0.07–0.20 Hz) frequency. The low frequency range was chosen because it is the most established range for examining transfer function analysis in humans (4). The fraction of output power that can be linearly related to input power at each frequency is denoted by the coherence function. The coherence function is similar to a correlation coefficient in that values approximating 0 may indicate a nonlinear relationship, such as severe extraneous noise in the signals or no relationship between signals, whereas a coherence value approaching 1 reflects a strong influence of BP to CBFv. Gain describes the magnitude of change in BP that is reflected by CBFv. A reduction in phase suggests that BP is driving CBFv, or that changes in CBFv rapidly occur after changes in BP. According to previous applications of this methodology, an intact pressure-flow relationship would be associated with reductions in gain and increases in phase. Conversely, the absence of an intact pressure-flow response would manifest as increases in gain, with reductions in phase. Transfer function metrics were conservatively analyzed using only band points with coherence greater than 0.5 arbitrary units (au) as per convention (35, 40). Finally, to provide insight into systemic regulation of perfusion pressure, spontaneous baroreflex sensitivity in the low-frequency (0.04–0.15 Hz) alpha index (αLF) was also calculated (42). Also, transfer function metrics for the PCA were not possible (owing to fragmenting of continuous data recordings) to calculate in an additional SCI file and two SCImido files.
Statistical Analysis
Following confirmation of normal distribution (Shapiro-Wilks test), SCI and AB individuals were compared using parametric (i.e., independent-samples t-tests) or nonparametric comparisons (i.e., related-samples Wilcoxon signed rank test, independent-samples Mann-Whitney U-test). Also, paired-sample t-tests were used to compare SCI and SCImido groups and to compare the within-group MCAv and PCAv responses. Bivariate correlations were also performed. P < 0.05 was considered significant unless otherwise reported. Two-way repeated measures ANOVA was used to compare the effect of midodrine on symptoms orthostatic tolerance over the tilt-stages in SCI. Data are reported as means ± SE. Previous data indicated a required sample size of 4–10 per group when comparing transfer function analysis between SCI and AB (49). Spontaneous transfer function analysis (TFA) metrics of dynamic pressure-flow relationships have been shown to have moderate to strong repeatability (i.e., intraclass correlation of 0.46–0.47) (49).
RESULTS
Able-Bodied Vs. Spinal Cord Injured
Homeostatic response to progressive tilt.
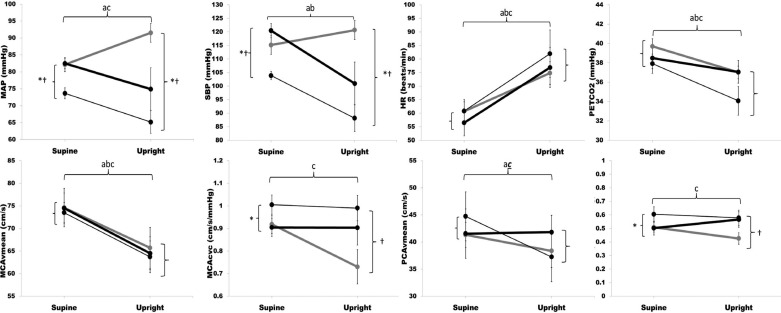
Steady-state changes in systemic and cerebral hemodynamics are presented in Fig. 2. Briefly, although BP was significantly lower in SCI during both supine and upright positions (P < 0.05), MCAv and PCAv were similar, indicating effective static CA. Also, BP increased significantly in AB, but decreased in SCI in response to tilt. Both MCAv and PCAv decreased similarly in SCI compared with AB (Fig. 2). Conductance in MCA and PCA decreased in response to tilt in AB (P <0.05), but did not change in SCI. Heart rate increased and PetCO2 decreased to a similar extent in both SCI and AB.
Fig. 2.
Steady-state hemodynamic responses to tilt: change in mean arterial pressure (MAP), systolic BP (SBP), heart rate (HR), partial pressure of end-tidal carbon dioxide (PetCO2), mean cerebral blood flow velocity of the middle cerebral artery (MCAvmean), mean cerebral blood flow velocity of the posterior cerebral artery (PCAvmean), cerebrovascular conductance of the middle cerebral artery (MCAcvc) and cerebrovascular conductance of the posterior cerebral artery (PCAcvc) in individuals with spinal cord injury (SCI; thin black line), individuals with SCI after midodrine administration (SCImido; thick black line), and able-bodied individuals (AB; thick gray line). Values are expressed as means ± SE. Markers denote significant differences between groups according to independent (SCI vs. AB) and paired samples (SCI vs. SCImido) t-tests (P < 0.05). aSCI supine vs. SCI upright; bSCImido supine vs. SCImido upright; cAB supine vs. AB upright; *SCI vs. SCImido; †AB vs. SCI; ctrend in AB supine vs. AB upright (P < 0.05).
Acute hemodynamic responses to orthostatic challenge.
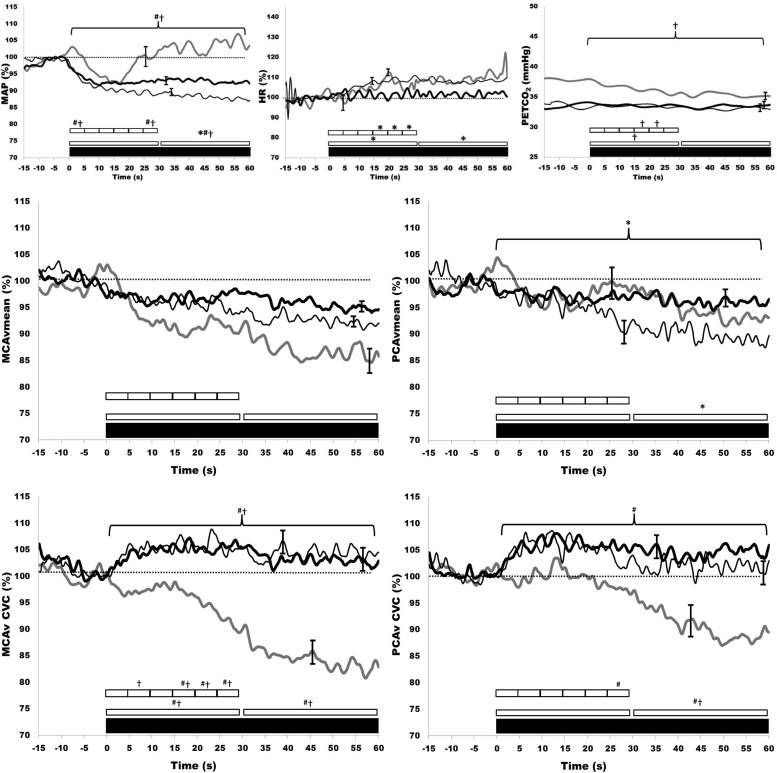
The acute hemodynamic response to tilt is presented in Fig. 3. Those with SCI had significantly lower MAP during the first 60 s of tilt. MCAv decreased from baseline in both AB and SCI (P < 0.001), but this response was not different between groups. MCAv was reduced during tilt in both SCI and AB (P < 0.001), but the magnitude of these reductions were not different between AB and SCI groups. PCAv was reduced in response to orthostatic challenge (both P < 0.001), but this was similar between AB and SCI. The tilt-induced decrease in MCA conductance was abolished in the SCI group throughout the 60 s after tilt. In SCI, MCA conductance was maintained at baseline levels in the 60 s after tilt, whereas in AB it consistently decreased over this time period. PCA conductance was also similar between SCI and AB throughout the first 30 s after tilt. On the other hand, from 30 to 60 s the tilt-induced decrease in PCA conductance was abolished in SCI. The heart rate response to tilt was similar in both SCI and AB. Pretilt PetCO2 was not significantly different between SCI and AB. PetCO2 decreased more in AB compared with SCI over the first 30 s but was similar from 30 to 60 s. Acute responses of MCAv and PCAv to upright tilt were not significantly different from each other within the SCI or AB groups.
Fig. 3.
Time-domain dynamic responses to tilt: change in MAP, HR, PetCO2, MCAvmean, PCAvmean, MCAcvc, and PCAcvc for 15 s before and 60 s after tilt in SCI (thin black line), SCImido (thick black line), and AB (thick gray line). Values are expressed as means ± SE. Markers denote significant differences between groups according to independent (SCI vs. AB) and paired (SCI vs. SCImido) sample t-tests (P < 0.05). *SCI vs. SCImido; †AB vs. SCI; #AB vs. SCImido. Note: for statistical purposes the 60-s period was binned into two 30-s periods, and the initial 30 s was binned in six 5-s periods of duration. For clarity, the largest SE is presented at the point of occurrence.
Transfer function metrics of dynamic cerebral autoregulation.
Transfer function metrics of the dynamic cerebral pressure-flow relationship in MCA and PCA are presented in Table 3. Power spectra for MAP and MCAv were lower in SCI compared with AB (P < 0.05). Also, upright low-frequency coherence for BP-MCAv and BP-PCAv was lower in SCI than in AB (both P < 0.001). Phase was not different between SCI and AB for MAP-MCAv or MAP-PCAv; however, SCI gain was increased for MAP-PCAv (P < 0.05; Table 3).
Table 3.
Transfer function metrics of mean arterial pressure and cerebral blood flow velocity in the supine and upright positions
| SCI |
SCImido |
AB |
||||
|---|---|---|---|---|---|---|
| Supine | Max Tilt | Supine | Max Tilt | Supine | Max Tilt | |
| MAP power spectrum, mmHg2/Hz−1 | 0.88 ± 0.61 | 0.89 ± 0.57* | 0.64 ± 0.62 | 0.79 ± 0.98 | 2.34 ± 1.77 | 10.65 ± 10.94‡ |
| MCA power spectrum, cm2·s−2·Hz−1 | 2.51 ± 1.60 | 0.98 ± 0.83* | 1.66 ± 1.13 | 1.28 ± 0.97 | 3.45 ± 3.28 | 9.26 ± 9.51 |
| MCA coherence, au | 0.64 ± 0.17 | 0.55 ± 0.19* | 0.54 ± 0.21 | 0.58 ± 0.21 | 0.65 ± 0.17 | 0.84 ± 0.09‡ |
| MCA gain, cm·s−1·mmHg−1 | 1.76 ± 0.96 | 1.16 ± 0.82 | 1.87 ± 0.68 | 1.40 ± 0.68‡ | 1.34 ± 0.63 | 1.16 ± 0.60 |
| MCA n-gain, %·mmHg−1 | 2.23 ± 0.91 | 3.93 ± 4.36 | 2.43 ± 0.88 | 2.22 ± 1.28 | 1.66 ± 0.53 | 1.71 ± 0.59 |
| MCA phase, rad | 0.46 ± 0.30† | 0.47 ± 0.39 | 0.74 ± 0.46 | 0.36 ± 0.28‡ | 0.67 ± 0.24 | 0.50 ± 0.13‡ |
| PCA power spectrum, cm2·s−2·Hz−1 | 1.01 ± 0.42 | 0.87 ± 0.46 | 1.20 ± 0.76 | 0.68 ± 0.66 | 1.27 ± 0.97 | 3.87 ± 5.36 |
| PCA coherence, au | 0.64 ± 0.15 | 0.59 ± 0.17* | 0.51 ± 0.21 | 0.52 ± 0.20 | 0.55 ± 0.14 | 0.76 ± 0.17‡ |
| PCA gain, cm·s−1·mmHg−1 | 1.01 ± 0.51 | 1.00 ± 0.51* | 1.32 ± 0.75 | 0.84 ± 0.49‡ | 0.87 ± 0.43 | 0.68 ± 0.37‡ |
| PCA n-gain, %·mmHg−11 | 2.17 ± 1.19 | 2.57 ± 1.56 | 2.94 ± 1.27 | 1.94 ± 1.02‡ | 1.98 ± 0.80 | 1.73 ± 0.66‡ |
| PCA phase, rad | 0.40 ± 0.27 | 0.55 ± 0.50 | 0.48 ± 0.19 | 0.26 ± 0.38 | 0.78 ± 0.34 | 0.50 ± 0.19‡ |
Significantly different from AB (P < 0 0.05); †significant different from SCImido (P < 0.05);
significantly different from supine (P < 0.05). Significant relationships are highlighted with bold text. Values are means ± SD.
Spinal Cord Injured Individuals With and Without Midodrine
Homeostatic response to progressive tilt.
The effect of midodrine on supine and upright steady-state measured hemodynamic variables are presented in Fig. 2. Supine and upright steady-state BPs were higher in SCI following midodrine; however, heart rate, PetCO2, MCAv, and PCAv values were unaltered.
Acute hemodynamic responses to tilt.
Midodrine did not influence the MAP response to tilt from 0 to 30 s. In contrast, from 0 to 60 s, the decline in MAP was mitigated with midodrine (Fig. 3). The MCAv response to tilt was not significantly different with or without midodrine. PCAv did not decline as much 30–60 s after tilt when SCI were administered midodrine (P < 0.05). Conductance of the MCA and PCA we not influenced by midodrine administration, and neither was PetCO2 (Fig. 3). Pretilt PetCO2 was not significantly different in SCI with or without midodrine. The acute responses of MCAv and PCAv to upright tilt were not significantly different from each other in SCI with or without midodrine. Rate pressure product was not related to PetCO2 in SCI before or after midodrine.
Transfer function metrics of cerebral pressure-flow relationships.
Midodrine did not substantially alter the dynamic cerebral pressure-flow relationships in the MCA and PCA (Table 3). Supine MAP-MCAv phase was increased with midodrine in SCI, whereas PCA metrics were unchanged. No upright-tilt transfer function metrics differed with or without midodrine.
Transfer Function Metrics and BP
In participants with SCI, αLF was significantly higher than it was in AB participants (30.0 ± 15.6 vs. 15.7 ± 10.7 ms/mmHg; P = 0.03). Midodrine did not result in significant changes in αLF in either the supine or upright position. In the SCI group, there was a positive relationship between upright mean BP with the MCA low-frequency gain at the last stage of fully tolerated tilt (r = 0.64, P < 0.05). In AB individuals only, there was a strong relationship between αLF and MCA low-frequency gain (r = 0.75, P = 0.01) and normalized gain (r = 0.68, P = 0.03).
Orthostatic Tolerance
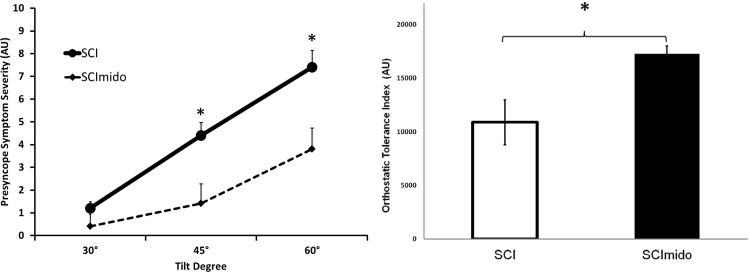
Symptoms of presyncope were significantly improved at 45° and 60° tilt with midodrine (Fig. 4). Those with SCI had an average 59% improvement in OTi (P = 0.003) during the midodrine tilt trial. There was no relationships between OTi with any transfer function metrics or static hemodynamic variables, (i.e., BP, MCAv/PCAv; mean values, absolute changes from supine, percent changes from supine) related to OTi.
Fig. 4.
Orthostatic tolerance in SCI and SCImido. Left: self-reported symptoms of presyncope (0–10 scale) (au, arbitrary units). Note: people who developed presyncope were given a 10/10 for symptom severity at subsequent stages. Right: calculated orthostatic tolerance index (OTi). Significant main effect of midodrine *Significant differences from post hoc t-tests before and after midodrine administration; P < 0.01. Data are presented as means ± SE.
Tonic Sympathetic Vasomotor Control and Transfer Function Metrics
In SCI participants, there was a negative relationship between upright low-frequency power of systolic BP with MCA low-frequency normalized gain (r = −0.67, P = 0.032), and a positive relationship between upright low-frequency power of systolic BP and MCA low-frequency phase (r = 0.65, P = 0.005). No relationships were evident in the AB group.
DISCUSSION
This is the first study to examine systemic and regional cerebral hemodynamics, as well as orthostatic tolerance in those with SCI, and the influence of midodrine administration. The main findings are as follows: 1) the steady-state and dynamic CBF response to tilt is similar in SCI compared with AB, indicating effective CA; 2) although midodrine does not influence the steady-state CBF response to tilt, it improved time-domain dynamic cerebral pressure-flow relationships (i.e., increased acute PCAv 30–60 s after tilt); 3) midodrine also resulted in an improved orthostatic tolerance in SCI; and 4) AB showed the previously reported inverse relationship between dynamic pressure-flow relationships and baroreflex sensitivity; however, those with SCI had an uncoupling of this association.
Static CA in AB and individuals with SCI.
The hemodynamic determinants of average blood flow (Q) through an organ is typically understood through Poiseuille's law, or the hemodynamic equivalent of Ohm's law, known as Darcy's law: Q = ΔP/R. In the context of the brain, ΔP is the cerebral perfusion pressure calculated from the difference between MAP and the effective downstream pressure of the cerebral circulation, and R is the cerebrovascular resistance (i.e., the inverse of conductance). This study showed that although the BP response to tilt was divergent (i.e., increasing in AB and decreasing in SCI), the MCA and PCA responses were well maintained. These results are largely consistent with several studies showing that static CA is preserved in those with SCI (33) and preganglionic autonomic failure (17). To maintain CBF, conductance during tilt was maintained at supine levels in SCI, whereas it decreased significantly in AB (Fig. 2). In support of this notion, early observations of surface vessels in the brain have confirmed that both large and small precapillary cerebral arteries undergo such autoregulatory-caliber adjustments in response to steady-state (and dynamic) increases and decreases in arterial BP (12, 13, 21). Such reflex adjustments in vascular resistance or conductance to steady-state alterations in BP are referred to as static CA.
Dynamic cerebral pressure-flow relationships in AB and individuals with SCI.
The dynamic cerebral pressure-flow relationships in the time domain showed that the acute (i.e., 0–25 s after tilt) BP and CBF response after hemodynamic challenge in SCI is similar to that in AB, whereas orthostatic exposure >30 s results in reduced BP and PCAv. BP dropped similarly between AB and SCI over the first 20 s after tilt; however, only in AB did it return back to baseline levels before 30 s. In SCI, BP continued to gradually drop in the 60 s after tilt. The failure of sympathetic vasomotor control to maintain BP and hence cerebral perfusion pressure in SCI appears to be partially mitigated by increased cerebrovascular conductance in both the MCA and PCA. As a result of the increased conductance, velocity is preserved in the MCA and PCA. It appears that time-domain cerebral pressure-flow relationships in SCI allow for maintained MCAv and PCAv during orthostatic challenges. Maintained cerebral perfusion in response to acute orthostatic challenge in SCI is remarkable considering recent work showing impaired MCAv in the 30 s after standing up with alpha-1 adrenoreceptor blockade (24). Although the BP response was blunted after alpha-1 adrenoreceptor blockade, Lewis et al. showed that mean MCAv was also reduced (24). As such, it may be that SCI-induced chronic sympathetic vasomotor decentralization leads to enhanced capacity to dynamically alter cerebrovascular conductance, compared with acute sympathetic vasomotor antagonism. Together, the steady-state and dynamic cerebral perfusion-pressure findings show that both MCAv and PCAv regulation is effective in those with SCI.
Frequency domain metrics derived through transfer function analysis of cerebral pressure-flow relationships showed a reduced MCA and PCA coherence, and increased PCA gain in the SCI group in the upright position. This finding is broadly consistent with a similar report in tetraplegic SCI (49), whereas these findings extend previous work (examining only the MCA) by showing increased gain in the PCA as well (37). However, it should be noted that transfer function analysis can be interpreted in two distinct manners. The traditional interpretation is to assume that these metrics reflect dynamic CA (see Methods for a specific interpretation of coherence, phase, and gain) (49, 53). Interpreted in this way, reduced coherence in the MCA/PCA would indicate more effective dynamic CA in those with SCI. However, by the other interpretation (i.e., the one we favor), reported impairments in the dynamic cerebral pressure-flow relationship may be due simply to a poor signal-to-noise ratio, which is a limitation of using TFA under spontaneous conditions (20, 28). Consistent with this view, reduced coherence in SCI as shown in two other studies (37, 49), may not indicate enhanced dynamic CA, as altered input power secondary to varying oscillation amplitude in given frequencies [as noted in mean BP power in SCI (Table 3)] is sufficient to alter coherence (5). Caution in the meaningful interpretation of select TFA metrics has been documented (40).
Effect of midodrine on static CA.
In agreement with two previous studies, the present investigation found that midodrine did not alter steady-state MCAv in those with SCI (44, 45). However, for the first time, this study has also illustrated that steady-state PCAv is not altered by midodrine administration in SCI. Although the steady-state BP response to the last tolerable stage of tilt was improved by midodrine, the MCAv, heart rate, and PetCO2 responses were not different. Interestingly, midodrine effectively mitigated the decline in PCAv that occurs in SCI after tilt (Fig. 3). Maintenance of posterior cerebral perfusion (as indexed by PCAv) after midodrine may explain two prior studies that reported marked improvements in orthostatic tolerance but limited influence on MCAv after midodrine administration in SCI (44, 45).
Effect of midodrine on dynamic cerebral pressure-flow relationships.
Our data suggest that PCA dynamic time-domain pressure-flow relationships in SCI became more similar to those of AB with when individuals with SCI were administered midodrine (i.e., the PCAv response improved), whereas MCA regulation was unchanged (Fig. 3). From a functional perspective, the current study largely supports recent work showing that posterior regional CBF control plays a crucial role in the development of syncope by showing that vertebral artery blood flow was better maintained compared with the internal carotid artery during orthostatic challenge (38). Transfer function analysis-derived metrics of dynamic cerebral pressure-flow velocity relationships in the MCA and PCA were largely not influenced by midodrine in SCI, however, MCA phase increased in SCI with midodrine (Table 3). Traditionally interpreted, increased PCA phase may indicate an improved dynamic cerebral pressure-flow regulation.
Orthostatic tolerance.
This study clearly showed for the first time, using both self-reported symptoms of presyncope and a calculated OTi, that orthostatic tolerance is markedly improved by midodrine in those with high-level SCI. One double-blind placebo-controlled study in AB individuals with neurally mediated syncope convincingly showed a reduction in episodes of syncope when using midodrine (43). Two studies, albeit using only self-reported symptoms of presyncope and not reporting any statistical analysis, also reported reductions in symptoms during head-up tilt with midodrine in individuals with SCI (44, 45). The current data indicate that in those with SCI, steady-state metrics do not relate to the development of presyncopal symptoms, which is not surprising given the marked heterogeneity with regard to the human hemodynamic response to orthostatic challenges (27).
In terms of orthostatic tolerance, it is reasonable to suggest a greater relative importance of CBF maintenance in the posterior cortex, because reductions in blood flow in the posterior region may cause an interruption of the blood supply to the medulla oblongata, which contains autonomic control centers, and discrete regions responsible for consciousness (39). Indeed, early work using the 133Xenon inhalation technique showed a relative redistribution of CBF away from the frontal lobe and toward the occipital lobe in response to orthostatic challenge (31). In those with SCI and orthostatic hypotension in general, PCA blood flow disruption likely plays a key role in the development of syncope, which appears to be mitigated by midodrine administration.
Cerebral pressure-flow relationships and baroreflex function.
Previous work has shown an inverse relationship between metrics of dynamic cerebral pressure-flow relationships and cardiac vagal baroreflex sensitivity, suggesting that at least in young people, individuals with the lowest capacity to autoregulate CBF were also those who mounted the greatest heart rate response to sudden changes in BP (and vice versa) (42). The present study replicated this relationship in young AB, but not SCI. Such relationships between cerebral pressure-flow metrics and baroreflex sensitivity are believed to be intrinsic, as supported by the intimately coupled cerebral regulatory centers for both cerebral autoregulation and baroreflex integration, and the clear evolutionary advantage of redundant hemodynamic control systems for the brain (18, 26). An uncoupling of these functions in those with SCI may be due to a variety of issues in SCI, including the drastically reduced BP (6), decentralization of sympathetic control cerebrovasculature (6, 23), and upregulation of vagal tone (7), all of which could alter transfer function metrics (1, 16, 36) (Table 3). Individuals with SCI and the lowest BP during tilt had the highest transfer function-derived metrics of cerebrovascular control, and the greatest regulatory capacity (i.e., lowest gain) was found in those with BP below the theorized autoregulatory threshold (i.e., mean BP <60 mmHg) (29). This unexpected finding of the highest autoregulation occurring in those with the lowest perfusion pressure generates additional skepticism that transfer function metrics of cerebral pressure and flow velocity validly measure dynamic CA (40).
Limitations
Transcranial Doppler was used for the assessment of CBF in this study, which required a consistent diameter to accurately reflect changes in flow. Indeed, two studies have shown that administration of similar alpha-1 agonists have not led to reductions in intracranial vessel diameter (14, 19), allowing the fair assumption of maintained MCA and PCA diameter with midodrine. Also, if vasoconstriction did occur with midodrine administration, it would be expected that MCAv and PCAv would increase greatly, which did not occur. With respect to vessels downstream of the MCA and PCA, the blood-brain barrier usually prevents intravascular catecholamines from binding to adrenergic receptors located in cerebral arterioles (25). Nevertheless, volumetric measured flow in the internal carotid artery, vertebral artery, and/or MCA is needed to further investigations of clinical cerebrovascular regulation. Because PCAv and MCAv were similar between groups, and this was the primary comparison being made in the present study (i.e., SCI vs. AB, not PCA vs. MCA), the choice to present either absolute or baseline values would not influence results from the present study. As such, we reported absolute values when comparing supine to baseline, and used relative changes when illustrating the dynamic response, because the use of absolute data would have precluded the summation of BP tilt contours. Because two of the individuals with SCI were injured >1 yr before testing, we correlated time since SCI with primary outcome metrics (i.e., orthostatic tolerance, change in BP/MCAv/PCAv during tilt). No metrics were related to time since injury. This was not a blinded, placebo-controlled trial. Although subjects were randomized with respect to the order of when they had the medication vs. control trial, participants were aware of the medications being provided. The participants were, however, completely blinded to the purpose of the study. The limitations of spontaneous linear TFA have been noted, including the well reported high variability in outcome measures (49). Future examination of dynamic pressure-flow relationships in SCI should consider the use of oscillatory lower body negative pressure to increase coherence between input and output and hence reliance on linear interpretation, and/or the use of nonlinear models (40). Although our group has shown that MCAv cerebrovascular reactivity is preserved in those with SCI, it was noted that the cerebrovascular resistance response was slightly reduced in response to hypocapnia in this population (49). Taking into consideration the similar decrease in PetCO2 noted in the present study, interpretation of our results should consider the fact that CBF is controlled by a number of factors outside of CA, including arterial CO2 concentration, and hence could have been influenced by a mitigated hypocapnic response. Similar caution should be employed when interpreting the midodrine results, because it in unknown whether midodrine administration influences cerebrovascular reactivity in those with SCI.
Conclusion
Independent of changes in transfer function metrics, midodrine led to improved PCAv responses after tilt and a 59% improvement in orthostatic tolerance. The vertebrobasilar region may be particularly susceptible to hypoperfusion in SCI, leading to increased orthostatic intolerance.
GRANTS
This work was supported by National Science and Engineering Research Council (Canada), Heart and Stroke Foundation of Canada, and Michael Smith Foundation for Health Research grants to A.A.P; by Paralyzed Veterans of America, Craig Neilson Foundation, Canadian Institute of Health Research, and Heart and Stroke Foundation of Canada grants to A.V.K.; and by a Canada Research Chair to P.N.A. D. E. R. Warburton was supported by salary awards from the Canadian Institutes of Health Research and Michael Smith Foundation for Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.A.P., A.V.K., P.N.A., and D.E.R.W. conception and design of research; A.A.P. and A.V.K. performed experiments; P.N.A. and D.E.R.W. analyzed data; A.A.P., A.V.K., P.N.A., and D.E.R.W. interpreted results of experiments; A.A.P. prepared figures; A.A.P., P.N.A., and D.E.R.W. drafted manuscript; A.A.P., A.V.K., P.N.A., and D.E.R.W. edited and revised manuscript; A.A.P., A.V.K., P.N.A., and D.E.R.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the individuals who participated in this study. We also acknowledge the statistical consultation of V. Noonan and G. Zhong. We especially acknowledge Dr. J. Taunton and GE Healthcare.
REFERENCES
- 1.Ainslie PN, Lucas SJ, Fan JL, Thomas KN, Cotter JD, Tzeng YC, Burgess KR. Influence of sympathoexcitation at high altitude on cerebrovascular function and ventilatory control in humans. J Appl Physiol 113: 1058–1067, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke 41: 2697–2704, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Azevedo E, Castro P, Santos R, Freitas J, Coelho T, Rosengarten B, Panerai R. Autonomic dysfunction affects cerebral neurovascular coupling. Clin Auton Res 21: 395–403, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Bernardi L, Leuzzi S, Radaelli A, Passino C, Johnston JA, Sleight P. Low-frequency spontaneous fluctuations of RR interval and blood pressure in conscious humans: a baroreceptor or central phenomenon? Clin Sci 87: 649–654, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Claassen JA, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol 106: 153–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 23: 1713–1725, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Claydon VE, Krassioukov AV. Clinical correlates of frequency analyses of cardiovascular control after spinal cord injury. Am J Physiol Heart Circ Physiol 294: H668–H678, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 44: 341–351, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Dawson SL, Blake MJ, Panerai RB, Potter JF. Dynamic but not static cerebral autoregulation is impaired in acute ischaemic stroke. Cerebrovasc Dis 10: 126–132, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987–1996. Stroke 31: 2307–2313, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schröck H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol 54: 582–590, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Fog M. Cerebral circulation: the reaction of the pial arteries to a fall in blood pressure. Arch Neurol Psychiatry 37: 351, 1937 [Google Scholar]
- 13.Fog M. Cerebral circulation: II. Reaction of pial arteries to increase in blood pressure. Arch Neurol Psychiatry 41: 260, 1939 [Google Scholar]
- 14.Greenfield JC, Jr, Tindall GT. Effect of norepinephrine, epinephrine, and angiotensin on blood flow in the internal carotid artery of man. J Clin Invest 47: 1672, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grobecker HF, Kees FK. Pharmacokinetic parameters and haemodynamic actions of midodrine in young volunteers. Int Angiol 12: 119–124, 1993 [PubMed] [Google Scholar]
- 16.Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. J Physiol 590 Pt 24: 6343–6352; 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetzel A, Reinhard M, Guschlbauer B, Braune S. Challenging cerebral autoregulation in patients with preganglionic autonomic failure. Clin Auton Res 13: 27–35, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Ishitsuka T, Iadecola C, Underwood MD, Reis DJ. Lesions of nucleus tractus solitarii globally impair cerebrovascular autoregulation. Am J Physiol Heart Circ Physiol 251: H269–H281, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Johnston WE, DeWitt DS, Vinten-Johansen J, Stump DA, Prough DS. Phenylephrine does not reduce cerebral perfusion during canine cardiopulmonary bypass. Anesth Analg 79: 14–18, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Katsogridakis E, Bush G, Fan L, Birch AA, Simpson DM, Allen R, Potter JF, Panerai RB. Detection of impaired cerebral autoregulation improves by increasing arterial blood pressure variability. J Cereb Blood Flow Metab 33: 519–523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol Heart Circ Physiol 234: H371–H383, 1978 [DOI] [PubMed] [Google Scholar]
- 22.Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res 152: 223–229, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Krassioukov A. Autonomic function following cervical spinal cord injury. Respir Physiol Neurobiol 169: 157–164, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Lewis NC, Ainslie PN, Atkinson G, Jones H, Grant EJ, Lucas SJ. Initial orthostatic hypotension and cerebral blood flow regulation: effect of alpha1-adrenoreceptor activity. Am J Physiol Regul Integr Comp Physiol 304: R147–R154, 2013 [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie ET, McCulloch J, O'Kean M, Pickard JD, Harper AM. Cerebral circulation and norepinephrine: relevance of the blood-brain barrier. Am J Physiol 231: 483–488, 1976 [DOI] [PubMed] [Google Scholar]
- 26.Nakai M. An increase in cerebral blood flow elicited by electrical stimulation of the solitary nucleus in rats with cervical cordotomy and vagotomy. Jpn J Physiol 35: 57, 1985 [DOI] [PubMed] [Google Scholar]
- 27.O'Leary DD, Hughson RL, Shoemaker JK, Greaves DK, Watenpaugh DE, Macias BR, Hargens AR. Heterogeneity of responses to orthostatic stress in homozygous twins. J Appl Physiol 102: 249–254, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Panerai RB, Dineen NE, Brodie FG, Robinson TG. Spontaneous fluctuations in cerebral blood flow regulation: contribution of PaCO2. J Appl Physiol 109: 1860–1868, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng 8: 42–59, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res 19: 197–211, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Passant U, Minthon L, Eng SV, Edvinsson L, Faldt R, Gustafson L. Redistribution of blood flow in the cerebral cortex of normal subjects during head-up postural change. Clin Auton Res 2: 119–124, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990 [PubMed] [Google Scholar]
- 33.Phillips AA, Ainslie PN, Krassioukov AV, Warburton DE. Regulation of cerebral blood flow after spinal cord injury. J Neurotrauma 30: 1551–1563, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Pinna GD, Maestri R, Raczak G, La Rovere MT. Measuring baroreflex sensitivity from the gain function between arterial pressure and heart period. Clin Sci 103: 81–88, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Purkayastha S, Saxena A, Eubank WL, Hoxha B, Raven PB. α-1 Adrenergic receptor control of the cerebral vasculature in humans at rest and during exercise. Exp Physiol 98: 451–461, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Sahota IS, Ravensbergen HR, McGrath MS, Claydon VE. Cerebrovascular responses to orthostatic stress after spinal cord injury. J Neurotrauma 29: 2446–2456, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH, Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol 97: 1272–1280, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Shin HK, Yoo KM, Chang HM, Caplan LR. Bilateral intracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol 56: 1353–1358, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Tzeng YC, Ainslie PN, Cooke WH, Peebles KC, Willie CK, MacRae BA, Smirl JD, Horsman HM, Rickards CA. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol 303: H658–H671, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Tzeng YC, Ainslie PN. Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. Eur J Appl Physiol. First published June 5, 2013; 10.1007/s00421-013-2667-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzeng YC, Lucas SJ, Atkinson G, Willie CK, Ainslie PN. Fundamental relationships between arterial baroreflex sensitivity and dynamic cerebral autoregulation in humans. J Appl Physiol 108: 1162–1168, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Ward CR, Gray JC, Gilroy JJ, Kenny RA. Midodrine: a role in the management of neurocardiogenic syncope. Heart 79: 45–49, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wecht JM, Radulovic M, Rosado-Rivera D, Zhang RL, Lafountaine MF, Bauman WA. Orthostatic effects of midodrine versus L-NAME on cerebral blood flow and the renin-angiotensin-aldosterone system in tetraplegia. Arch Phys Med Rehabil 92: 1789–1795, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Wecht JM, Rosado-Rivera D, Handrakis JP, Radulovic M, Bauman WA. Effects of midodrine hydrochloride on blood pressure and cerebral blood flow during orthostasis in persons with chronic tetraplegia. Arch Phys Med Rehabil 91: 1429–1435, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Willie CK, Cowan EC, Ainslie PN, Taylor CE, Smith KJ, Sin PY, Tzeng YC. Neurovascular coupling and distribution of cerebral blood flow during exercise. J Neurosci Methods 198: 270–273, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590: 3261–75, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson LC, Cotter JD, Fan JL, Lucas RA, Thomas KN, Ainslie PN. Cerebrovascular reactivity and dynamic autoregulation in tetraplegia. Am J Physiol Regul Integr Comp Physiol 298: R1035–R1042, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN, Low PA. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology 51: 120–124, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Wu JC, Chen YC, Liu L, Chen TJ, Huang WC, Cheng H, Tung-Ping S. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology 78: 1051–1057, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Zhang R, Wilson TE, Witkowski S, Cui J, Crandall CG, Levine BD. Inhibition of nitric oxide synthase does not alter dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 286: H863–H869, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998 [DOI] [PubMed] [Google Scholar]