Abstract
Anemia and the need for transfusion of packed red blood cells (PRBCs) are common in preterm infants. PRBC transfusion increases the oxygen carrying capacity of hemoglobin and may result in higher rates of organ dysfunction. To determine whether PRBC transfusion in preterm infants is associated with an increased incidence of bronchopulmonary dysplasia (BPD), this retrospective study was performed on neonates with birth weights ≤ 1,500 g or gestational age ≤ 32 weeks admitted from August, 2008 to November, 2013. Infants who received PRBC transfusion before the diagnosis of BPD and those who did not receive PRBC transfusion or received PRBC transfusion after diagnosis of BPD were compared for incidence of BPD and other morbidities. Of 231 preterm infants, 137 received PRBC transfusion before BPD was diagnosed (group 1) and 94 did not (group 2). The incidence of BPD was significantly higher in group 1 than in group 2 (37.2% vs. 2.1%, P < 0.00001). After adjusting for potential risk factors, the adjusted odds ratio for BPD was 9.80 (95% confidence interval, 1.70–56.36; P = 0.01). This study demonstrated an association between PRBC transfusion and BPD in preterm infants. A cautious approach to PRBC transfusion in these infants is warranted.
Anemia requiring transfusion of packed red blood cells (PRBCs) is relatively common in preterm infants. PRBC transfusion is typically applied based on clinical judgment and evidence-based guidelines that can vary among hospitals, because reliable markers of reduced tissue oxygen delivery have yet to be identified. Both restrictive and liberal guidelines are practiced1,2,3. Most symptoms of reduced tissue oxygen delivery in preterm infants, such as tachycardia, slow weight gain, apnea, and lactic acidosis, are the direct result of chronic anemia in these patients. PRBC transfusion can improve these symptoms and increase the hematocrit (Hct) value4,5,6,7. However, transfusion also may introduce some inflammatory mediators, such as interleukin (IL)-1β, IL-8, tumor necrosis factor-α, and monocyte chemoattractant protein8,9. Furthermore, the ability of RBCs to deliver oxygen may be diminished by changes in the cell membranes that alter RBC deformability and decrease their ability to scavenge nitric oxide as well as by biochemical changes such as a reduction in 2,3-diphosphoglycerate levels10,11. Together, these factors can lead to higher rates of organ dysfunction and morbidity such as necrotizing enterocolitis (NEC) and bronchopulmonary dysplasia (BPD). Therefore, it is important for clinicians treating preterm infants to understand the potential risks of PRBC transfusion, particularly BPD.
In this study, we hypothesized that very low birth weight (VLBW) or gestational age (GA) ≤ 32 weeks of premature infants who receive a PRBC transfusion have an increased risk of developing BPD. We also assessed whether the age at first PRBC transfusion and number of PRBC transfusions influence the incidence of BPD.
Patients and Methods
Study setting
The study population comprised live-born neonates with a BW of 500–1,500 g or GA of 22–32 weeks who were admitted to the neonatal intensive care unit of Hangzhou First People's Hospital from August, 2008 to November, 2013. We excluded infants with major congenital anomalies, those who died before 28 days of life, or those for whom pulmonary outcome data were missing. This study was approved by the ethics committees of Hangzhou First People's Hospital, and the database of all patients' data was permitted to use by the ethics committees of Hangzhou First People's Hospital. Written informed consent was obtained from all their parents when patients were be hospitalized.
PRBC transfusions were based upon standard transfusion guidelines described by Roseff et al.12: (1) Hematocrit (Hct) < 35% in an infant with a fraction of inspired oxygen (FiO2) > 30% who was on continuous positive airway pressure/intermittent mandatory ventilation with mean airway pressure ≥ 6 cm H2O; (2) Hct < 30% in an infant with an FiO2 < 30% who was on continuous positive airway pressure and/or intermittent mandatory ventilation with mechanical ventilation with mean airway pressure < 6 cm H2O; had significant apnea or bradycardia (>6 episodes in 12 hr or 2 episodes in 24 hr requiring bag and mask ventilation while receiving therapeutic doses of methylxanthines); had significant tachycardia or tachypnea (heart rate > 180 beats/min for 24 hr; respiratory rate > 80 breaths/min for 24 hr); and exhibited slow weight gain (<10 g/day over 4 days while receiving ≥100 kcal/kg/day). PRBC transfusion included 20 ml/kg PRBCs over 4 to 6 hours. Furosemide, 0.5 mg/kg, was given after the transfusion. Patients' vital signs were recorded every 15 min by the nursing staff. No corticosteroids or vitamin A were used to prevent BPD.
Study design
This study was an observational cohort study to assess the impact of PRBC transfusion on BPD as well as other neonatal morbidities in premature infants. The patients were divided into two groups: group 1 who received PRBC transfusions before the diagnosis of BPD or undiagnosed BPD and group 2 who did not received PRBC transfusion or were given PRBC transfusion after diagnosis of BPD. The number of transfusions in group 1 stats up to 36 weeks' PMA.
Data collection
Data collected from the neonatal database included BW, GA, gender, type of delivery and Apgar scores at 1, 5, and 10 min. Maternal factors such as clinical chorioamnionitis, pregnancy-induced hypertension (PIH), diabetes, and prenatal steroid administration were collected. Neonatal factors such as sepsis (documented as clinical sepsis or positive blood culture), pneumonia, patent ductus arteriosus (PDA), days of mechanical ventilation, days of supplemental oxygen, need for supplemental oxygen or mechanical ventilation at 28 days and 36 weeks' post-menstrual age (PMA), length of hospital stay as well as the final outcome (discharge home or death) were collected. BPD was defined as oxygen dependency for at least 28 days and further classified into the following three subgroups at 36 weeks' PMA: mild BPD (no oxygen requirement), moderate BPD (FiO2 < 30%), and severe BPD (FiO2 ≥ 30% and/or positive pressure assistance)13. The target oxygen saturation was set as 88%–93% for this patient population. We recorded the age at first PRBC transfusion and the number of PRBC transfusions. To obtain data missing from the database, we reviewed patients' medical records.
Statistical analyses
The incidence of BPD was compared between groups 1 and 2. Univariate analysis was performed to assess the incidence of BPD according to each risk factor in both groups 1 and 2. Either χ2 test or the two-sided Fisher's exact test was used to compare proportions, and the two-sample Student t test or the Wilcoxon rank-sum test was used to compare means. Risk factors that were significant at P < 0.2 from the univariate analysis were subjected to multivariate logistic regression analysis to derive the adjusted odds ratio (OR) and 95% confidence interval (CI) for the BPD rate for groups 1 and 2. For secondary outcomes measured on a continuous scale, we used analysis of covariance. Statistical significance was set at P < 0.05. All statistical analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC, USA).
Results
During the study period, 281 infants were treated with a BW ≤ 1,500 g or GA ≤ 32 weeks. We excluded 36 infants due to either death or refusal of treatment prior to 36 weeks' PMA and 14 patients for incomplete data. Of the remaining 231 patients, 137 received PRBC transfusion (group 1) and 94 did not (group 2). All patients with BPD received PRBC transfusion before diagnosis of BPD in group 1 and two patients with BPD didn't receive PRBC transfusion in group 2. The clinical characteristics are presented in Table 1. Gender, cord blood gas parameters, and Apgar scores were similar between the two groups. There were no differences in prenatal steroid use, incidence of maternal PIH, diabetes, infection, and premature rupture of membranes between the two groups. There were no differences in the incidence of neonatal pneumonia, intra-uterine growth restriction (IUGR), PDA, sepsis, and ureaplasma urealyticum (UU) positivity in urine between the two groups. The patients in group 1 had a lower GA [30 weeks (range, 29–31 weeks) vs. 31 weeks (range, 31–32 weeks), respectively; P < 0.0001] and BW (1316.9 ± 184.6 g vs. 1539.6 ± 172.6 g, respectively; P < 0.00001) than those in group 2. More surfactant was used in group 1 than in group 2 (78.1% vs 50.0%, P < 0.0001). Aminophylline use was more often in group 1 than in group 2 (70.8% vs 31.9%, respectively; P < 0.0001). Mechanical ventilation (MV) for more than 1 week was used significantly more often in group 1 than in group 2 (17.5% vs 5.3%, respectively; P = 0.009). The rate of NEC was significantly greater in group 1 than in group 2 (24.8% vs. 6.4%, respectively; P = 0.0007).
Table 1. Comparison of demographic and clinical characteristics between patients with and without PRBC transfusions.
| PRBC transfusion (group 1) | No PRBC transfusion (group 2) | P value | |
|---|---|---|---|
| Number of patients | 137 | 94 | |
| Birth weight, g | 1316.9 ± 184.6 | 1539.6 ± 172.6 | <0.00001 |
| Gestational age, weeks | 30(29–31) | 31(31–32) | <0.0001 |
| Intra-uterine growth restriction | 37 (27.0%) | 30 (31.9%) | 0.42 |
| Male | 80 (58.4%) | 49 (52.1%) | 0.35 |
| Apgar score at 1 min | 7(5–8) | 8(6–8) | 0.24 |
| Apgar score at 5 min | 8(6–9) | 8(7–8) | 0.87 |
| Apgar score at 10 min | 8(7–9) | 8(8–8) | 0.84 |
| Vaginal delivery | 40 (29.2%) | 33 (35.1%) | 0.34 |
| Prenatal steroids | 112 (81.8%) | 81 (86.2%) | 0.37 |
| Maternal pregnancy-induced hypertension | 35 (25.5%) | 31 (33.0%) | 0.22 |
| Maternal diabetes | 5 (3.6%) | 2 (2.1%) | 0.51 |
| Chorioamnionitis | 18 (13.1%) | 9 (9.6%) | 0.41 |
| Premature rupture of membranes | 40 (31.9%) | 32 (34.0%) | 0.44 |
| Cord blood pH | 7.29 ± 0.06 | 7.29 ± 0.07 | 1.0 |
| Ureaplasma urealyticum positive in urine | 14 (10.2%) | 12 (12.8%) | 0.55 |
| Surfactant | 107 (78.1%) | 47 (50.0%) | <0.0001 |
| Aminophylline | 100 (73.0%) | 30 (31.9%) | <0.0001 |
| Mechanical ventilation ≥ 1 week | 24 (17.5%) | 5 (5.3%) | 0.009 |
| Nasal continuous positive airway pressure ≥ 1 week | 12 (8.8%) | 7 (7.4%) | 0.72 |
| Pneumonia | 17 (12.4%) | 8 (8.5%) | 0.35 |
| Sepsis | 48 (35.0%) | 24 (25.5%) | 0.13 |
| Patent ductus arteriosus | 18 (13.1%) | 8 (8.5%) | 0.28 |
| Ibuprofen for patent ductus arteriosus | 7 (5.1%) | 0 (0.0%) | 0.10 |
| Patent ductus arteriosus ligation | 1 (0.7%) | 0 (0.0%) | 0.66 |
| Necrotizing enterocolitis | 34 (24.8%) | 6 (6.4%) | 0.0007 |
| Bronchopulmonary dysplasia | 51 (37.2%) | 2 (2.1%) | <0.00001 |
| Mild | 36 (26.3%) | 2 (2.1%) | 0.0002 |
| Moderate/severe | 15 (10.9%) | 0 (0.0%) | 0.03 |
| Length of hospital stay, weeks | 43.3 ± 8.2 | 34.7 ± 4.0 | <0.00001 |
| Patients discharged alive | 133 (97.1%) | 94 (100.0%) | 0.22 |
Primary outcomes
The factors associated with the incidence of BPD in preterm infants in univariate analyses included GA, BW, PRBC transfusion, surfactant use, aminophylline use, NEC, and MV ≥ 1 week. Sepsis was also a potential confounding factor. After adjustments for significant differences in these risk factors, using multivariate logistic regression, PRBC transfusion was found to have a statistically significant association with an increased BPD rate. The results in Table 1 show that the BPD rate was higher in group 1 than in group 2 (37.2% vs. 2.1%, respectively; OR 27.28; 95% CI 6.44–115.49; P < 0.00001), and the incidence of mild BPD (oxygen requirement resolved by 36 weeks) and moderate/severe BPD (oxygen requirement not resolved by 36 weeks) were also higher in group 1 than in group 2 (mild: 26.3% vs. 2.1%, P = 0.0002; moderate/severe: 10.9% vs. 0.0%, P = 0.03). After adjusting for differences in the significant risk factors, GA, BW, surfactant use, aminophylline use, sepsis, NEC, and MV ≥ 1 week, the adjusted OR for BPD was 9.80 (95% CI 1.70–56.36; P = 0.01; Table 2).
Table 2. Summary table of logistic regression with BPD as the dependent variable and GA, BW, transfusion PRBC, sepsis, aminophylline use, NEC, no surfactant use, and MV ≥ 1 week as independent variables.
| Adjusted OR | 95% CI | P value | |
|---|---|---|---|
| Gestational age, weeks | 0.95 | (0.67, 1.36) | 0.80 |
| Birth weight, g | 0.99 | (0.99, 1.00) | 0.05 |
| No surfactant | 0.04 | (0.00, 0.45) | 0.007 |
| Mechanical ventilation ≥ 1 week | 4.85 | (1.46, 16.06) | 0.009 |
| Sepsis | 2.51 | (0.99, 6.32) | 0.05 |
| Aminophylline | 3.71 | (1.05, 13.05) | 0.04 |
| Necrotizing enterocolitis | 0.70 | (0.24, 2.04) | 0.51 |
| Transfusion | 9.80 | (1.70, 56.36) | 0.01 |
Early PRBC transfusion, number of PRBC transfusions, and BPD outcome
We observed that the incidence of BPD was higher with younger age at first PRBC transfusion. For further analysis, we chose 3 weeks as a cut-off for the division of infants in group 1 into early transfusion and late transfusion subgroups. The early transfusion group received the first PRBC transfusion at <3 weeks, and the late transfusion group received PRBC at ≥3 weeks of age. The median age at first PRBC transfusion was 15 days in the early transfusion subgroup and 25 days in late transfusion subgroup (P < 0.0001). The BPD rate was 54.7% (41/75) in the early transfusion group compared to 16.1% (10/62) in the late transfusion group (P < 0.0001; Table 3).
Table 3. Comparison of risk factors associated with BPD between early and late PRBC transfusion groups.
| Early transfusion group | Late transfusion group | P value | |
|---|---|---|---|
| Number of patients | 75 | 62 | |
| Age of first transfusion, weeks | 15 (12–17) | 25 (22–29) | <0.0001 |
| Birth weight, g | 1303.8 ± 260.5 | 1332.7 ± 218.3 | 0.48 |
| Surfactant | 66 (88.0%) | 44 (71.0%) | 0.02 |
| Mechanical ventilation ≥ 1 week | 18 (24.0%) | 6 (9.7%) | 0.03 |
| Sepsis | 35 (46.7%) | 13 (21.0%) | 0.002 |
| Aminophylline | 60 (80.0%) | 45 (72.6%) | 0.31 |
| Bronchopulmonary dysplasia | 41 (54.7%) | 10 (16.1%) | <0.0001 |
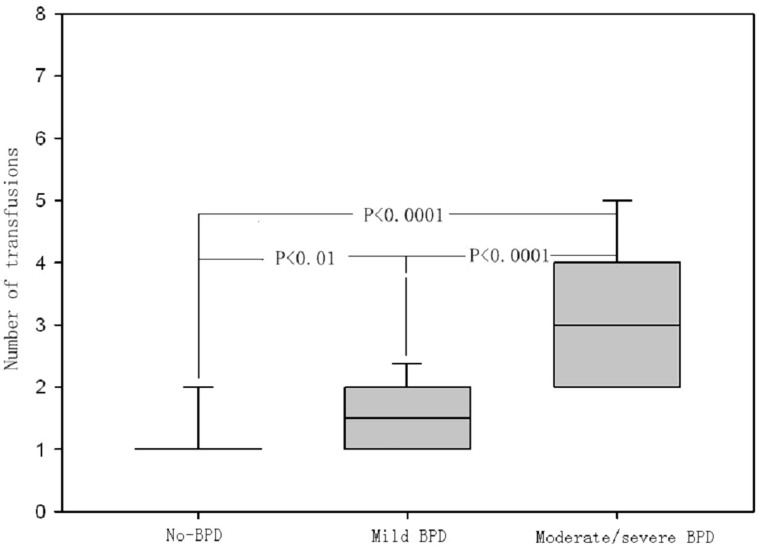
We compared potential risk factors associated with BPD between the early transfusion and late transfusion groups according to the results of logistic regression analysis (Table 2). There was no significant difference between BW among the two groups. The early transfusion group had higher rates of surfactant usage (P = 0.02), sepsis (P = 0.002), and MV usage for ≥1 week (P = 0.03); Table 3). After adjusting for differences in the significant risk factors, BW, surfactant use, aminophylline use, sepsis, and MV ≥ 1 week, the adjusted OR of early transfusion for BPD was 5.01 (95% CI 1.75–14.32; P = 0.002), meanwhile, the adjusted OR of number of PRBC transfusions for BPD was 2.91 (95% CI 1.34–6.31; P = 0.006; Table 4). In addition, compared to infants in group 1 who never developed BPD, the median number of transfusions was higher in infants with both mild BPD (oxygen requirement resolved by 36 weeks) and moderate/severe BPD (oxygen requirement not resolved by 36 weeks) [median, no-BPD vs mild BPD vs moderate/severe BPD: 1 (1–1) vs 1.5 (1–2) vs 3 (2–4); Figure 1].
Table 4. Summary table of logistic regression with BPD as the dependent variable in PRBC transfusion (group 1) and early RBC transfusion, number of transfusions, BW, surfactant use, sepsis, aminophylline use, and MV ≥ 1 week as independent variables.
| Adjusted OR | 95% CI | P value | |
|---|---|---|---|
| Birth weight, g | 0.99 | (0.99, 1.000) | 0.04 |
| Surfactant | >1000 | (<0.001, >1000) | 0.94 |
| Mechanical ventilation ≥ 1 week | 2.83 | (0.58, 13.72) | 0.19 |
| Sepsis | 1.72 | (0.62, 4.78) | 0.29 |
| Aminophylline | 7.52 | (1.38, 40.95) | 0.01 |
| Early PRBC transfusion | 5.01 | (1.75, 14.32) | 0.002 |
| Number of transfusions | 2.91 | (1.34, 6.31) | 0.006 |
Figure 1. Comparison of the number of PRBC transfusions in the non BPD group, the mild BPD group, and the moderate/severe BPD group.
Discussion
The present study identified an association between PRBC transfusion and BPD in preterm infants. The association between PRBC transfusions and BPD persisted even after adjustment for confounding variables including gestational age, BW, surfactant use, and days of ventilation in a logistic regression analysis.
An association between PRBC transfusion and BPD has been reported previously14,15,16,17. These previous retrospective studies reported BPD as a secondary outcome while primarily focusing on the effects of transfusions and ferritin levels related to the number of blood transfusions in all infants. The definition used for BPD included patients requiring any oxygen concentration at 36 weeks' PMA. In a study on 98 infants with a GA < 34 weeks, Cooke et al. reported a higher incidence of BPD in the PRBC transfusion group15. Valieva et al. studied the effects of transfusions in extremely low birth weight infants and found that the incidence of BPD is significantly associated with the number of transfusions at day 28 of life, but this relationship disappeared by 36 weeks' PMA14. A recent retrospective study on 50 infants with VLBW demonstrated an increase in neonatal morbidities, including BPD, in infants who received PRBC transfusions but the study failed to adjust for other risk factors, such as BW, GA, and small for GA16. The present study included a larger number of patients (231 patients) than previous studies and showed a significant increase in the BPD rate among infants who received PRBC transfusions after adjusting for other potential risk factors.
The significant association of PRBC transfusion and BPD could possibly be explained by several mechanisms. The iron produced consequent to the breakdown of heme released from transfused erythrocytes could promote free radical generation, infection, and fibrosis18. Heme is broken down by heme oxygenase (HO) to iron, CO, and bilirubin. Under normal circumstances, the products of HO activity are beneficial to the organism, but with excessive HO activity, the products are potentially harmful18. In addition, transfusion-related acute lung injury (TRALI) has been reported in children19,20. TRALI is defined as “a new episode of acute lung injury that occurs during or within 6 hours of a completed transfusion, which is not temporally related to a competing etiology for acute lung injury21”. PRBC transfusions may have an impact on the lungs probably through an acute inflammatory response. The acute inflammatory injury in lungs makes it difficult to wean from ventilator support smoothly or may increase the need for long-term supplemental oxygen. The persistence of lung inflammation contributes greatly to BPD, including alteration of the lung's ability to repair and inhibition of secondary septation, alveolarization, and normal vascular development22. We observed that some infants in the PRBC transfusion group still needed mechanical ventilation, and the ventilator parameters could not be reduced in these patients. TRALI may be responsible for this fact. Recently, transfusion-related immune modulation was linked to inflammatory cytokine production and immunoactivation in the endothelium after PRBC transfusion in preterm infants9. Infants who received PRBC transfusion at younger age showed a higher incidence of BPD in the present study, and this could be due to iron overload and production of inflammatory cytokines that promote injury in the immature lung.
Our study firstly investigated the correlation of age at first PRBC transfusion and number of PRBC transfusions with the outcome, BPD. The highest incidence of BPD was observed in infants who received PRBC transfusion within the first 3 weeks of life. This may suggest that the lung in preterm infant is vulnerable to injury at earlier age. Furthermore, a greater number of PRBC transfusions correlated with BPD and its severity. Although it is likely that sicker infants received more transfusions, multiple transfusions caused more harm to the lungs through inflammatory cytokines and heme.
In our patient population, the common causes of anemia were phlebotomy loss and anemia of prematurity. Phlebotomy losses can be minimized by using bedside, in-line analyzers or noninvasive monitoring devices, implementing discriminating blood drawing schedules, and delaying cord ligation23,24,25,26. The ability of early use of erythropoietin to reduce the incidence of BPD and requirement of blood transfusion has been described previously27,28. Furthermore, the use of supplemental iron and implementation of more stringent transfusion guidelines can also decrease the need for transfusion29,30.
The present study has some limitations. Apneic episodes were recorded by the nursing staff, which may have introduced some bias. The loss of some preterm infants to follow-up (14) might have affected the final results to some extent. Although we adjusted for known confounding factors for BPD, other factors that can influence BPD could not be ruled out entirely. In addition, we did not randomize infants for treatment with PRBC transfusions. It is likely that sicker infants received more transfusions and were at greater risk of later morbidities. Because of the inherent limitations of a retrospective study, we believe that a randomized prospective study would be able to more strongly explore these associations.
Conclusions
This study demonstrated an association between PRBC transfusion and an increased incidence of BPD in preterm infants. The incidence of BPD was higher with earlier PRBC transfusions (within 3 weeks of life). Greater numbers of PRBC transfusions also increased the incidence of BPD and its severity.
Author Contributions
X.M.H. conceived the idea and designed the research with Z.Q.Z. Z.Q.Z. analyzed the data and wrote the manuscript. Z.Q.Z. and H.L. supplied theoretical background for the explanation. All the authors contributed to discussion of the results.
Acknowledgments
The authors wish to thank the Hangzhou Science and Technology Development Project (grant number 201108331302) and the Open Foundation of Key Laboratory of Reproductive Genetics, Ministry of Education/Key Laboratory for Diagnosis and Therapy of Neonatal Diseases, China (grant number 2012-RG/ND-0004).
References
- Luban N. L. Management of anemia in the newborn. Early Hum Dev. 84, 493–498 (2008). [DOI] [PubMed] [Google Scholar]
- Bell E. F. et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 115, 1685–1691 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpalani H. et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 149, 301–307 (2005). [DOI] [PubMed] [Google Scholar]
- DeMaio J. G., Harris M. C., Deuber C. & Spitzer A. R. Effect of blood transfusion on apnea frequency in growing premature infants. J Pediatr. 114, 1039–1041 (1989). [DOI] [PubMed] [Google Scholar]
- Stute H., Greiner B. & Linderkamp O. Effect of blood transfusion on cardio respiratory abnormalities in preterm infants. Arch Dis Child Fetal Neonatal Ed. 72, 194–196 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westkamp E. et al. Blood transfusion in anemic infants with apnea of prematurity. Biol Neonate. 82, 228–232 (2002). [DOI] [PubMed] [Google Scholar]
- Keyes W. G., Donohue P. K., Spivak J. L., Jones M. D. & Oski F. A. Assessing the need for transfusion of premature infants and role of hematocrit, clinical signs, and erythropoietin level. Pediatrics. 84, 412–417 (1989). [PubMed] [Google Scholar]
- Ho J., Sibbald W. J. & Chin Y. I. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 31(suppl), 687–697 (2003). [DOI] [PubMed] [Google Scholar]
- Keir A. K., McPhee A. J., Andersen C. C. & Stark M. J. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res. 73, 75–79 (2013). [DOI] [PubMed] [Google Scholar]
- Donadee C. et al. Nitric oxidescavenging by red blood cell microparticles and cellfree hemoglobin as a mechanism for the red cell storage lesion. Circulation. 124, 465–476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinmouth A. et al. Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion. 46, 2014–2027 (2006). [DOI] [PubMed] [Google Scholar]
- Roseff S. D., Luban N. L. & Manno C. S. Guidelines for assessing appropriateness of pediatric transfusion. Transfusion. 42, 1398–1412 (2002). [DOI] [PubMed] [Google Scholar]
- Ehrenkranz R. A. et al. Validation of the national institutes of health consensus definition of bronchopulmonary dysplasia. Pediatrics. 116, 1353–1360 (2005). [DOI] [PubMed] [Google Scholar]
- Valieva O. A., Strandjord T. P., Mayock D. E. & Juul S. E. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 155, 331–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. W., Drury J. A., Yoxall C. W. & James C. Blood transfusion and chronic lung disease in preterm infants. Eur J Pediatr. 156, 47–50 (1997). [DOI] [PubMed] [Google Scholar]
- Jeon G. W. & Sin J. B. Risk factors of transfusion in anemia of very low birth weight infants. Yonsei Med J. 54, 366–373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Fang J., Su H. & Chen M. Risk factors for bronchopulmonary dysplasia in neonates born at ≤1500 g (1999–2009). Pediatr Int. 53, 915–920 (2011). [DOI] [PubMed] [Google Scholar]
- Collard K. J. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses. 66, 355–364 (2006). [DOI] [PubMed] [Google Scholar]
- Gauvin F. et al. Transfusion-related acute lung injury in the Canadian paediatric population. Paediatr Child Health. 17, 235–239 (2012). [PMC free article] [PubMed] [Google Scholar]
- Lieberman L., Petraszko T., Yi Q. L., Hannach B. & Skeate R. Transfusion-related lung injury in children: a case series and review of the literature. Transfusion. 54, 57–64 (2014). [DOI] [PubMed] [Google Scholar]
- Kleinman S. et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 44, 1774–1789 (2004). [DOI] [PubMed] [Google Scholar]
- Ryan R. M., Ahmed Q. & Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol. 34, 174–190 (2008). [DOI] [PubMed] [Google Scholar]
- Widness J. A. et al. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 115, 1299–1306 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan A. et al. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol. 25, 21–25 (2005). [DOI] [PubMed] [Google Scholar]
- Lin J. C. et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics. 106, 19 (2000). [DOI] [PubMed] [Google Scholar]
- Sweet D. G. et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants- 2013 update. Neonatology. 103, 353–368 (2013). [DOI] [PubMed] [Google Scholar]
- Rayjada N. et al. Decrease in Incidence of Bronchopulmonary Dysplasia with Erythropoietin Administration in Preterm Infants: A Retrospective Study. Neonatology. 102, 287–292 (2012). [DOI] [PubMed] [Google Scholar]
- Ohlsson A. & Aher S. M. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 9, CD004863 (2012). [DOI] [PubMed] [Google Scholar]
- Türker G., Sarper N., Gökalp A. S. & Usluer H. The effect of early recombinant erythropoietin and enteral iron supplementation on blood transfusion in preterm infants. Am J Perinatol. 22, 449–455 (2005). [DOI] [PubMed] [Google Scholar]
- Franz A. R. & Pohlandt F. Red blood cell transfusions in very and extremely low birthweight infants under restrictive transfusion guidelines: is exogenous erythropoietin necessary? Arch Dis Child Fetal Neonatal Ed. 84, 96–100 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]