Abstract
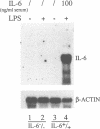
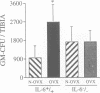
Interleukin-6 (IL-6) is a multifunctional cytokine whose circulating levels are under physiological conditions below detection, but whose production is rapidly and strongly induced by several pathological and inflammatory stimuli. IL-6 has been implicated in a number of cell functions connected to immunity and hematopoiesis. Recently, it has been proposed to act as a stimulator of osteoclast formation and activity, in particular following estrogen depletion. The purpose of this study was to gain additional insights into the role of IL-6 during development, as well as in physiological and pathological conditions. We report here that IL-6 deficient mice generated by gene targeting are viable and do not present any evident phenotypic abnormality. However, analysis of bone metabolism revealed a specific bone phenotype. IL-6 deficient female mice have a normal amount of trabecular bone, but higher rates of bone turnover than control littermates. Estrogen deficiency induced by ovariectomy causes in wild type animals a significant loss of bone mass together with an increase in bone turnover rates. Strikingly, ovariectomy does not induce any change in either bone mass or bone remodeling rates in the IL-6 deficient mice. These findings indicate that IL-6 plays an important role in the local regulation of bone turnover and, at least in mice, appears to be essential for the bone loss caused by estrogen deficiency.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bain S. D., Bailey M. C., Celino D. L., Lantry M. M., Edwards M. W. High-dose estrogen inhibits bone resorption and stimulates bone formation in the ovariectomized mouse. J Bone Miner Res. 1993 Apr;8(4):435–442. doi: 10.1002/jbmr.5650080407. [DOI] [PubMed] [Google Scholar]
- Brakenhoff J. P., Hart M., Aarden L. A. Analysis of human IL-6 mutants expressed in Escherichia coli. Biologic activities are not affected by deletion of amino acids 1-28. J Immunol. 1989 Aug 15;143(4):1175–1182. [PubMed] [Google Scholar]
- Burger R., Gramatzki M. Responsiveness of the interleukin (IL)-6-dependent cell line B9 to IL-11. J Immunol Methods. 1993 Jan 14;158(1):147–148. doi: 10.1016/0022-1759(93)90266-a. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dempster D. W., Lindsay R. Pathogenesis of osteoporosis. Lancet. 1993 Mar 27;341(8848):797–801. doi: 10.1016/0140-6736(93)90570-7. [DOI] [PubMed] [Google Scholar]
- Eriksen E. F., Colvard D. S., Berg N. J., Graham M. L., Mann K. G., Spelsberg T. C., Riggs B. L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988 Jul 1;241(4861):84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girasole G., Jilka R. L., Passeri G., Boswell S., Boder G., Williams D. C., Manolagas S. C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992 Mar;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Akira S., Taga T., Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990 Dec;11(12):443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- Horowitz M. C. Cytokines and estrogen in bone: anti-osteoporotic effects. Science. 1993 Apr 30;260(5108):626–627. doi: 10.1126/science.8480174. [DOI] [PubMed] [Google Scholar]
- Hughes F. J., Howells G. L. Interleukin-6 inhibits bone formation in vitro. Bone Miner. 1993 Apr;21(1):21–28. doi: 10.1016/s0169-6009(08)80117-1. [DOI] [PubMed] [Google Scholar]
- Ishimi Y., Miyaura C., Jin C. H., Akatsu T., Abe E., Nakamura Y., Yamaguchi A., Yoshiki S., Matsuda T., Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990 Nov 15;145(10):3297–3303. [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Komm B. S., Terpening C. M., Benz D. J., Graeme K. A., Gallegos A., Korc M., Greene G. L., O'Malley B. W., Haussler M. R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988 Jul 1;241(4861):81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- Michalevicz R., Lifshitz D., Revel M. Interferon beta 2/interleukin-6 and interleukin-3 synergize in stimulating proliferation of human early hematopoietic progenitor cells. Scanning Microsc. 1989 Dec;3(4):1143–1150. [PubMed] [Google Scholar]
- Morrone G., Ciliberto G., Oliviero S., Arcone R., Dente L., Content J., Cortese R. Recombinant interleukin 6 regulates the transcriptional activation of a set of human acute phase genes. J Biol Chem. 1988 Sep 5;263(25):12554–12558. [PubMed] [Google Scholar]
- Ohsaki Y., Takahashi S., Scarcez T., Demulder A., Nishihara T., Williams R., Roodman G. D. Evidence for an autocrine/paracrine role for interleukin-6 in bone resorption by giant cells from giant cell tumors of bone. Endocrinology. 1992 Nov;131(5):2229–2234. doi: 10.1210/endo.131.5.1425421. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Brown C., Puscheck E., Friedrich E., Slatopolsky E., Maggio D., McCracken R., Avioli L. V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987 Dec;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Paul S. R., Bennett F., Calvetti J. A., Kelleher K., Wood C. R., O'Hara R. M., Jr, Leary A. C., Sibley B., Clark S. C., Williams D. A. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7512–7516. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Raisz L. G. Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med. 1988 Mar 31;318(13):818–828. doi: 10.1056/NEJM198803313181305. [DOI] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Rodgers J. B., Monier-Faugere M. C., Malluche H. Animal models for the study of bone loss after cessation of ovarian function. Bone. 1993 May-Jun;14(3):369–377. doi: 10.1016/8756-3282(93)90166-8. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Kaspar P., Brunet L. J., Bhatt H., Gadi I., Köntgen F., Abbondanzo S. J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992 Sep 3;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Suematsu S., Matsusaka T., Matsuda T., Ohno S., Miyazaki J., Yamamura K., Hirano T., Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Cytokine receptors and signal transduction. FASEB J. 1992 Dec;6(15):3387–3396. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- Tanabe O., Akira S., Kamiya T., Wong G. G., Hirano T., Kishimoto T. Genomic structure of the murine IL-6 gene. High degree conservation of potential regulatory sequences between mouse and human. J Immunol. 1988 Dec 1;141(11):3875–3881. [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]