Abstract
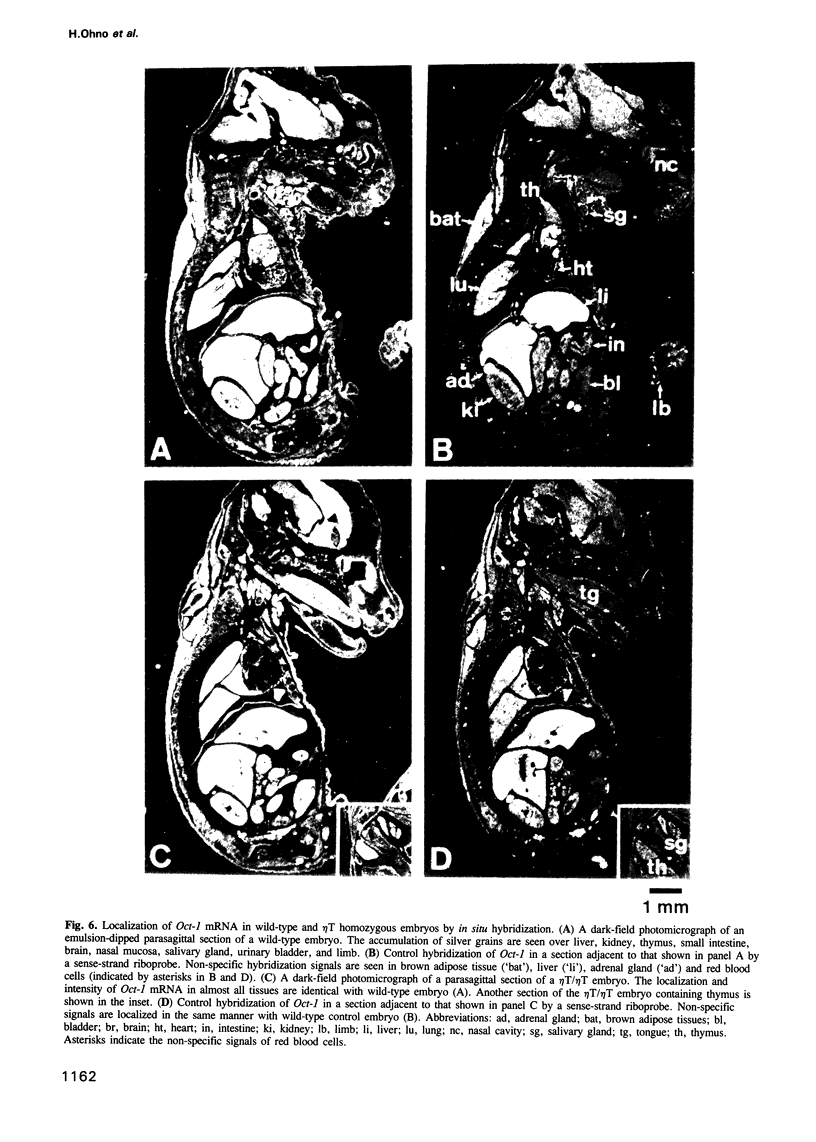
CD3 zeta and eta chains are components of the T cell antigen receptor (TCR) complex and are transcribed from a common gene by alternative splicing. TCR complexes containing the zeta eta dimer have been thought to mediate different functions than complexes containing the zeta 2 dimer. To analyze the role of eta in the development and function of T cells, we generated eta-deficient mice without affecting zeta by gene targeting in embryonic stem cells. Homozygous mutant embryos developed normally. Unexpectedly, however, these mice exhibited high mortality soon after birth for unknown reason(s). Analysis of surviving homozygous animals revealed that the development and function of T cells were normal in the absence of the eta chain. Recently, the zeta/eta locus was reported to encode a transcription factor, Oct-1, on the opposite DNA strand. Our targeting strategy resulted in modulation of Oct-1 transcription--reduction of the authentic Oct-1 mRNA and induction of aberrant transcripts. Although differences in tissue distribution and DNA binding capacity of Oct-1 between wild-type and eta-deficient mice were not evident from in situ hybridization and gel shift analysis, the high mortality in the eta-deficient strain may well be due to the disturbance of Oct-1 transcription by the mutation in the zeta/eta locus. Such possible complexities have to be taken into account in the interpretation of gene targeting experiments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
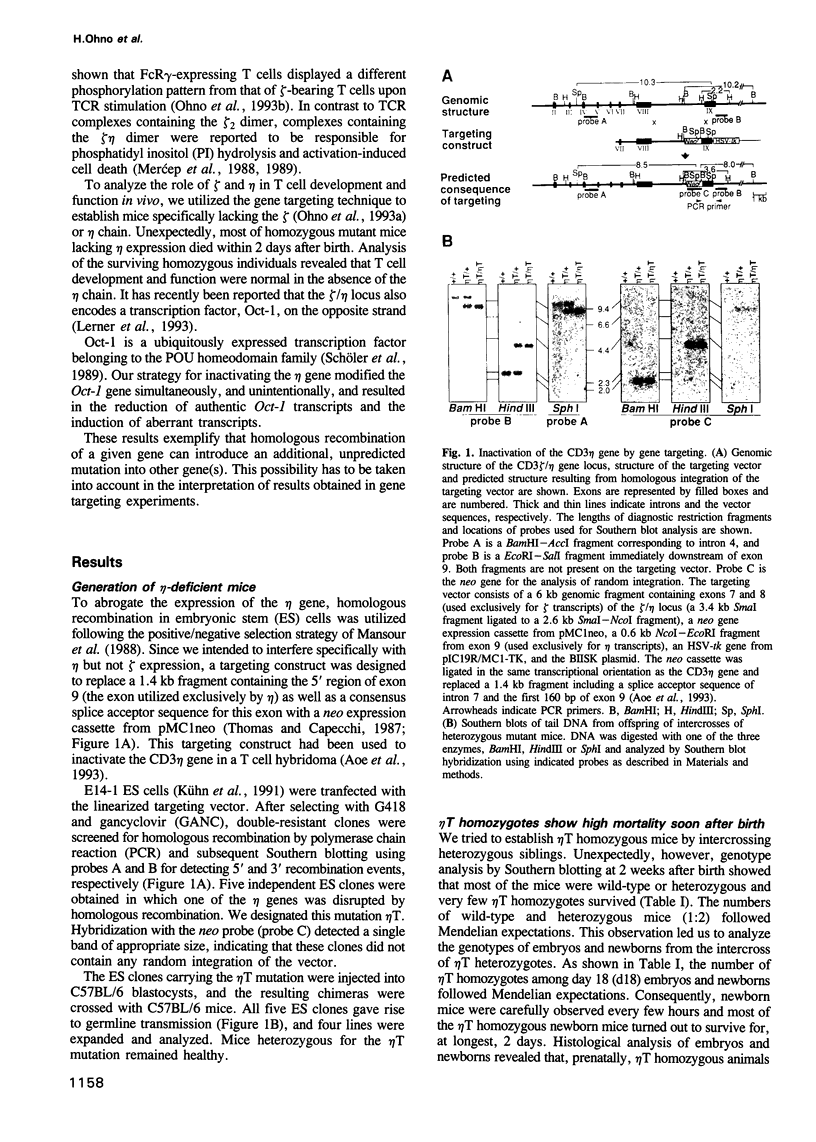
- Aoe T., Ohno H., Sonta S., Saito T. Functional inactivation of the CD3 zeta chain in a T cell hybridoma by in vitro gene targeting. Int Immunol. 1993 Jul;5(7):725–733. doi: 10.1093/intimm/5.7.725. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Maandag E. R., van Roon M., van der Lugt N. M., van der Valk M., Hooper M. L., Berns A., te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992 Sep 24;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- Clayton L. K., Bauer A., Jin Y. J., D'Adamio L., Koyasu S., Reinherz E. L. Characterization of thymus-derived lymphocytes expressing Ti alpha-beta CD3 gamma delta epsilon zeta-zeta, Ti alpha-beta CD3 gamma delta epsilon eta-eta or Ti alpha-beta CD3 gamma delta epsilon zeta-zeta/zeta-eta antigen receptor isoforms: analysis by gene transfection. J Exp Med. 1990 Oct 1;172(4):1243–1253. doi: 10.1084/jem.172.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., D'Adamio L., Howard F. D., Sieh M., Hussey R. E., Koyasu S., Reinherz E. L. CD3 eta and CD3 zeta are alternatively spliced products of a common genetic locus and are transcriptionally and/or post-transcriptionally regulated during T-cell development. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5202–5206. doi: 10.1073/pnas.88.12.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Karvelas M., Nossal G. J., Ye Z. S., Jacks T., Baltimore D. Oct-2, although not required for early B-cell development, is critical for later B-cell maturation and for postnatal survival. Genes Dev. 1993 Apr;7(4):570–582. doi: 10.1101/gad.7.4.570. [DOI] [PubMed] [Google Scholar]
- Davis A. C., Wims M., Spotts G. D., Hann S. R., Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993 Apr;7(4):671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Hildebrandt M., Nellen W. Differential antisense transcription from the Dictyostelium EB4 gene locus: implications on antisense-mediated regulation of mRNA stability. Cell. 1992 Apr 3;69(1):197–204. doi: 10.1016/0092-8674(92)90130-5. [DOI] [PubMed] [Google Scholar]
- Hussey R. E., Clayton L. K., Diener A., McConkey D. J., Howard F. D., Rodewald H. R., D'Adamio L., Dallenbach F., Stein H., Schmidt E. V. Overexpression of CD3 eta during thymic development does not alter the negative selection process. J Immunol. 1993 Feb 15;150(4):1183–1194. [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Clayton L. K., Howard F. D., Koyasu S., Sieh M., Steinbrich R., Tarr G. E., Reinherz E. L. Molecular cloning of the CD3 eta subunit identifies a CD3 zeta-related product in thymus-derived cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3319–3323. doi: 10.1073/pnas.87.9.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. S., van Lingen B., Papaioannou V. E., Spiegelman B. M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993 Jul;7(7B):1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Khochbin S., Lawrence J. J. An antisense RNA involved in p53 mRNA maturation in murine erythroleukemia cells induced to differentiate. EMBO J. 1989 Dec 20;8(13):4107–4114. doi: 10.1002/j.1460-2075.1989.tb08595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. W. An antisense mRNA directs the covalent modification of the transcript encoding fibroblast growth factor in Xenopus oocytes. Cell. 1989 Nov 17;59(4):687–696. doi: 10.1016/0092-8674(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Koyasu S., D'Adamio L., Arulanandam A. R., Abraham S., Clayton L. K., Reinherz E. L. T cell receptor complexes containing Fc epsilon RI gamma homodimers in lieu of CD3 zeta and CD3 eta components: a novel isoform expressed on large granular lymphocytes. J Exp Med. 1992 Jan 1;175(1):203–209. doi: 10.1084/jem.175.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal G. W., Armstrong B. C., Battey J. F. N-myc mRNA forms an RNA-RNA duplex with endogenous antisense transcripts. Mol Cell Biol. 1990 Aug;10(8):4180–4191. doi: 10.1128/mcb.10.8.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989 Mar;9(3):1128–1136. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Lerner A., D'Adamio L., Diener A. C., Clayton L. K., Reinherz E. L. CD3 zeta/eta/theta locus is colinear with and transcribed antisense to the gene encoding the transcription factor Oct-1. J Immunol. 1993 Sep 15;151(6):3152–3162. [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992 Jan 3;255(5040):79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- Love P. E., Shores E. W., Johnson M. D., Tremblay M. L., Lee E. J., Grinberg A., Huang S. P., Singer A., Westphal H. T cell development in mice that lack the zeta chain of the T cell antigen receptor complex. Science. 1993 Aug 13;261(5123):918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Malissen M., Gillet A., Rocha B., Trucy J., Vivier E., Boyer C., Köntgen F., Brun N., Mazza G., Spanopoulou E. T cell development in mice lacking the CD3-zeta/eta gene. EMBO J. 1993 Nov;12(11):4347–4355. doi: 10.1002/j.1460-2075.1993.tb06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
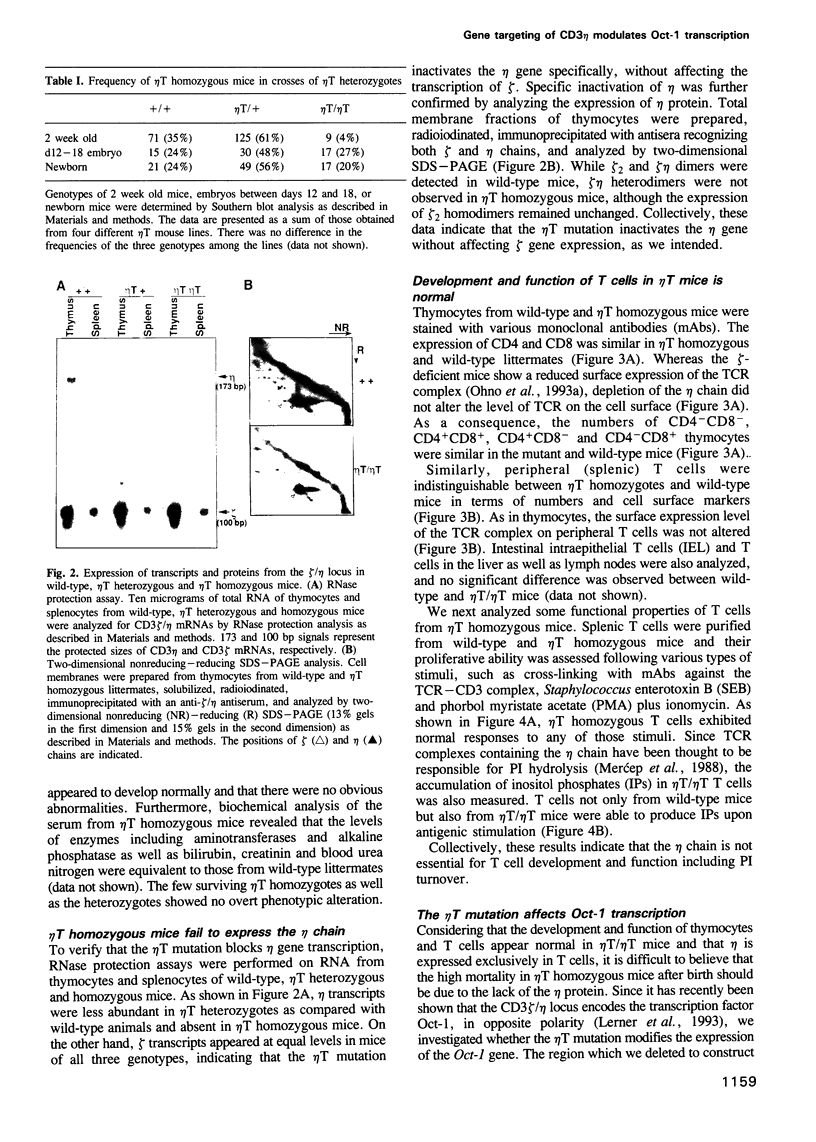
- Merćep M., Bonifacino J. S., Garcia-Morales P., Samelson L. E., Klausner R. D., Ashwell J. D. T cell CD3-zeta eta heterodimer expression and coupling to phosphoinositide hydrolysis. Science. 1988 Oct 28;242(4878):571–574. doi: 10.1126/science.2845582. [DOI] [PubMed] [Google Scholar]
- Merćep M., Weissman A. M., Frank S. J., Klausner R. D., Ashwell J. D. Activation-driven programmed cell death and T cell receptor zeta eta expression. Science. 1989 Dec 1;246(4934):1162–1165. doi: 10.1126/science.2531464. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H., O'Shea J. J., Longo D. L., Loeffler C. M., McVicar D. W., Ochoa A. C. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992 Dec 11;258(5089):1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- Ohno H., Aoe T., Ra C., Yamamoto T., Saito T. TCR isoform containing the Fc receptor gamma chain exhibits structural and functional differences from isoform containing CD3 zeta. Int Immunol. 1993 Nov;5(11):1403–1411. doi: 10.1093/intimm/5.11.1403. [DOI] [PubMed] [Google Scholar]
- Ohno H., Aoe T., Taki S., Kitamura D., Ishida Y., Rajewsky K., Saito T. Developmental and functional impairment of T cells in mice lacking CD3 zeta chains. EMBO J. 1993 Nov;12(11):4357–4366. doi: 10.1002/j.1460-2075.1993.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Saito T. CD3 zeta and eta chains are produced by alternative splicing from a common gene. Int Immunol. 1990;2(11):1117–1119. doi: 10.1093/intimm/2.11.1117. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Seki S., Abo T., Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4-8- double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990 Jul 1;172(1):7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff D. G., Ra C. S., Frank S. J., Klausner R. D., Kinet J. P. Family of disulphide-linked dimers containing the zeta and eta chains of the T-cell receptor and the gamma chain of Fc receptors. Nature. 1990 Sep 13;347(6289):189–191. doi: 10.1038/347189a0. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Blau H. M. Genetic complementation reveals a novel regulatory role for 3' untranslated regions in growth and differentiation. Cell. 1993 Mar 26;72(6):903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- Romeo C., Amiot M., Seed B. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor zeta chain. Cell. 1992 Mar 6;68(5):889–897. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- Rudnicki M. A., Braun T., Hinuma S., Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992 Oct 30;71(3):383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Saga Y., Yagi T., Ikawa Y., Sakakura T., Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992 Oct;6(10):1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Saito T., Weiss A., Miller J., Norcross M. A., Germain R. N. Specific antigen-Ia activation of transfected human T cells expressing murine Ti alpha beta-human T3 receptor complexes. Nature. 1987 Jan 8;325(7000):125–130. doi: 10.1038/325125a0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Weissman A. M., Robey F. A., Berkower I., Klausner R. D. Characterization of an anti-peptide antibody that recognizes the murine analogue of the human T cell antigen receptor-T3 delta-chain. J Immunol. 1986 Nov 15;137(10):3254–3258. [PubMed] [Google Scholar]
- Schöler H. R., Hatzopoulos A. K., Balling R., Suzuki N., Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989 Sep;8(9):2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T., Aicher W. K., Fujihashi K., Yamamoto M., McGhee J. R., Bluestone J. A., Kiyono H. Novel function for intestinal intraepithelial lymphocytes. Murine CD3+, gamma/delta TCR+ T cells produce IFN-gamma and IL-5. J Immunol. 1991 Dec 1;147(11):3736–3744. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Volk R., Köster M., Pöting A., Hartmann L., Knöchel W. An antisense transcript from the Xenopus laevis bFGF gene coding for an evolutionarily conserved 24 kd protein. EMBO J. 1989 Oct;8(10):2983–2988. doi: 10.1002/j.1460-2075.1989.tb08448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]







