Abstract
Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (OGCs) is very rare, less than 1% of all pancreatic malignancies, and shows worse prognosis than that of invasive ductal adenocarcinoma of the pancreas. We present a case of en bloc resection for a huge undifferentiated carcinoma with OGCs that invaded the stomach and transverse mesocolon. A 67-year female was admitted for left upper quadrant pain and computed tomography demonstrated a mass occupying the lesser sac and abutting the stomach and pancreas. There were no distant metastases and the patient underwent subtotal pancreatectomy with splenectomy, total gastrectomy, and segmental resection of the transverse colon. Histopathological examination confirmed an 11 cm-sized undifferentiated carcinoma of the pancreas with OGCs. Immunohistochemical staining revealed reactivity with pan-cytokeratin in adenocarcinoma component, with vimentin in neoplastic multi-nucleated cells, with CD45/CD68 in OGCs, and with p53 in tumor cells, respectively. The patient had suffered from multiple bone metastases and survived 9 mo after surgery. This case supports the ductal epithelial origin of undifferentiated carcinoma with OGCs and early diagnosis could result in favorable surgical outcomes. Investigations on the surgical role and prognostic factors need to be warranted in this tumor.
Keywords: Carcinoma, Giant cell, Pancreas, Prognosis, Treatment outcome
Core tip: Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells (OGCs) is very rare and shows a poor prognosis. A 67-year female underwent subtotal pancreatectomy, total gastrectomy, and segmental resection of the transverse colon for a mass occupying the lesser sac and abutting the stomach and pancreas. Histopathological examination confirmed an 11cm-sized undifferentiated carcinoma of the pancreas with OGCs. The patient had suffered from multiple bone metastases and survived 9 mo after surgery. This case supports the ductal epithelial origin of undifferentiated carcinoma with OGCs and early diagnosis could result in favorable surgical outcomes.
INTRODUCTION
Undifferentiated carcinoma of the pancreas is a rare and aggressive tumor and a variety of other terms, such as osteoclast-like, osteoclastic or pleomorphic giant cell tumor, anaplastic carcinoma, pleomorphic (giant cell) carcinoma, sarcomatoid carcinoma, and spindle cell carcinoma, have been used to describe this type of tumor. These various terms are put together into a single category designated as undifferentiated carcinoma of the pancreas in the current WHO classification of the tumours despite their histological differences[1]. In addition, as undifferentiated carcinoma of the pancreas may accompany osteoclast-like giant cells (OGCs) that are suggested to be a reactive non-neoplastic histiocytic origin, this WHO Classification separates undifferentiated carcinoma with OGCs from plain undifferentiated carcinoma, implying there are a few clinical and histopathological distinctions between them.
Undifferentiated carcinoma of the pancreas has been reported a rare tumor[2,3] and OGCs-accompanying undifferentiated carcinoma of the pancreas an extremely rare tumor, less than 1% of all pancreatic malignancies[4]. This very rare undifferentiated carcinoma of the pancreas with OGCs shows worse prognosis than that of invasive ductal adenocarcinoma of the pancreas[5-8], because it is frequently found to be unresectable at diagnosis due to advanced stages[9,10] and tends to early recur even after complete surgical resection[4,11,12]. Correspondingly, median or average survival of patients with undifferentiated carcinoma of the pancreas with OGCs has been reported less than 1 year with few exceptions[4,7,13-16].
There have been relatively few reports, primarily based on case reports, regarding the clinical and histopathological features of this fatal tumor in literatures. In this report, we present a case of a huge undifferentiated carcinoma of the pancreas with OGCs which directly invaded the stomach and transverse mesocolon but was successfully en bloc resected, and review the literature with emphasis on the histogenesis and surgical outcomes.
CASE REPORT
A 67-year female was admitted to the department of gastroenterology for left upper quadrant pain of two months. The pain was aggravated by diet but no nausea or vomiting was reported. The patient presented weigh loss of about 3 kg over two months. She had no noticeable past medical history and underwent total vaginal hysterectomy and appendectomy 10 years ago. No allergies or significant social or family history was noted. She had taken medicine for esophagitis for two months. On physical examination, chronically ill-looking appearance was observed and vaguely palpable abdominal mass with mild deep tenderness was detected.
Abnormal laboratory results were decreased hemoglobin at 11.3 g/dL (reference range, 12.0 to 16.0 g/dL), elevated amylase at 157 U/L (reference range, 28 to 100 U/L), and elevated CA 19-9 at 73.2 U/mL (reference range, 0 to 37 U/mL). Lipase, bilirubin and transaminases were normal.
Gastric endoscopy demonstrated a huge extrinsically compressing mass mainly against the lesser curvature side of the antrum and body. CT scans showed an about 10cm-sized huge mass occupying the lesser sac, involving parenchyma of the pancreatic body and neck portion, and abutting the gastric posterior wall and duodenal second portion (Figure 1A). This mass looked like arising from the pancreas and compressing the main hepatic artery and portal vein. Endoscopic ultrasonography (EUS) revealed a heterogeneous and poorly demarcated mass with small central cystic lesions between the pancreas body and stomach; the mass seemed to be originated from the pancreas body and abutted on the gastric wall; portal vein and common bile duct were intact and pancreatic duct dilatation was not definite. EUS-guided fine needle aspiration demonstrated malignant tumor, suggestive of undifferentiated carcinoma of the pancreas. As no definite distant metastases were found on PET-CT, the patient was transferred for surgery.
Figure 1.

Huge undifferentiated carcinoma with osteoclast-like giant cells. A: Computed tomography scans revealed the tumor occupying the lesser sac; B: Tumor arose from the pancreas body and invaded directly the stomach wall and transverse mesocolon.
On intraabdominal exploration, no metastatic peritoneal nodules were detected. The mass arose from the pancreas and directly invaded the lesser curvature of stomach and the mid portion of transverse mesocolon. Fine dissection was initiated between the mass and major hepatic inflow vessels in order to investigate curative resectability. After confirming complete dissection between them, further dissection proceeded and finally en bloc resection was performed through subtotal pancreatectomy with splenectomy, total gastrectomy, and segmental resection of the transverse colon (Figure 1B).
Histopathological examination confirmed an 11cm-sized undifferentiated carcinoma of the pancreas with OGCs, which extended beyond the pancreas to the stomach wall and transverse mesocolon. The tumor was predominantly composed of spindle-shaped, highly pleomorphic, neoplastic, mono- or multinucleated cells, as well as non-neoplastic multinucleated OGCs, sporadically intermingled with neoplastic cells (Figure 2A). Characteristically, ductal adenocarcinoma component was found in some areas, providing evidence of ductal cell origin of this undifferentiated carcinoma with OGCs (Figure 2B). Additional reports of extensive hemorrhage and necrosis of the tumor (up to 80%), negative resection margins, no lymphovascular invasion, and nodal metastases (2/30) were given. Immunohistochemical staining revealed reactivity with pan-cytokeratin in adenocarcinoma component, with vimentin in undifferentiated carcinoma component (neoplastic multinucleated cells), with CD45/CD68 in OGCs, and with p53 in tumor cells, respectively. Non-neoplastic OGCs did not show reactivity with vimentin or p53 (Figure 3).
Figure 2.

Histopathological characteristics. A: Tumor was composed of highly pleomorphic neoplastic cells and non-neoplastic osteoclast-like giant cells. Hematoxylin and eosin (H and E), × 100; B: Ductal adenocarcinoma component was also found in some areas. H and E, × 40.
Figure 3.
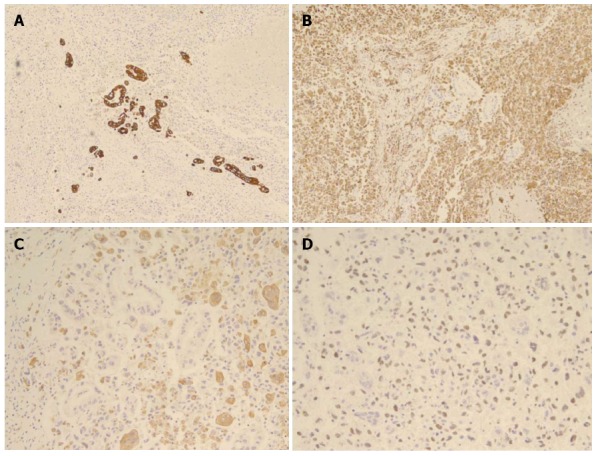
Immunohistochemical staining. A: Reactivity with cytokeratin in ductal adenocarcinoma component; B: Reactivity with vimentin in pleomorphic neoplastic cells; C: Reactivity with CD68 in osteoclast-like giant cells; D: Reactivity with p53 in tumor cells.
The patient commenced soft diet on postoperative day (POD) 6th and was discharged on POD 19th without significant complications. She had been followed free of recurrence for a half year. Seven months after surgery the patient complained lower back pain and lumbar MRI revealed multiple mass lesions of myeloinfiltrative pattern in the thoracolumbar spines and pelvic bones. She had suffered from multiple bone metastases and showed 9-mo survival after surgery.
DISCUSSION
Undifferentiated carcinoma of the pancreas with OGCs is characterized by a dual component of undifferentiated carcinoma cells (neoplastic mono- or multinucleated cells) and multinucleated OGCs, mimicking giant cell tumor of bone. Although there have been few reports suggesting origins of undifferentiated carcinoma of the pancreas from acinar cells, mesenchymal cells, undifferentiated precursor or stem cells, mucinous cystic neoplasms, and ductal cells[9], a duct epithelial origin is now established and this tumor has been recognized as a variant of ductal adenocarcinoma of the pancreas in the most recent WHO Classification[1]. Verbeke et al[17] confirmed the duct epithelial origin of undifferentiated carcinoma of the pancreas in their letter to the editor by demonstration of (1) foci of conventional ductal adenocarcinoma component; (2) occasional association with mucinous cystic neoplasia; (3) cytokeratin expression in at least some of the pleomorphic tumour cells; and (4) K-ras mutations in the pleomorphic tumour cells, with identical mutations identified in associated foci of conventional ductal adenocarcinoma or intraductal neoplastic lesions. In this case, histopathological analysis of the resected specimen revealed the coexistence of adenocarcinoma component and undifferentiated carcinoma component with reactivity with vimentin, suggesting that the tumor originated from pancreatic ductal cells with mesenchymal differentiation. These data provide evidence to support the epithelial origin of these neoplastic components.
Non-neoplastic OGCs in undifferentiated carcinoma of the pancreas is the histopathological hallmark. OGCs present in this tumor are consistently found to be of a reactive mesenchymal nature, characterized by the lack of morphological atypia, proliferative activity, and K-ras and p53 abnormalities[17]. In contrast to the established origin of undifferentiated carcinoma of the pancreas, there have been controversies regarding the origin of OGCs. Proposed origins of OGCs have included epithelial, histiocytic, or mesenchymal metaplasia[18]. However, their nuclear features, lack of reactivity with epithelial markers, and CD68 and lysozyme reactivity are indicative of a histiocytic origin[4]. These characteristic giant cells were suggested to result from fusion of mononuclear histiocytes/marcophages attracted by growth or chemotactic factors produced by the neoplastic cells[19]. The presence of OGCs in this tumor may reflect clinical significance, for example, a better prognosis or response to adjuvant therapy compared to undifferentiated carcinoma without OGCs. In this context, the most recent WHO Classification might have separated undifferentiated carcinoma with OGCs from undifferentiated carcinoma without OGCs, though comments on clinical differences between both tumors lack[20]. Some authors reported that undifferentiated carcinoma with OGCs might have a more favorable prognosis than pancreatic ductal adenocarcinoma[21] or undifferentiated carcinoma without OGCs[22,23].
A few cases diagnosed with undifferentiated carcinoma of the pancreas with OGCs have been reported and are summarized in Table 1. Surgical outcomes in patients with this unusual tumor have been disappointing. They are even worse than those of fatal invasive ductal adenocarcinoma of the pancreas[5-7]. Although few long-term survivors have been reported, even more than 10-year survivors[4,7,29], most of the patients in case reports showed early recurrence and rapid progression even after complete surgical resection and died of tumor within 1 year[4,11,12]. The patient in this case also suffered from multiple bone metastases without definite evidence of local recurrence and survived only 9 mo after curative resection. Concerning the surgical role, there have been no reports in undifferentiated carcinoma of the pancreas with OGCs. Instead, some authors recommended appropriate surgery for pleomorphic carcinoma of the pancreas with favorable characteristics, absence of invasion of adjacent organs and distant metastases[30], but others did not due to poor surgical outcomes[31]. Interestingly, in situ as well as early-stage undifferentiated carcinomas of the pancreas with OGCs have been reported[9,16,23]. The tumor size ranged from 2.0 to 5.3 cm and postoperative outcomes, described in only one case[16], were satisfactory with no evidence of tumor recurrence or metastasis. Accordingly, early diagnosis and subsequent complete resection, although it is exceedingly uncommon, could be only chance to cure this fatal tumor. Tumor size tends to be small in an early stage and smaller-sized undifferentiated carcinomas with OGCs have showed favorable surgical outcomes in some cases[14,16]. However, overall tumor size has not been reported to be a reliable prognostic indicator[7], because patients with even a large undifferentiated carcinoma could show long-term survival[4]. Investigations on the surgical role and prognostic factors need to be warranted based on multi-center cooperation and large cohort of patients with undifferentiated carcinoma with OGCs.
Table 1.
Surgical cases diagnosed with undifferentiated carcinoma of the pancreas with osteoclast-like giant cells
| Ref. | Year | Case | Age (yr) | Sex | Location | Size (cm) | Operations | Outcomes |
| Molberg et al[4] | 1998 | 7 | 43-88 | 5F, 2M | Head, tail | 5-14 | PD, DP | DOC 1 mo-NED 14 yr |
| Carvounis et al[24] | 2003 | 1 | 70 | F | Head and neck | 7 | PD | AWD 9 mo |
| Bedioui et al[14] | 2004 | 1 | 72 | F | Head | 6 | PD | NED 18 mo |
| Charfi et al[25] | 2006 | 1 | 65 | F | Tail | 10 | DP | DOD 12 mo |
| Tezuka et al[16] | 2006 | 1 | 68 | F | Head | 0.7 | PD | NED 22 mo |
| Jang et al[10] | 2006 | 1 | 75 | M | Body and tail | 18 | Palliative DP | NA |
| Hirano et al[12] | 2008 | 1 | 26 | F | Body and tail | 11 | DP | NED 8 mo |
| Manduch et al[26] | 2009 | 1 | 66 | M | Head | 9.5 | PD | DOD 12 mo |
| Daum et al[27] | 2010 | 1 | 71 | F | Body and tail | 17 | DP | NA |
| Mannan et al[22] | 2010 | 1 | 40 | F | Head and neck | 4 | PD | NA |
| Maksymov et al[23] | 2011 | 1 | 68 | F | Uncinate process | 2 | PD | NED 14 mo |
| Wada et al[11] | 2011 | 1 | 59 | M | Tail | 14 | DP and total gastrectomy | DOD 4 mo |
| Hur et al[28] | 2011 | 1 | 77 | F | Tail | 10 | DP and left hemicolectomy | DOC 3 mo |
| Yoshioka et al[13] | 2012 | 1 | 74 | F | Body | NA | DP | DOD 19 mo |
M: Male; F: Female; PD: Pancreaticoduodenectomy; DP: Distal pancreatectomy; DOC: Died of other causes; NED: No evidence of disease; AWD: Alive with disease; DOD: Died of disease; NA: Not available.
In summary, undifferentiated carcinoma of the pancreas with OGCs originates from duct epithelial cells and is now recognized as a variant of ductal adenocarcinoma of the pancreas. The present case supports evidence of tumor origin from pancreatic ductal cells. Non-neoplastic OGCs in undifferentiated carcinoma derive from histiocytic lineage and may reflect better clinical courses compared to tumor without OGCs. Surgical outcomes in patients with undifferentiated carcinoma with OGCs have been disappointing. Early diagnosis and subsequent complete resection could be only chance to cure this fatal tumor but tumor size may not prognostic indicator.
ACKNOWLEDGMENTS
The author thanks Professor Jai Hyang Go (Department of Pathology, Dankook University College of Medicine) for her pathologic diagnosis and helpful advice.
COMMENTS
Case characteristics
A 67-year female complained left upper quadrant pain, lasting 2 mo and aggravated by diet.
Clinical diagnosis
The patient presented 3 kg-weight loss over two months and vaguely palpable abdominal mass with mild deep tenderness was detected.
Differential diagnosis
Malignant tumor was suspected and abdominal computed tomography (CT) scan was firstly performed.
Laboratory diagnosis
CBC, liver function profile, pancreatic enzymes, and tumor markers were tested and resultantly amylase and CA 19-9 were slightly (about two fold) elevated.
Imaging diagnosis
CT scan showed an about 10 cm-sized huge mass arising from the pancreas, compressing the adjacent major vessels, and abutting the gastric posterior wall and duodenal second portion.
Pathologic diagnosis
Endoscopic ultrasonography-guided fine needle aspiration biopsy demonstrated malignant tumor, suggestive of undifferentiated carcinoma of the pancreas.
Treatment
The patient underwent en bloc resection through subtotal pancreatectomy with splenectomy, total gastrectomy, and segmental resection of the transverse colon, but later she refused adjuvant therapy.
Related reports
Other contents related to this tumor include ductal epithelial origin of undifferentiated carcinoma component, histiocytic origin of osteoclast-like giant cells (OGCs), more favorable prognosis than that of invasive ductal adenocarcinoma or undifferentiated carcinoma without OGCs, and excellent surgical outcomes in cases of diagnosis at early stages.
Term explanation
OGCs are non-neoplastic, reactive, multinucleated cells and similar to those seen in giant cell tumor of bone.
Experiences and lessons
Though an aggressive surgery for selected cases of undifferentiated carcinoma of the pancreas with OCGs may prolong survival duration, surgical role in this tumor is to be established.
Peer review
This article is an interesting case report for a very rare tumor. To complement weakness of case report, the author added a table summarizing all cases diagnosed with this tumor.
Footnotes
P- Reviewers: Aydin U, Kalaitzakis E S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
References
- 1.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2010. [Google Scholar]
- 2.Chen J, Baithun SI. Morphological study of 391 cases of exocrine pancreatic tumours with special reference to the classification of exocrine pancreatic carcinoma. J Pathol. 1985;146:17–29. doi: 10.1002/path.1711460103. [DOI] [PubMed] [Google Scholar]
- 3.Cubilla AL, Fitzgerald PJ. Morphological patterns of primary nonendocrine human pancreas carcinoma. Cancer Res. 1975;35:2234–2248. [PubMed] [Google Scholar]
- 4.Molberg KH, Heffess C, Delgado R, Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279–1287. doi: 10.1002/(sici)1097-0142(19980401)82:7<1279::aid-cncr10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Zou XP, Yu ZL, Li ZS, Zhou GZ. Clinicopathological features of giant cell carcinoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2004;3:300–302. [PubMed] [Google Scholar]
- 6.Tschang TP, Garza-Garza R, Kissane JM. Pleomorphic carcinoma of the pancreas: an analysis of 15 cases. Cancer. 1977;39:2114–2126. doi: 10.1002/1097-0142(197705)39:5<2114::aid-cncr2820390528>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Paal E, Thompson LD, Frommelt RA, Przygodzki RM, Heffess CS. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129–140. doi: 10.1053/adpa.2001.25404. [DOI] [PubMed] [Google Scholar]
- 8.Yonemasu H, Takashima M, Nishiyama KI, Ueki T, Yao T, Tanaka M, Tsuneyoshi M. Phenotypical characteristics of undifferentiated carcinoma of the pancreas: a comparison with pancreatic ductal adenocarcinoma and relevance of E-cadherin, alpha catenin and beta catenin expression. Oncol Rep. 2001;8:745–752. doi: 10.3892/or.8.4.745. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann F, Esposito I, Michalski CW, Herpel E, Friess H, Schirmacher P. Early undifferentiated pancreatic carcinoma with osteoclastlike giant cells: direct evidence for ductal evolution. Am J Surg Pathol. 2007;31:1919–1925. doi: 10.1097/PAS.0b013e318067bca8. [DOI] [PubMed] [Google Scholar]
- 10.Jang HW, Park WK, Chang JC, Kim JW, Bae YK, Choi JH, Yun SS, Lee DS. [Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas] Korean J Gastroenterol. 2006;48:355–359. [PubMed] [Google Scholar]
- 11.Wada T, Itano O, Oshima G, Chiba N, Ishikawa H, Koyama Y, Du W, Kitagawa Y. A male case of an undifferentiated carcinoma with osteoclast-like giant cells originating in an indeterminate mucin-producing cystic neoplasm of the pancreas. A case report and review of the literature. World J Surg Oncol. 2011;9:100. doi: 10.1186/1477-7819-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano H, Morita K, Tachibana S, Okimura A, Fujisawa T, Ouchi S, Nakasho K, Ueyama S, Nishigami T, Terada N. Undifferentiated carcinoma with osteoclast-like giant cells arising in a mucinous cystic neoplasm of the pancreas. Pathol Int. 2008;58:383–389. doi: 10.1111/j.1440-1827.2008.02240.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshioka M, Uchinami H, Watanabe G, Takahashi T, Nakagawa Y, Andoh H, Yoshioka T, Nanjo H, Yamamoto Y. Effective use of gemcitabine in the treatment of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas with portal vein tumor thrombus. Intern Med. 2012;51:2145–2150. doi: 10.2169/internalmedicine.51.7670. [DOI] [PubMed] [Google Scholar]
- 14.Bedioui H, Ksantini R, Sassi K, Nouira K, Chebbi F, Fteriche F, Jouini M, Haouet S, Ammous A, Kacem M, et al. [Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. A case report] Ann Chir. 2004;129:526–529. doi: 10.1016/j.anchir.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Valmary S, Seulin P, Lamant-Rochaix L, Pradère B, Selves J. [Non-differentiated carcinoma with osteoclast-like giant cells of the pancreas] Ann Pathol. 2003;23:240–243. [PubMed] [Google Scholar]
- 16.Tezuka K, Yamakawa M, Jingu A, Ikeda Y, Kimura W. An unusual case of undifferentiated carcinoma in situ with osteoclast-like giant cells of the pancreas. Pancreas. 2006;33:304–310. doi: 10.1097/01.mpa.0000235303.11734.2a. [DOI] [PubMed] [Google Scholar]
- 17.Verbeke CS, Menon KV. Osteoclast-like giant cell tumour of the pancreas: an undifferentiated carcinoma of duct epithelial origin. Pancreatology. 2006;6:254; author reply 254. doi: 10.1159/000091963. [DOI] [PubMed] [Google Scholar]
- 18.Gao L, Li ZS, Jin ZD, Man XH, Zhang MH, Zhu MH. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas diagnosed by endoscopic ultrasonography-guided fine-needle aspiration. Chin Med J (Engl) 2009;122:1598–1600. [PubMed] [Google Scholar]
- 19.Newbould MJ, Benbow EW, Sene A, Young M, Taylor TV. Adenocarcinoma of the pancreas with osteoclast-like giant cells: a case report with immunocytochemistry. Pancreas. 1992;7:611–615. doi: 10.1097/00006676-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Fukushima N, Hruban RH, Kato Y, klimstra DS, Kloppel G, Shimizu M, Terris B. Ductal adenocarcinoma variants and mixed neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2010. pp. 294–295. [Google Scholar]
- 21.Deckard-Janatpour K, Kragel S, Teplitz RL, Min BH, Gumerlock PH, Frey CF, Ruebner BH. Tumors of the pancreas with osteoclast-like and pleomorphic giant cells: an immunohistochemical and ploidy study. Arch Pathol Lab Med. 1998;122:266–272. [PubMed] [Google Scholar]
- 22.Mannan R, Khanna M, Bhasin TS, Misra V, Singh PA. Undifferentiated carcinoma with osteoclast-like giant cell tumor of the pancreas: a discussion of rare entity in comparison with pleomorphic giant cell tumor of the pancreas. Indian J Pathol Microbiol. 2010;53:867–868. doi: 10.4103/0377-4929.72016. [DOI] [PubMed] [Google Scholar]
- 23.Maksymov V, Khalifa MA, Bussey A, Carter B, Hogan M. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement. A case report and literature review. JOP. 2011;12:170–176. [PubMed] [Google Scholar]
- 24.Carvounis EE, Smyrniotis V, Chatziioannou A, Paphitis A. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. Int J Gastrointest Cancer. 2003;33:103–106. doi: 10.1385/IJGC:33:2-3:103. [DOI] [PubMed] [Google Scholar]
- 25.Charfi S, Khabir A, Frikha F, Boudawara TS. [Non-differentiated carcinoma with osteoclast-like giant cells of the pancreas: a case report] Cancer Radiother. 2006;10:152–154. doi: 10.1016/j.canrad.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Manduch M, Dexter DF, Jalink DW, Vanner SJ, Hurlbut DJ. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: report of a case with osteochondroid differentiation. Pathol Res Pract. 2009;205:353–359. doi: 10.1016/j.prp.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Daum O, Ferdova E, Kural T, Grossmann P, Nemcova J, Mukensnabl P, Michal M. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells masquerading as (extra)gastrointestinal stromal tumor: potential diagnostic pitfall. Pathol Int. 2010;60:59–61. doi: 10.1111/j.1440-1827.2009.02470.x. [DOI] [PubMed] [Google Scholar]
- 28.Hur YH, Kim HH, Seoung JS, Seo KW, Kim JW, Jeong YY, Lee JH, Koh YS, Kim JC, Kim HJ, et al. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. J Korean Surg Soc. 2011;81:146–150. doi: 10.4174/jkss.2011.81.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suda K, Takase M, Oyama T, Mitsui T, Horike S. An osteoclast-like giant cell tumor pattern in a mucinous cystadenocarcinoma of the pancreas with lymph node metastasis in a patient surviving over 10 years. Virchows Arch. 2001;438:519–520. doi: 10.1007/s004280100404. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Hirohashi K, Tanaka H, Uenishi T, Shuto T, Kubo S, Kinoshita H. Resectable Pleomorphic Giant Cell Carcinoma of the Pancreas. Int J Gastrointest Cancer. 2001;29:63–68. [PubMed] [Google Scholar]
- 31.Yamaguchi K, Nakamura K, Shimizu S, Yokohata K, Morisaki T, Chijiiwa K, Tanaka M. Pleomorphic carcinoma of the pancreas: reappraisal of surgical resection. Am J Gastroenterol. 1998;93:1151–1155. doi: 10.1111/j.1572-0241.1998.351_e.x. [DOI] [PubMed] [Google Scholar]


