Abstract
Thrombocytopenia is a common complication in liver disease and can adversely affect the treatment of liver cirrhosis, limiting the ability to administer therapy and delaying planned surgical/diagnostic procedures because of an increased risk of bleeding. Multiple factors, including splenic sequestration, reduced activity of the hematopoietic growth factor thrombopoietin, bone marrow suppression by chronic hepatitis C virus infection and anti-cancer agents, and antiviral treatment with interferon-based therapy, can contribute to the development of thrombocytopenia in cirrhotic patients. Of these factors, the major mechanisms for thrombocytopenia in liver cirrhosis are (1) platelet sequestration in the spleen; and (2) decreased production of thrombopoietin in the liver. Several treatment options, including platelet transfusion, interventional partial splenic embolization, and surgical splenectomy, are now available for severe thrombocytopenia in cirrhotic patients. Although thrombopoietin agonists and targeted agents are alternative tools for noninvasively treating thrombocytopenia due to liver cirrhosis, their ability to improve thrombocytopenia in cirrhotic patients is under investigation in clinical trials. In this review, we propose a treatment approach to thrombocytopenia according to our novel concept of splenic volume, and we describe the current management of thrombocytopenia due to liver cirrhosis.
Keywords: Liver cirrhosis, Thrombocytopenia, Thrombopoietin, Partial splenic embolization, Splenectomy
Core tip: The major mechanisms for thrombocytopenia in liver cirrhosis are (1) platelet sequestration in the spleen; and (2) decreased production of thrombopoietin in the liver. For thrombocytopenia that is caused by platelet sequestration in the spleen, partial splenic embolization or laparoscopic splenectomy are effective. Thrombopoietin agonists and targeted agents are alternative tools for noninvasively treating thrombocytopenia due to decreased thrombopoietin production, although their ability to improve thrombocytopenia is under investigation in clinical trials. In this review, we describe the current management of thrombocytopenia due to liver cirrhosis, and we propose the novel concept of using the splenic volume to discern the primary cause of thrombocytopenia due to liver cirrhosis.
INTRODUCTION
Thrombocytopenia is a common complication in liver disease, and liver disease-related thrombocytopenia is often defined as a platelet count < 100 × 109/L, including moderate (less than 100 × 109/L) and severe (less than 50 × 109/L) thrombocytopenia. Although clinically significant spontaneous bleeding does not usually occur until the platelet count is less than 10 × 109/L-20 × 109/L, cirrhotic patients with or without cancers often require numerous medical and/or surgical procedures during diagnosis and therapy. The presence of thrombocytopenia can aggravate surgical or traumatic bleeding and can also significantly complicate routine patient care, such as liver biopsy, antiviral therapy, and medically indicated or elective surgery for cirrhotic patients, resulting in delayed or cancelled medical management and affecting the administration of effective treatment for several conditions (e.g., antiviral therapy for chronic hepatitis C virus (HCV) infection or cancer chemotherapy). Indeed, the degree of thrombocytopenia has been shown to be a useful prognostic marker in cirrhotic patients because the finding of severe thrombocytopenia (< 50 × 109/L) in liver disease can be associated with significant morbidity[1,2]. Additionally, a decreased platelet count can often be a diagnostic clue to unsuspected cirrhosis and to the presence of esophageal varices[3-6]. Multiple factors, including splenic sequestration, reduced activity of the hematopoietic growth factor thrombopoietin (TPO), cirrhotic coagulopathy, cirrhotic bone marrow suppression by chronic HCV infection and anti-cancer agents, and antiviral treatment with interferon (IFN)-based therapy, can contribute to the development of thrombocytopenia in cirrhotic patients. Of these factors, the major mechanisms for thrombocytopenia in liver cirrhosis are (1) platelet sequestration in the spleen; and (2) decreased production of TPO in the liver. Historically, thrombocytopenia has been thought to arise from the increased pooling of platelets in an enlarged spleen (splenomegaly). While the normal splenic volume has been reported to range from 50-200 mL[7], splenomegaly sometimes increases it to even more than 1000 mL. Platelet sequestration is seen in congestive splenomegaly due to cirrhosis-induced portal hypertension and is characterized by a redistribution of platelets from the circulating pool to the splenic pool[8]. However, the interventional and/or surgical treatments aimed at reversing portal hypertension do not always correct thrombocytopenia in the clinical setting. Indeed, decreased platelet production has been noted, even in patients without splenomegaly[9], suggesting that other factors are involved in thrombocytopenia due to liver cirrhosis. Platelets are derived from megakaryocytes, and TPO is known to be a potent cytokine that regulates megakaryocyte and platelet production[10,11]. TPO, which is primarily produced in the liver but is also produced, to a lesser extent, in the bone marrow and kidney[12,13], binds to the TPO receptor (c-Mpl), which is expressed on the surface of stem cells, megakaryocyte progenitor cells, megakaryocytes, and platelets. Experimentally, when TPO or its receptor (c-Mpl) has been “knocked-out” by homologous recombination in mice, the megakaryocyte and platelet masses are reduced to approximately 10% of the normal value, even though the animals are healthy and do not spontaneously bleed[14-16]. Cirrhotic patients with thrombocytopenia have lower circulating TPO levels than do cirrhotic patients with normal platelet counts, possibly as a result of diminished TPO production[17]. Interestingly, following successful liver transplantation or splenic embolization, the TPO levels appear to normalize, suggesting that decreased TPO production in the liver may also contribute to thrombocytopenia in cirrhotic patients[17-19]. This review describes the current management of thrombocytopenia in cirrhotic patients and also proposes a treatment approach for thrombocytopenia based on using the splenic volume to distinguish among the major causes of thrombocytopenia (splenic and other mechanisms, such as decreased TPO production).
MANAGEMENT OF THROMBOCYTOPENIA DUE TO LIVER CIRRHOSIS
Several treatment options, including platelet transfusion, interventional splenic artery embolization, and surgical splenectomy, are now available for thrombocytopenia in cirrhotic patients. Therapeutic options to safely and effectively raise the platelet level can have significant effects on the care of cirrhotic patients. Specifically, an increase in platelet levels can significantly reduce the need for platelet transfusions and facilitate the use of IFN-based antiviral therapy and other medically indicated treatments in cirrhotic patients. Recently, treatments such as interventional management using partial splenic embolization (PSE) and surgical splenectomy have often attempted to correct splenomegaly-associated thrombocytopenia as the only current tool that noninvasively improves thrombocytopenia is the administration of platelet infusions. For example, antiviral therapy against HCV using peginterferon (Peg-IFN) alfa-2a plus ribavirin was discontinued in up to 2.6% of patients because of laboratory abnormalities, including thrombocytopenia, and 3%-5% of patients receiving Peg-IFN alfa-2a or alfa-2b plus ribavirin required dose modification because of thrombocytopenia[20,21]. In contrast, the management of thrombocytopenia using PSE or splenectomy prior to antiviral therapy successfully enables avoidance of discontinuation because of thrombocytopenia[22,23]. Recently, a low platelet count has been reported to be a predictor of liver atrophy and long-term mortality in patients on a liver transplant waiting list[24]. Additionally, experimental studies have shown that platelets can promote liver regeneration and improve liver fibrosis[25-27]. Indeed, PSE has a clinically improved prognostic outcome in cirrhotic patients[28]. Although invasive procedures such as PSE and splenectomy are occasionally underused due to potential interference with treatment options, including liver transplantation, aggressive rather than passive management for thrombocytopenia may improve long-tern survival in cirrhotic patients. Although TPO agonists and targeted agents are alternative tools for noninvasively treating thrombocytopenia due to liver cirrhosis, their ability to improve thrombocytopenia in cirrhotic patients is under investigation in clinical trials.
Platelet transfusion
Patients with platelet counts below 50 × 109/L may benefit from prophylactic transfusions to increase platelet counts before procedures. Guidelines for when to use platelet transfusions are available, but the relevance of these published guidelines for liver cirrhosis is unclear. The American Society of Clinical Oncology recommends platelet transfusions for cancer patients with platelet counts of 10 × 109/L-20 × 109/L, depending on the type of cancer[29]. Currently, there is no consensus on the appropriate threshold values for prophylactic platelet transfusions in cirrhotic patients. Complications and limitations of platelet transfusion include febrile nonhemolytic and allergic reactions, the need for hospitalization, iron overload (with chronic transfusions), the risk of infection, platelet refractoriness due to HLA alloimmunization (occurring in up to 40% of patients), and cost[30,31]. Furthermore, platelet transfusions do not ensure a hemostatic platelet level, especially when the risk of bleeding is high[29]. While red blood cells have a lifespan of approximately 120 d, transfused platelets have a shorter life span and will need to be re-dosed within 3-4 d if given for prophylaxis.
Agents targeting the TPO receptor
TPO plays an important role in regulating thrombopoiesis. The decrease in TPO production or activity in cirrhotic patients suggests that TPO can serve as a rational therapeutic target to stimulate platelet production. Several promising novel agents that stimulate TPO and increase platelet levels have been under development for the prevention and/or treatment of thrombocytopenia. Several types of TPO agonists and targeted agents, such as recombinant TPO, interleukin (IL)-11, and TPO mimetrics (peptide and nonpeptide TPO receptor agonists), have been evaluated (Table 1).
Table 1.
Thrombopoietin-receptor agonists for the treatment of thrombocytopenia
| Agent | Target disease | Dose (route) |
| Recombinant human thrombopoietin | ||
| rhTPO | Withdrawn from clinical use | (intravenous) |
| PEG-rHuMGDF | Withdrawn from clinical use | (subcutaneous) |
| rhIL-11 | Chemotherapy-induced TCP[40,42] | 50 μg/kg per day (subcutaneous) |
| TCP in patients with cirrhosis[43] | 50 μg/kg per day (subcutaneous) | |
| TPO mimetrics (peptide TPO receptor agonists and nonpeptide TPO receptor agonists) | ||
| Romiplostim | ITP[47-50] | 0.2-10 μg/kg once a week (subcutaneous) |
| Myelodysplastic syndrome[51-53] | Once a week (subcutaneous) | |
| HCV-related TCP[54,97] | 2 μg/kg once a week (subcutaneous) | |
| Eltrombopag | ITP[55,56] | 50 mg once daily (oral) |
| HCV-related TCP | 25 mg once daily (oral) | |
| E5501 | HCV-related TCP (phase II; NCT00914927) | |
TPO: Thrombopoietin; PEG-rHuMGDF: Pegylated recombinant human megakaryocyte growth and development factor; rhIL-11: Recombinant human interleukin-11; TCP: Thrombocytopenia; ITP: Immune thrombocytopenic purpura; HCV: Hepatitis C virus.
Recombinant TPO and other thrombopoietic agents
Two forms of recombinant human TPO were evaluated in clinical trials and shown to increase megakaryopoiesis and thrombopoiesis: recombinant human TPO (rhTPO) and its pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF). rhTPO is a glycosylated form of TPO consisting of the full-length, native human amino acid sequence. rhTPO has been shown to be a potent stimulator of megakaryocyte growth and platelet production, and it is biologically active in reducing the thrombocytopenia of nonmyeloablative chemotherapy[11]. Although rhTPO could significantly increase platelet counts, the reduction of thrombocytopenia was not always accompanied by a decrease in transfusions[32]. In addition, rhTPO failed to demonstrate the clinical benefits of time to platelet recovery or platelet transfusion requirements in stem cell transplantation or leukemia chemotherapy[11]. Thus, the role of rhTPO in the treatment of thrombocytopenia was limited, and the clinical development of rhTPO was halted. PEG-rHuMGDF is an N-terminal TPO derivative that was pegylated to extend its half-life and retain its TPO activity[11,33]. In initial trials in patients undergoing chemotherapy, PEG-rHuMGDF treatment increased the median platelet nadir counts and enhanced recovery in a dose-dependent manner[34-36]. However, some subjects, including normal platelet donors treated with PEG-rHu-MGDF, developed neutralizing antibodies that cross-reacted with and inactivated endogenous TPO, resulting in extremely severe thrombocytopenia[32,37,38]. The clinical development of PEG-rHuMGDF by Amgen was stopped in 1998 due to this side effect[11].
IL-3, -6, and -11
The interleukins IL-3, IL-6, and IL-11 produce a significant stimulation of platelet production. However, the clinical uses of recombinant IL-3 or IL-6 are severely limited by their proinflammatory properties, which induce flu-like symptoms, including hypotension, fatigue, and myalgias[39]. However, recombinant human IL-11 (rhIL-11) was approved by the United States Food and Drug Administration for use in the prevention of chemotherapy-induced thrombocytopenia[40]. IL-11 acts synergistically with IL-3, TPO, and stem cell factors to increase the number and maturation of megakaryocytic progenitors[41]. IL-11 has also been shown to increase spleen megakaryocyte colony-forming cells and spleen colony-forming units in mice[41]. In patients with cancer who were receiving chemotherapy, a subcutaneous injection of rhIL-11 at a dose of 25-50 μg/kg per day increased their platelet levels and reduced their need for platelet transfusions[40,42]. In cirrhotic patients (Child A and B) with thrombocytopenia, a daily subcutaneous injection of rhIL-11 at 50 μg/kg per day improved platelet counts within 6 to 8 d from the treatment initiation, and the platelet counts doubled in 89% of the cirrhotic patients, achieving > 80 × 109/L in 78%[43]. However, rhIL-11 can cause significant toxicity, including edema, fluid retention, cardiovascular events, and, in some studies, myalgias and arthralgias[44]. Therefore, the use of rhIL-11 in cirrhotic patients requires careful attention due to the high frequency of regimen-related toxicity[43].
Mimetics (TPO peptide and nonpeptide mimetics)
Recently, great interest has been focused on the development of TPO peptide and nonpeptide mimetics. These mimetics are designed to bind to the TPO receptor but have no sequence homology with endogenous TPO. Two TPO receptor agonists (Romiplostim and Eltrombopag) are currently available.
Romiplostim is a 60 kDa molecule that is composed of 4 TPO-mimetic peptides that are attached via glycine bridges to an IgG heavy-chain Fc molecule[45]. This agent increased the platelet count in healthy individuals[46] and in patients with immune thrombocytopenia (ITP)[47-50] and myelodysplastic syndrome[51-53]. Romiplostim has been evaluated in patients with HCV-related thrombocytopenia. In patients with chronic liver disease and severe thrombocytopenia secondary to HCV infection, preoperative Romiplostim administration increased platelet counts to a level acceptable for elective surgical interventions without postoperative bleeding episodes[54]. Romiplostim is currently approved by the FDA only for the treatment of chronic ITP in adults and is administered weekly as a subcutaneous injection at a dose of 1 to 10 mg/kg.
Eltrombopag is a small-molecule nonpeptide oral platelet growth factor that acts as a TPO-R agonist. The binding of eltrombopag to the transmembrane domain of the TPO receptor activates intracellular signal transduction pathways that stimulate megakaryocyte proliferation and differentiation and increase platelet counts in a dose-dependent manner in healthy subjects and patients with chronic ITP[55,56]. Eltrombopag is FDA-approved for the treatment of adults with ITP and is administered orally at a dose of 25 to 75 mg per day (lower doses in patients of Asian ancestry). One potential advantage of Eltrombopag that makes it superior to the TPO peptide (Romiplostim) is that Eltrombopag may be administered orally. A phase II, multicenter, randomized trial of daily Eltrombopag in patients with HCV-associated thrombocytopenia and compensated liver disease showed that, after 4 wk of therapy, platelet count increases to > 100 × 109/L were achieved in 75%, 79%, and 95% of patients treated with 30 mg, 50 mg, and 75 mg Eltrombopag, respectively, compared to 0% of placebo patients (P < 0.001)[57]. None of the patients with liver disease had a worsening of their liver function tests[57]. Additionally, significantly more patients in the Eltrombopag treatment groups completed 12 wk of antiviral therapy (36%, 53%, and 65% in the 30-mg, 50-mg, and 75-mg groups, respectively), compared with 6% of placebo patients[57]. The latest study revealed that Eltrombopag administration prior to elective invasive procedures reduced the need for platelet transfusions in patients with chronic liver disease compared with placebo, with platelet transfusion frequencies of 28% and 81%, respectively[58]. Nonetheless, the use of Eltrombopag has been reported to be associated with an increased incidence of portal-vein thrombosis compared to placebo, at 4% and 1%, respectively[58]. Collectively, although rhIL-11 and TPO mimetics are alternative tools for noninvasively treating thrombocytopenia due to liver cirrhosis, the evidence for their ability to improve thrombocytopenia is mainly based on patients with ITP; few studies have evaluated cirrhotic patients. A further investigation of their usefulness for the treatment of thrombocytopenia due to liver cirrhosis is expected through clinical trials.
INTERVENTIONAL MANAGEMENT
PSE
PSE is an interventional, non-surgical procedure that was developed to treat hypersplenism secondary to hepatic disease. PSE for hypersplenism can be carried out with almost no blood loss under local anesthesia. In 1973, Maddison[59] proposed total splenic embolization using interventional techniques instead of splenectomy for the treatment of hypersplenism. Initially, patients with complete splenic embolization had severe complications, including splenic abscess, splenic rupture, serious pneumonia, sepsis, hematoma, bleeding, pancreatic and hepatic infarction, and death. Thus, an important limitation of complete splenic infarction was the high incidence of morbidity. Six years later, Spigos et al[60] developed PSE and treated 13 patients with PSE, antibiotic coverage, and post-embolization pain control, which were performed safely and effectively. A significant reduction in both the morbidity and mortality rates was obtained by PSE compared with complete splenic embolization.
As for the clinical effects, PSE for hypersplenism has been reported to achieve prolonged improvement in blood cell counts[61,62]. The platelet count rises after PSE and then reaches the peak value in 1-2 wk. The platelet counts stabilize in 2 mo at approximately 2-fold higher than their value before PSE, slightly less than the peak value. This PSE-derived clinical benefit is mainly due to the resolution of the platelet sequestration in the spleen. As an alternative possible mechanism of increasing the platelet counts after PSE, Hidaka et al[19] reported that TPO production, the score of megakaryocytes with platelet production, and an index of platelet production by megakaryocytes in the bone marrow were restored after PSE in cirrhotic patients but not in patients with idiopathic portal hypertension. In addition to the prolonged improvement in blood cell counts, several studies have reported PSE-associated fringe benefits, such as individual liver functional improvement[61,63]. The mechanism of these liver functional changes is not well elucidated but may be related to increases in hepatic arterial and superior mesenteric vasculature blood flow after embolization[64]. Porter et al[65] used a video dilution technique to demonstrate that the splenic arterial flow was reduced from 19% to 3% post-embolization, whereas there was a concomitant increase in the hepatic arterial flow from 3% to 15% and an increase in the superior mesenteric blood flow from 6% to 19%. However, Mukaiya et al[66] reported that PSE decreased splenic arterial flow and reduced splenic vein pressure without altering portal blood flow by a thermodilution method. Bárcena et al[67] reported that PSE could resolve splenic artery steal syndrome, which resulted in improved graft function in liver-transplanted patients.
PSE, like laparoscopic splenectomy, has recently been highlighted and widely applied to improve thrombocytopenia in cirrhotic patients prior to the administration of Peg-IFN and ribavirin for the treatment of HCV infections. PSE prior to IFN-based therapy, compared with no PSE, produced advantageous maintenance of higher platelet counts and an increase in adherence to Peg-IFN[23,68]. PSE can also be adapted to improve esophagogastric varices and portal hypertension in cirrhotic patients. In other medical treatments, such as hepatectomy for hepatocellular carcinoma[69], chemotherapy for cancer, and orthotopic liver transplantation[70,71], clinical benefits, such as improved thrombocytopenia and neutropenia, have been reported for PSE as a pre- and post-treatment procedure.
Splenic infarction ratio or splenic infarcted volume?
Classically, the splenic infarction ratio ranges from 50% to 80%; this range has been used routinely as the target of PSE. While the splenic infarction rate correlated positively with increases in the platelet count, no therapeutic differences were found in patients with splenic infarction rates of 50%, 70% and 80%. Therefore, more reliable predictive factors of the increase in platelet counts after PSE and the recommended extent of splenic infarction in PSE for liver cirrhosis are needed. Recently, contrast-enhanced computed tomography (CT) scanning has enabled us to accurately measure the area of splenic infarction. A previous study by the present authors proposed the novel concept that infarcted splenic volume, and not the splenic infarction rate, is a determinant factor for increases in the platelet count after PSE and that an infarcted splenic volume of greater than 388 mL could induce a sufficient increase in the platelet count at 1 year after PSE [formula of the increased platelet counts at 1 year = 2.19 + 0.01 × infarcted splenic volume (mL), R2 = 0.203][72]. This easy prediction method would help to determine the necessary infarcted splenic volume or spleen embolization ratio during PSE. As an adequate preoperative splenic volume and infarcted splenic volume are required for an effective increase in platelet counts after PSE, the PSE for cirrhotic patients with small spleens, such as those smaller than 400 mL, must embolize nearly the entire volume of the spleen (388 mL = 97% of 400 mL). Based on the above concept, which applies to cirrhotic patients with preoperative splenic volumes < 400 mL, a laparoscopic splenectomy, which can remove the total spleen, has been recommended to obtain as great an increase in the platelet counts as possible[62,72]. In cirrhotic patients with preoperative splenic volumes greater than 400 mL, a prolonged increase in the platelet counts could be achieved by PSE with sufficient infarcted splenic volume. As for the upper limit of infarcted splenic volume in a single PSE, however, there is evidence that a massive infarcted splenic volume of greater than 540 mL in a single PSE is a significant risk factor for severe complications, such as splenic abscess, refractory ascites, or pleural effusion post PSE[73]. Although recent advances in interventional radiology have further decreased the side-effects of PSE and have greatly expanded the indications of PSE, the morbidity rate of PSE for hypersplenism has still been shown to fall within the range of 0% to 17%[28,73-76]. As for mortality, some studies have reported that PSE for hypersplenism is associated with no deaths[28,76,77], whereas others have reported a mortality rate ranging from 1% to 12%[73-75,78].
A relapse in thrombocytopenia is occasionally observed in patients following PSE. In general, the residual splenic volume will decrease gradually over a period of months after PSE. In contrast, a re-enlargement of the residual splenic area after PSE, accompanied by a relapse of thrombocytopenia, has been reported in some cases[62]. Indeed, in cirrhotic patients with massive splenomegaly above 700 mL, the non-infarcted splenic volume plays an important role in long-term platelet increases[62]. In cases of relapse after PSE, laparoscopic splenectomy as a salvage treatment can provide a sufficient increase in platelet counts[79]. However, for an inexperienced surgeon, splenectomy following PSE is very difficult because of the inflammatory reaction around the spleen. As an alternative procedure, repeated PSE might be a safe and effective strategy against the relapse of thrombocytopenia post PSE. Furthermore, Child-Pugh class C is known as a significant risk factor of severe complications after PSE[73]. Additionally, in patients with Child-Pugh class C and massive splenomegaly above 1000 mL, repeated PSE may be a safe and effective strategy to achieve a sufficient infarcted splenic volume and a smaller non-infarcted splenic volume. Therefore, depending on the preoperative splenic volume, laparoscopic splenectomy (preoperative splenic volume < 400 mL), a single PSE (400 mL < preoperative splenic volume < 700 mL), or repeated PSE (preoperative splenic volume > 700 mL) may be recommended[62]. This new approach, based on the preoperative splenic volume, will help us to select a suitable operative procedure for thrombocytopenia preoperatively, as shown in Table 2.
Table 2.
Approaches to treat thrombocytopenia induced by liver cirrhosis based on preoperative splenic volume[62]
| Splenic volume | SV < 400 mL | 400 mL ≤ SV ≤ 700 mL | 700 mL< SV |
| Procedure | L-splenectomy | L-splenectomy | L-splenectomy |
| or | or | ||
| Single PSE | Repeated PSE | ||
| Target in PSE | Infarcted splenic area | Non-infarcted splenic area | |
| (infarcted splenic volume) | (infarcted splenic ratio and non-infarcted splenic volume) |
SV: Splenic volume; L-Splenectomy: Laparoscopic splenectomy; PSE: Partial splenic embolization. Modified and adapted from reference[62].
Thus, the volume of the embolized spleen has been found to be critical in PSE. If the embolization volume is too small, then the therapy will not be effective, and if it is too large, then the risk of serious complications is increased significantly. Therefore, the quantitative evaluation of the embolized volume during PSE is both desirable and useful. The blood flow rate has traditionally been used as an indicator of the embolized volume, but the results have largely depended on the experience of the operator. Making an accurate estimation of the embolized splenic volume during PSE is still difficult. Further developments in the technique of real time assessment for embolized splenic volume are expected to produce a safer and more effective PSE.
SURGICAL MANAGEMENT
Splenectomy
As a surgical option, open splenectomy has been performed for hypersplenism since the 1950s. Since the first report of laparoscopic splenectomy for ITP by Delaitre and Maignien[80] in 1991, recent advances in laparoscopic surgical techniques have enabled the performance of laparoscopic splenectomy, even for hypersplenism, with advantages over conditional open splenectomy that include less blood loss, less pain, a shorter hospital stay, better cosmetic outcomes, and fewer surgery-related complications[81-83]. Laparoscopic splenectomy can also be performed as a hand-assisted laparoscopic splenectomy (HALS) in cirrhotic patients with hypersplenism. Several studies have demonstrated that HALS is more appropriate than total laparoscopic splenectomy in cirrhotic patients with portal hypertension as laparoscopic splenectomy is technically difficult due to the splenomegaly, well-developed collateral circulation, and the increased risk of bleeding caused by thrombocytopenia. Ohta et al[84] reported that portal hypertension and severe liver dysfunction were independent risk factors for massive intraoperative bleeding during laparoscopic splenectomy. Furthermore, massive intraoperative hemorrhage, which is usually difficult to control by laparoscopic procedures alone, is a risk factor for serious postoperative morbidity in patients with liver dysfunction. The surgeon’s use of one hand in HALS can control a sudden massive intraoperative hemorrhage and can easily mobilize a huge spleen, which is likely to result in less intraoperative blood loss and shorter operative times in comparison to conventional laparoscopic splenectomy.
Similar to PSE, laparoscopic splenectomy has been performed for hypersplenism-induced thrombocytopenia in patients with cirrhosis. Yoshida et al[85] reported that the platelet count after splenectomy in cirrhotic patients can be predicted based on preoperative clinical characteristics [the increased platelet count at 1 mo (× 109/L) = 6.320 + 0.011 (preoperative splenic volume) - 0.004 (lymphocyte count/μL) + 2.25 (preoperative platelet count × 109/L), R2 = 0.336]. Thus, similar to PSE, the laparoscopic splenectomy-derived clinical benefit for thrombocytopenia depends on the preoperative splenic volume, indicating that preoperative splenic volume will help physicians to discern the primary cause of thrombocytopenia due to liver cirrhosis among splenic and other mechanisms, such as decreased TPO production (Figure 1).
Figure 1.
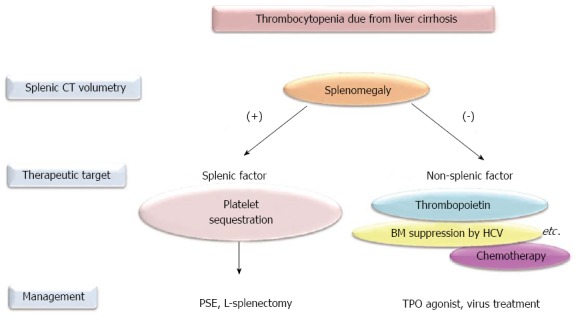
Approaches to treat thrombocytopenia due to liver cirrhosis according to splenic volume. Thrombocytopenia is defined as fewer than 100 × 109/L. CT: Computed tomography; PSE: Partial splenic embolization; L-splenectomy: Laparoscopic splenectomy; BM: Bone marrow; HCV: Hepatitis C virus; TPO: Thrombopoietin.
The morbidity and mortality rates following laparoscopic splenectomy for hypersplenism are 11%-36% and 0%, respectively[86-88]. Among the severe complications that can occur after splenectomy, portal and splenic vein thrombosis (PSVT) and overwhelming post-splenectomy infection (OPSI) are well known adverse events. The former originates from hemodynamic changes that occur post-splenectomy, such as a complete lack of splenic vein flow following decreased portal vein flow. Recent advances in diagnostic modalities, such as enhanced CT or Doppler ultrasonography, reveal that PSVT is a common complication after splenectomy, despite the endogenous coagulopathy of cirrhosis[89-92]. Previous studies demonstrated that massive splenomegaly and the splenic vein diameter were significant risk factors for PSVT after laparoscopic splenectomy in cirrhotic patients[93,94]. Both the spleen size and splenic vein diameter are thought to be proportional to the splenic venous flow and pressure, suggesting that the rapid drop in the flow of the splenic vein after splenectomy contributes to thrombus formation. As the clinical manifestations of PSVT are nonspecific, asymptomatic PSVT is diagnosed in 52.5% (21/40) of patients[94]. Among the types of PSVT, portal vein thrombosis (PVT) and mesenteric vein thrombus extending from the distal splenic vein should be treated with anti-coagulation therapy (Figure 2). Kawanaka et al[95] reported a 36% incidence of PVT after splenectomy in cirrhotic patients and a low antithrombin III (AT-III) activity; any further decreases in this activity were associated with PVT. They showed that treatment with AT-III concentrates [prophylactic administration of AT-III concentrates (1500 U/d)] on postoperative days 1, 2, and 3 was likely to prevent the development of PVT after laparoscopic splenectomy[95].
Figure 2.
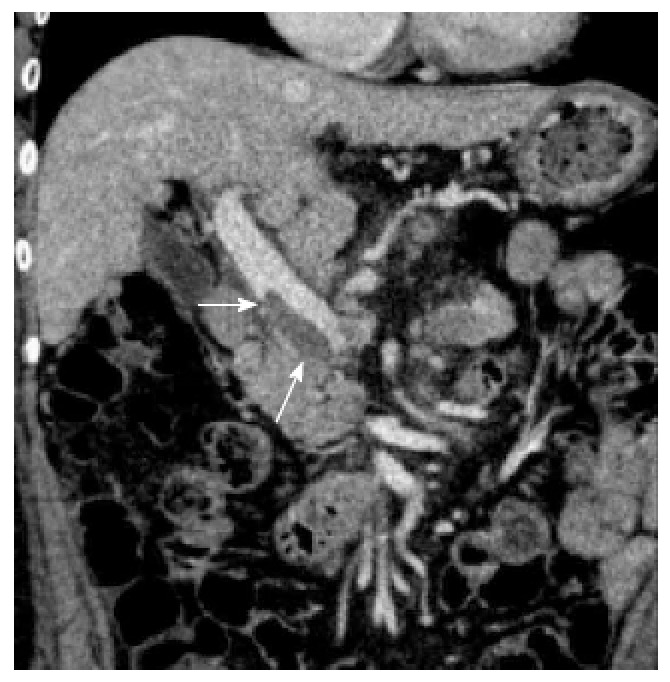
Portal vein thrombosis after splenectomy (arrow).
Overwhelming post-splenectomy infection
The spleen contains many macrophages (part of the reticuloendothelial system), which are immune cells that phagocytose (eat) and destroy bacteria. Since overwhelming post-splenectomy infection (OPSI) was first described by King and Schumaker[96] in 1952, OPSI has become another well-known adverse event post-splenectomy. OPSI originates mainly from encapsulated bacteria, such as Streptococcus pneumoniae, Hemophilus influenzae, and Neisseria meningitidis. Because capsules made of polysaccharides (sugars) permit bacteria to evade phagocytosis by macrophages, opsonization is required for the complete phagocytosis of an encapsulated bacterium. The spleen produces immunoglobulin M antibodies and complements, both of which can be used to opsonize bacteria. Thus, the spleen plays an important role in both the tagging of bacteria for destruction and the actual destruction of the bacteria through phagocytosis. As infecting bacteria cannot be adequately opsonized in conditions such as post-splenectomy, this infection becomes more severe. OPSI is a rare but rapidly fatal infection that occurs in patients following the removal of the spleen. Therefore, post-splenectomy patients require immunizations (pneumococcal conjugate vaccine, Hib vaccine, and the meningococcal vaccine) against pathogens that normally require opsonization for phagocytosis by the splenic macrophages.
CONCLUSION
The major mechanisms of thrombocytopenia in liver cirrhosis are (1) platelet sequestration in the spleen; and (2) decreased production of TPO in the liver. The concept of splenic volume helps us to discern the primary cause of thrombocytopenia due to liver cirrhosis among splenic sequestration and other mechanisms, such as decreased TPO production. For thrombocytopenia caused by platelet sequestration in the spleen, either PSE or laparoscopic splenectomy is effective against thrombocytopenia in cirrhotic patients. The choice between PSE and splenectomy depends upon the splenic volume, the intention of the treatment (required increase in platelet counts), and the conditions of the patient (whether general anesthesia is available) (Table 3). For thrombocytopenia caused by a decreased production of TPO, TPO agonists and targeted agents may represent alternative tools for noninvasive treatment of thrombocytopenia due to liver cirrhosis in the near future.
Table 3.
Comparison between partial splenic embolization and laparoscopic splenectomy for thrombocytopenia caused by liver cirrhosis
| Procedure | PSE | L-splenectomy (HALS) |
| Invasiveness | + | +++ |
| (no transfusion) | (rarely with major bleeding) | |
| (local anesthesia) | (general anesthesia) | |
| Platelet increase | ++ | +++ |
| Specific complication | Splenic abscess | Portal thrombosis, OPSI |
| Available in case with HCC | Synchronous TACE | Synchronous RFA or hepatectomy |
PSE: Partial splenic embolization; L-Splenectomy: Laparoscopic splenectomy; HALS: Hand-assisted laparoscopic splenectomy; OPSI: Overwhelming post-splenectomy infection; HCC: Hepatocellular carcinoma; TACE: Trans-arterial chemoembolization; RFA: Radio frequency ablation.
Footnotes
Supported by Grant-in-Aid for Young Scientists, Ministry of Education, Culture, Sports, Science and Technology of Japan, No. 24791434 (to Hayashi H) and Takeda Science Foundation, Japan (to Hayashi H)
P- Reviewers: Abraham P, Lonardo A, Syam AF, Tanaka T S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–1007. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14 Suppl D:60D–66D. doi: 10.1155/2000/617428. [DOI] [PubMed] [Google Scholar]
- 3.Cozzolino G, Lonardo A, Francica G, Amendola F, Cacciatore L. Differential diagnosis between hepatic cirrhosis and chronic active hepatitis: specificity and sensitivity of physical and laboratory findings in a series from the Mediterranean area. Am J Gastroenterol. 1983;78:442–445. [PubMed] [Google Scholar]
- 4.Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V, et al. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200–1205. doi: 10.1136/gut.52.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Runyon BA. A Primer on Detecting Cirrhosis and Caring for These Patients without Causing Harm. Int J Hepatol. 2011;2011:801983. doi: 10.4061/2011/801983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udell JA, Wang CS, Tinmouth J, FitzGerald JM, Ayas NT, Simel DL, Schulzer M, Mak E, Yoshida EM. Does this patient with liver disease have cirrhosis? JAMA. 2012;307:832–842. doi: 10.1001/jama.2012.186. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko J, Sugawara Y, Matsui Y, Ohkubo T, Makuuchi M. Normal splenic volume in adults by computed tomography. Hepatogastroenterology. 2002;49:1726–1727. [PubMed] [Google Scholar]
- 8.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest. 1966;45:645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Suárez J, Burgaleta C, Hernanz N, Albarran F, Tobaruela P, Alvarez-Mon M. HCV-associated thrombocytopenia: clinical characteristics and platelet response after recombinant alpha2b-interferon therapy. Br J Haematol. 2000;110:98–103. doi: 10.1046/j.1365-2141.2000.02132.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaushansky K. Thrombopoietin. N Engl J Med. 1998;339:746–754. doi: 10.1056/NEJM199809103391107. [DOI] [PubMed] [Google Scholar]
- 11.Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100:3457–3469. doi: 10.1182/blood.V100.10.3457. [DOI] [PubMed] [Google Scholar]
- 12.Nomura S, Ogami K, Kawamura K, Tsukamoto I, Kudo Y, Kanakura Y, Kitamura Y, Miyazaki H, Kato T. Cellular localization of thrombopoietin mRNA in the liver by in situ hybridization. Exp Hematol. 1997;25:565–572. [PubMed] [Google Scholar]
- 13.Qian S, Fu F, Li W, Chen Q, de Sauvage FJ. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. 1998;92:2189–2191. [PubMed] [Google Scholar]
- 14.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 15.Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- 16.de Sauvage FJ, Carver-Moore K, Luoh SM, Ryan A, Dowd M, Eaton DL, Moore MW. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J Exp Med. 1996;183:651–656. doi: 10.1084/jem.183.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peck-Radosavljevic M, Zacherl J, Meng YG, Pidlich J, Lipinski E, Längle F, Steininger R, Mühlbacher F, Gangl A. Is inadequate thrombopoietin production a major cause of thrombocytopenia in cirrhosis of the liver? J Hepatol. 1997;27:127–131. doi: 10.1016/s0168-8278(97)80291-7. [DOI] [PubMed] [Google Scholar]
- 18.Rios R, Sangro B, Herrero I, Quiroga J, Prieto J. The role of thrombopoietin in the thrombocytopenia of patients with liver cirrhosis. Am J Gastroenterol. 2005;100:1311–1316. doi: 10.1111/j.1572-0241.2005.41543.x. [DOI] [PubMed] [Google Scholar]
- 19.Hidaka H, Kokubu S, Saigenji K, Isobe Y, Maeda T. Restoration of thrombopoietin production after partial splenic embolization leads to resolution of thrombocytopenia in liver cirrhosis. Hepatol Res. 2002;23:265. doi: 10.1016/s1386-6346(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 20.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 21.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 22.Akahoshi T, Tomikawa M, Kawanaka H, Furusyo N, Kinjo N, Tsutsumi N, Nagao Y, Hayashi J, Hashizume M, Maehara Y. Laparoscopic splenectomy with interferon therapy in 100 hepatitis-C-virus-cirrhotic patients with hypersplenism and thrombocytopenia. J Gastroenterol Hepatol. 2012;27:286–290. doi: 10.1111/j.1440-1746.2011.06870.x. [DOI] [PubMed] [Google Scholar]
- 23.Tahara H, Takagi H, Sato K, Shimada Y, Tojima H, Hirokawa T, Ohyama T, Horiuchi K, Naganuma A, Arai H, et al. A retrospective cohort study of partial splenic embolization for antiviral therapy in chronic hepatitis C with thrombocytopenia. J Gastroenterol. 2011;46:1010–1019. doi: 10.1007/s00535-011-0407-9. [DOI] [PubMed] [Google Scholar]
- 24.Bleibel W, Caldwell SH, Curry MP, Northup PG. Peripheral platelet count correlates with liver atrophy and predicts long-term mortality on the liver transplant waiting list. Transpl Int. 2013;26:435–442. doi: 10.1111/tri.12064. [DOI] [PubMed] [Google Scholar]
- 25.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 26.Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–376. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe M, Murata S, Hashimoto I, Nakano Y, Ikeda O, Aoyagi Y, Matsuo R, Fukunaga K, Yasue H, Ohkohchi N. Platelets contribute to the reduction of liver fibrosis in mice. J Gastroenterol Hepatol. 2009;24:78–89. doi: 10.1111/j.1440-1746.2008.05497.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Takesue M, Yoshida K, Kuboki M, Yamamoto S. Improved prognosis of cirrhosis patients with esophageal varices and thrombocytopenia treated by endoscopic variceal ligation plus partial splenic embolization. Dig Dis Sci. 2006;51:352–358. doi: 10.1007/s10620-006-3137-8. [DOI] [PubMed] [Google Scholar]
- 29.Schiffer CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M, Goldstein M, Hume H, McCullough JJ, McIntyre RE, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1519–1538. doi: 10.1200/JCO.2001.19.5.1519. [DOI] [PubMed] [Google Scholar]
- 30.McCullough J. Current issues with platelet transfusion in patients with cancer. Semin Hematol. 2000;37:3–10. doi: 10.1016/s0037-1963(00)90047-7. [DOI] [PubMed] [Google Scholar]
- 31.Perrotta PL, Snyder EL. Non-infectious complications of transfusion therapy. Blood Rev. 2001;15:69–83. doi: 10.1054/blre.2001.0151. [DOI] [PubMed] [Google Scholar]
- 32.Vadhan-Raj S, Patel S, Bueso-Ramos C, Folloder J, Papadopolous N, Burgess A, Broemeling LD, Broxmeyer HE, Benjamin RS. Importance of predosing of recombinant human thrombopoietin to reduce chemotherapy-induced early thrombocytopenia. J Clin Oncol. 2003;21:3158–3167. doi: 10.1200/JCO.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Vadhan-Raj S, Cohen V, Bueso-Ramos C. Thrombopoietic growth factors and cytokines. Curr Hematol Rep. 2005;4:137–144. [PubMed] [Google Scholar]
- 34.Basser RL, Rasko JE, Clarke K, Cebon J, Green MD, Grigg AP, Zalcberg J, Cohen B, O’Byrne J, Menchaca DM, et al. Randomized, blinded, placebo-controlled phase I trial of pegylated recombinant human megakaryocyte growth and development factor with filgrastim after dose-intensive chemotherapy in patients with advanced cancer. Blood. 1997;89:3118–3128. [PubMed] [Google Scholar]
- 35.Basser RL, Rasko JE, Clarke K, Cebon J, Green MD, Hussein S, Alt C, Menchaca D, Tomita D, Marty J, et al. Thrombopoietic effects of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) in patients with advanced cancer. Lancet. 1996;348:1279–1281. doi: 10.1016/S0140-6736(96)04471-6. [DOI] [PubMed] [Google Scholar]
- 36.Hunt P, Li YS, Nichol JL, Hokom MM, Bogenberger JM, Swift SE, Skrine JD, Hornkohl AC, Lu H, Clogston C. Purification and biologic characterization of plasma-derived megakaryocyte growth and development factor. Blood. 1995;86:540–547. [PubMed] [Google Scholar]
- 37.Basser RL, O’Flaherty E, Green M, Edmonds M, Nichol J, Menchaca DM, Cohen B, Begley CG. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood. 2002;99:2599–2602. doi: 10.1182/blood.v99.7.2599. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, Kuter DJ. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–3248. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 39.Vial T, Descotes J. Clinical toxicity of cytokines used as haemopoietic growth factors. Drug Saf. 1995;13:371–406. doi: 10.2165/00002018-199513060-00006. [DOI] [PubMed] [Google Scholar]
- 40.Tepler I, Elias L, Smith JW, Hussein M, Rosen G, Chang AY, Moore JO, Gordon MS, Kuca B, Beach KJ, et al. A randomized placebo-controlled trial of recombinant human interleukin-11 in cancer patients with severe thrombocytopenia due to chemotherapy. Blood. 1996;87:3607–3614. [PubMed] [Google Scholar]
- 41.Neben TY, Loebelenz J, Hayes L, McCarthy K, Stoudemire J, Schaub R, Goldman SJ. Recombinant human interleukin-11 stimulates megakaryocytopoiesis and increases peripheral platelets in normal and splenectomized mice. Blood. 1993;81:901–908. [PubMed] [Google Scholar]
- 42.Gordon MS, McCaskill-Stevens WJ, Battiato LA, Loewy J, Loesch D, Breeden E, Hoffman R, Beach KJ, Kuca B, Kaye J, et al. A phase I trial of recombinant human interleukin-11 (neumega rhIL-11 growth factor) in women with breast cancer receiving chemotherapy. Blood. 1996;87:3615–3624. [PubMed] [Google Scholar]
- 43.Ghalib R, Levine C, Hassan M, McClelland T, Goss J, Stribling R, Seu P, Patt YZ. Recombinant human interleukin-11 improves thrombocytopenia in patients with cirrhosis. Hepatology. 2003;37:1165–1171. doi: 10.1053/jhep.2003.50160. [DOI] [PubMed] [Google Scholar]
- 44.Cantor SB, Elting LS, Hudson DV, Rubenstein EB. Pharmacoeconomic analysis of oprelvekin (recombinant human interleukin-11) for secondary prophylaxis of thrombocytopenia in solid tumor patients receiving chemotherapy. Cancer. 2003;97:3099–3106. doi: 10.1002/cncr.11447. [DOI] [PubMed] [Google Scholar]
- 45.Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med. 2009;60:193–206. doi: 10.1146/annurev.med.60.042307.181154. [DOI] [PubMed] [Google Scholar]
- 46.Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. 2004;76:628–638. doi: 10.1016/j.clpt.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser JS, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355:1672–1681. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
- 48.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 49.Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X, Berger DP. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–1899. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 50.Bussel JB, Buchanan GR, Nugent DJ, Gnarra DJ, Bomgaars LR, Blanchette VS, Wang YM, Nie K, Jun S. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118:28–36. doi: 10.1182/blood-2010-10-313908. [DOI] [PubMed] [Google Scholar]
- 51.Perez Ruixo JJ, Doshi S, Wang YM, Mould DR. Romiplostim dose-response in patients with myelodysplastic syndromes. Br J Clin Pharmacol. 2013;75:1445–1454. doi: 10.1111/bcp.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenberg PL, Garcia-Manero G, Moore M, Damon L, Roboz G, Hu K, Yang AS, Franklin J. A randomized controlled trial of romiplostim in patients with low- or intermediate-risk myelodysplastic syndrome receiving decitabine. Leuk Lymphoma. 2013;54:321–328. doi: 10.3109/10428194.2012.713477. [DOI] [PubMed] [Google Scholar]
- 53.Kantarjian H, Fenaux P, Sekeres MA, Becker PS, Boruchov A, Bowen D, Hellstrom-Lindberg E, Larson RA, Lyons RM, Muus P, et al. Safety and efficacy of romiplostim in patients with lower-risk myelodysplastic syndrome and thrombocytopenia. J Clin Oncol. 2010;28:437–444. doi: 10.1200/JCO.2009.24.7999. [DOI] [PubMed] [Google Scholar]
- 54.Moussa MM, Mowafy N. Preoperative use of romiplostim in thrombocytopenic patients with chronic hepatitis C and liver cirrhosis. J Gastroenterol Hepatol. 2013;28:335–341. doi: 10.1111/j.1440-1746.2012.07246.x. [DOI] [PubMed] [Google Scholar]
- 55.Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357:2237–2247. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 56.Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, Erickson-Miller CL. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. Blood. 2007;109:4739–4741. doi: 10.1182/blood-2006-11-057968. [DOI] [PubMed] [Google Scholar]
- 57.McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, Berg T, Gordon SC, Campbell FM, Theodore D, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227–2236. doi: 10.1056/NEJMoa073255. [DOI] [PubMed] [Google Scholar]
- 58.Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, Jeffers L, McHutchison J, Chen PJ, Han KH, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367:716–724. doi: 10.1056/NEJMoa1110709. [DOI] [PubMed] [Google Scholar]
- 59.Maddison F. Embolic therapy of hypersplenism. Invest Radiol. 1973;8:280–281. [Google Scholar]
- 60.Spigos DG, Jonasson O, Mozes M, Capek V. Partial splenic embolization in the treatment of hypersplenism. AJR Am J Roentgenol. 1979;132:777–782. doi: 10.2214/ajr.132.5.777. [DOI] [PubMed] [Google Scholar]
- 61.Tajiri T, Onda M, Yoshida H, Mamada Y, Taniai N, Kumazaki T. Long-term hematological and biochemical effects of partial splenic embolization in hepatic cirrhosis. Hepatogastroenterology. 2002;49:1445–1448. [PubMed] [Google Scholar]
- 62.Hayashi H, Beppu T, Okabe K, Masuda T, Okabe H, Ishiko T, Baba H. Therapeutic factors considered according to the preoperative splenic volume for a prolonged increase in platelet count after partial splenic embolization for liver cirrhosis. J Gastroenterol. 2010;45:554–559. doi: 10.1007/s00535-009-0185-9. [DOI] [PubMed] [Google Scholar]
- 63.Hirai K, Kawazoe Y, Yamashita K, Kumagai M, Tanaka M, Sakai T, Inoue R, Eguchi S, Majima Y, Abe M. Transcatheter partial splenic arterial embolization in patients with hypersplenism: a clinical evaluation as supporting therapy for hepatocellular carcinoma and liver cirrhosis. Hepatogastroenterology. 1986;33:105–108. [PubMed] [Google Scholar]
- 64.Nishida O, Moriyasu F, Nakamura T, Ban N, Miura K, Sakai M, Uchino H, Miyake T. Interrelationship between splenic and superior mesenteric venous circulation manifested by transient splenic arterial occlusion using a balloon catheter. Hepatology. 1987;7:442–446. doi: 10.1002/hep.1840070305. [DOI] [PubMed] [Google Scholar]
- 65.Porter BA, Frey CF, Link DP, Lantz BM, Pimstone NR. Splenic embolization monitored by the video dilution technique. AJR Am J Roentgenol. 1983;141:1063–1065. doi: 10.2214/ajr.141.5.1063. [DOI] [PubMed] [Google Scholar]
- 66.Mukaiya M, Hirata K, Yamashiro K, Katsuramaki T, Kimura H, Denno R. Changes in portal hemodynamics and hepatic function after partial splenic embolization (PSE) and percutaneous transhepatic obliteration (PTO) Cancer Chemother Pharmacol. 1994;33 Suppl:S37–S41. doi: 10.1007/BF00686666. [DOI] [PubMed] [Google Scholar]
- 67.Bárcena R, Moreno A, Foruny JR, Moreno A, Sánchez J, Gil-Grande L, Blázquez J, Nuño J, Fortún J, Rodriguez-Gandía MA, et al. Improved graft function in liver-transplanted patients after partial splenic embolization: reversal of splenic artery steal syndrome? Clin Transplant. 2006;20:517–523. doi: 10.1111/j.1399-0012.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 68.Foruny JR, Blázquez J, Moreno A, Bárcena R, Gil-Grande L, Quereda C, Pérez-Elías MJ, Moreno J, Sánchez J, Muriel A, et al. Safe use of pegylated interferon/ribavirin in hepatitis C virus cirrhotic patients with hypersplenism after partial splenic embolization. Eur J Gastroenterol Hepatol. 2005;17:1157–1164. doi: 10.1097/00042737-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu T, Tajiri T, Yoshida H, Yokomuro S, Mamada Y, Taniai N, Kawano Y, Takahashi T, Arima Y, Aramaki T, et al. Hand-assisted laparoscopic hepatectomy after partial splenic embolization. Surg Endosc. 2003;17:1676. doi: 10.1007/s00464-003-4210-4. [DOI] [PubMed] [Google Scholar]
- 70.Firat A, Boyvat F, Moray G, Aytekin C, Karakayali H, Haberal M. Comparison of two different percutaneous splenic artery interventions in the treatment of hypersplenism: preliminary report. Transplant Proc. 2005;37:1094–1098. doi: 10.1016/j.transproceed.2004.12.171. [DOI] [PubMed] [Google Scholar]
- 71.Sockrider CS, Boykin KN, Green J, Marsala A, Mladenka M, McMillan R, Zibari GB. Partial splenic embolization for hypersplenism before and after liver transplantation. Clin Transplant. 2002;16 Suppl 7:59–61. doi: 10.1034/j.1399-0012.16.s7.9.x. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi H, Beppu T, Masuda T, Mizumoto T, Takahashi M, Ishiko T, Takamori H, Kanemitsu K, Hirota M, Baba H. Predictive factors for platelet increase after partial splenic embolization in liver cirrhosis patients. J Gastroenterol Hepatol. 2007;22:1638–1642. doi: 10.1111/j.1440-1746.2007.05090.x. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi H, Beppu T, Okabe K, Masuda T, Okabe H, Baba H. Risk factors for complications after partial splenic embolization for liver cirrhosis. Br J Surg. 2008;95:744–750. doi: 10.1002/bjs.6081. [DOI] [PubMed] [Google Scholar]
- 74.N'Kontchou G, Seror O, Bourcier V, Mohand D, Ajavon Y, Castera L, Grando-Lemaire V, Ganne-Carrie N, Sellier N, Trinchet JC, et al. Partial splenic embolization in patients with cirrhosis: efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol. 2005;17:179–184. doi: 10.1097/00042737-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Sakai T, Shiraki K, Inoue H, Sugimoto K, Ohmori S, Murata K, Takase K, Nakano T. Complications of partial splenic embolization in cirrhotic patients. Dig Dis Sci. 2002;47:388–391. doi: 10.1023/a:1013786509418. [DOI] [PubMed] [Google Scholar]
- 76.Han MJ, Zhao HG, Ren K, Zhao DC, Xu K, Zhang XT. Partial splenic embolization for hypersplenism concomitant with or after arterial embolization of hepatocellular carcinoma in 30 patients. Cardiovasc Intervent Radiol. 1997;20:125–127. doi: 10.1007/s002709900119. [DOI] [PubMed] [Google Scholar]
- 77.Sangro B, Bilbao I, Herrero I, Corella C, Longo J, Beloqui O, Ruiz J, Zozaya JM, Quiroga J, Prieto J. Partial splenic embolization for the treatment of hypersplenism in cirrhosis. Hepatology. 1993;18:309–314. [PubMed] [Google Scholar]
- 78.Alwmark A, Bengmark S, Gullstrand P, Joelsson B, Lunderquist A, Owman T. Evaluation of splenic embolization in patients with portal hypertension and hypersplenism. Ann Surg. 1982;196:518–524. doi: 10.1097/00000658-198211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hashimoto N, Akahoshi T, Tomikawa M, Kawanaka H, Konishi K, Uehara H, Kinjo N, Korenaga D, Takenaka K, Maehara Y. Value of laparoscopic splenectomy as salvage treatment for relapsed thrombocytopenia after partial splenic arterial embolization. Dig Surg. 2010;27:515–520. doi: 10.1159/000320331. [DOI] [PubMed] [Google Scholar]
- 80.Delaitre B, Maignien B. Laparoscopic splenectomy--technical aspects. Surg Endosc. 1992;6:305–308. doi: 10.1007/BF02498866. [DOI] [PubMed] [Google Scholar]
- 81.Owera A, Hamade AM, Bani Hani OI, Ammori BJ. Laparoscopic versus open splenectomy for massive splenomegaly: a comparative study. J Laparoendosc Adv Surg Tech A. 2006;16:241–246. doi: 10.1089/lap.2006.16.241. [DOI] [PubMed] [Google Scholar]
- 82.Zhu JH, Wang YD, Ye ZY, Zhao T, Zhu YW, Xie ZJ, Liu JM. Laparoscopic versus open splenectomy for hypersplenism secondary to liver cirrhosis. Surg Laparosc Endosc Percutan Tech. 2009;19:258–262. doi: 10.1097/SLE.0b013e3181a6ec7c. [DOI] [PubMed] [Google Scholar]
- 83.Ahad S, Gonczy C, Advani V, Markwell S, Hassan I. True benefit or selection bias: an analysis of laparoscopic versus open splenectomy from the ACS-NSQIP. Surg Endosc. 2013;27:1865–1871. doi: 10.1007/s00464-012-2727-0. [DOI] [PubMed] [Google Scholar]
- 84.Ohta M, Nishizaki T, Matsumoto T, Shimabukuro R, Sasaki A, Shibata K, Matsusaka T, Kitano S. Analysis of risk factors for massive intraoperative bleeding during laparoscopic splenectomy. J Hepatobiliary Pancreat Surg. 2005;12:433–437. doi: 10.1007/s00534-005-1027-7. [DOI] [PubMed] [Google Scholar]
- 85.Yoshida D, Nagao Y, Tomikawa M, Kawanaka H, Akahoshi T, Kinjo N, Uehara H, Hashimoto N, Hashizume M, Maehara Y. Predictive factors for platelet count after laparoscopic splenectomy in cirrhotic patients. Hepatol Int. 2011:Epub ahead of print. doi: 10.1007/s12072-011-9315-6. [DOI] [PubMed] [Google Scholar]
- 86.Hashizume M, Tomikawa M, Akahoshi T, Tanoue K, Gotoh N, Konishi K, Okita K, Tsutsumi N, Shimabukuro R, Yamaguchi S, et al. Laparoscopic splenectomy for portal hypertension. Hepatogastroenterology. 2002;49:847–852. [PubMed] [Google Scholar]
- 87.Kercher KW, Carbonell AM, Heniford BT, Matthews BD, Cunningham DM, Reindollar RW. Laparoscopic splenectomy reverses thrombocytopenia in patients with hepatitis C cirrhosis and portal hypertension. J Gastrointest Surg. 2004;8:120–126. doi: 10.1016/j.gassur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe Y, Horiuchi A, Yoshida M, Yamamoto Y, Sugishita H, Kumagi T, Hiasa Y, Kawachi K. Significance of laparoscopic splenectomy in patients with hypersplenism. World J Surg. 2007;31:549–555. doi: 10.1007/s00268-006-0504-8. [DOI] [PubMed] [Google Scholar]
- 89.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 90.Shah NL, Northup PG, Caldwell SH. A clinical survey of bleeding, thrombosis, and blood product use in decompensated cirrhosis patients. Ann Hepatol. 2012;11:686–690. [PubMed] [Google Scholar]
- 91.Ferro D, Angelico F, Caldwell SH, Violi F. Bleeding and thrombosis in cirrhotic patients: what really matters? Dig Liver Dis. 2012;44:275–279. doi: 10.1016/j.dld.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 92.Northup PG, Caldwell SH. New concepts of coagulation and bleeding in liver disease. Intern Emerg Med. 2010;5:3–6. doi: 10.1007/s11739-009-0345-1. [DOI] [PubMed] [Google Scholar]
- 93.Kinjo N, Kawanaka H, Akahoshi T, Tomikawa M, Yamashita N, Konishi K, Tanoue K, Shirabe K, Hashizume M, Maehara Y. Risk factors for portal venous thrombosis after splenectomy in patients with cirrhosis and portal hypertension. Br J Surg. 2010;97:910–916. doi: 10.1002/bjs.7002. [DOI] [PubMed] [Google Scholar]
- 94.Danno K, Ikeda M, Sekimoto M, Sugimoto T, Takemasa I, Yamamoto H, Doki Y, Monden M, Mori M. Diameter of splenic vein is a risk factor for portal or splenic vein thrombosis after laparoscopic splenectomy. Surgery. 2009;145:457–464; discussion 465-466. doi: 10.1016/j.surg.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 95.Kawanaka H, Akahoshi T, Kinjo N, Konishi K, Yoshida D, Anegawa G, Yamaguchi S, Uehara H, Hashimoto N, Tsutsumi N, et al. Impact of antithrombin III concentrates on portal vein thrombosis after splenectomy in patients with liver cirrhosis and hypersplenism. Ann Surg. 2010;251:76–83. doi: 10.1097/SLA.0b013e3181bdf8ad. [DOI] [PubMed] [Google Scholar]
- 96.King H, Shumacker HB. Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952;136:239–242. doi: 10.1097/00000658-195208000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Voican CS, Naveau S, Perlemuter G. Successful antiviral therapy for hepatitis C virus-induced cirrhosis after an increase in the platelet count with romiplostim: two case reports. Eur J Gastroenterol Hepatol. 2012;24:1455–1458. doi: 10.1097/MEG.0b013e328357d5f2. [DOI] [PubMed] [Google Scholar]


