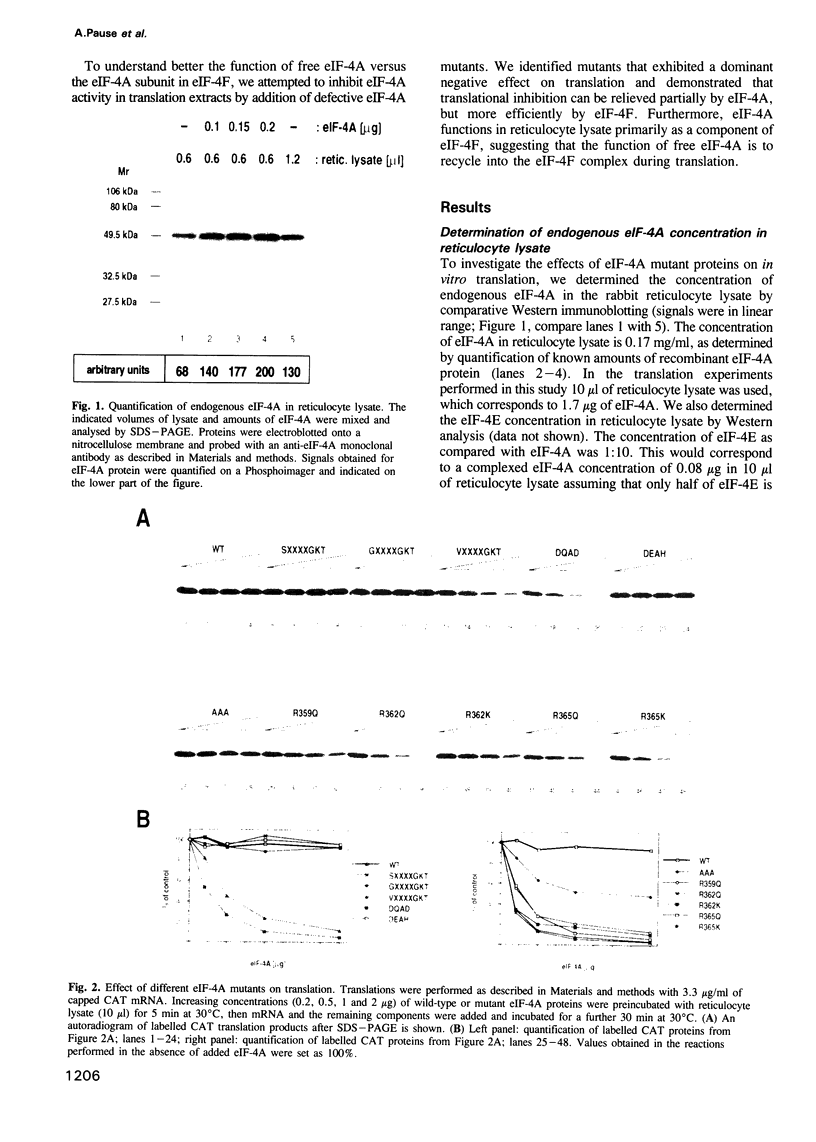
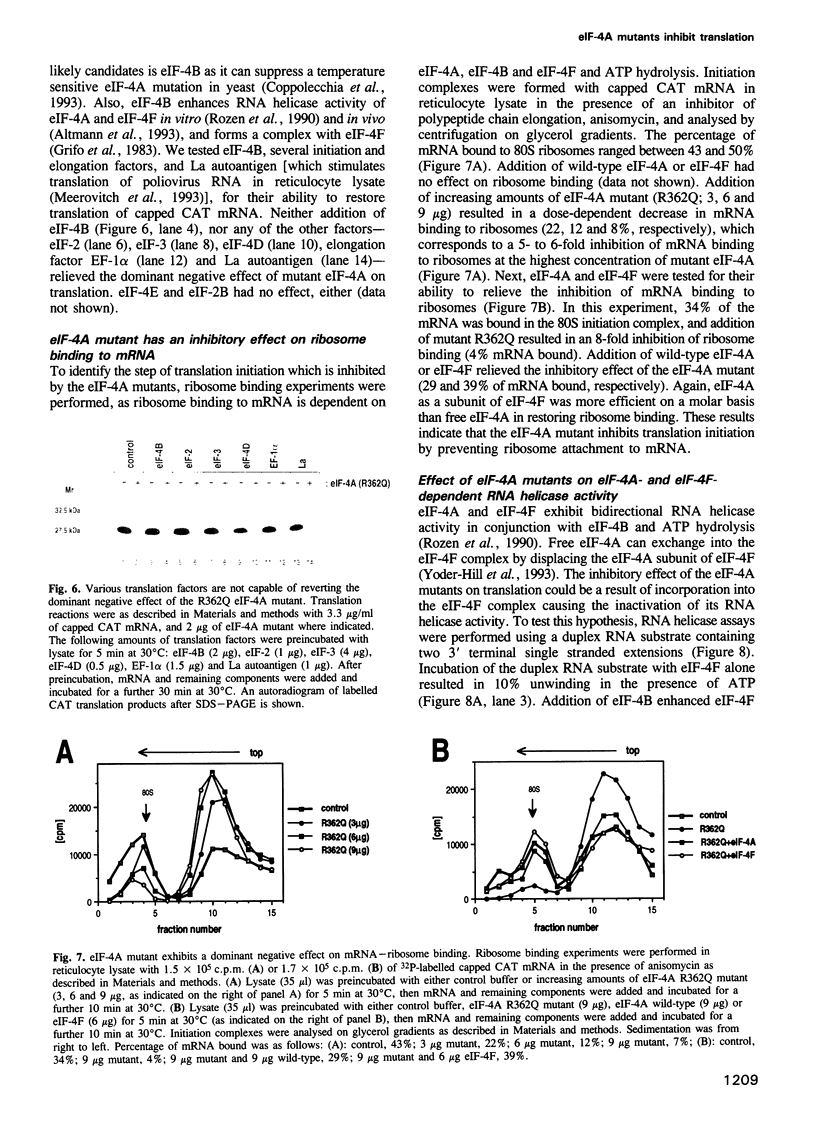
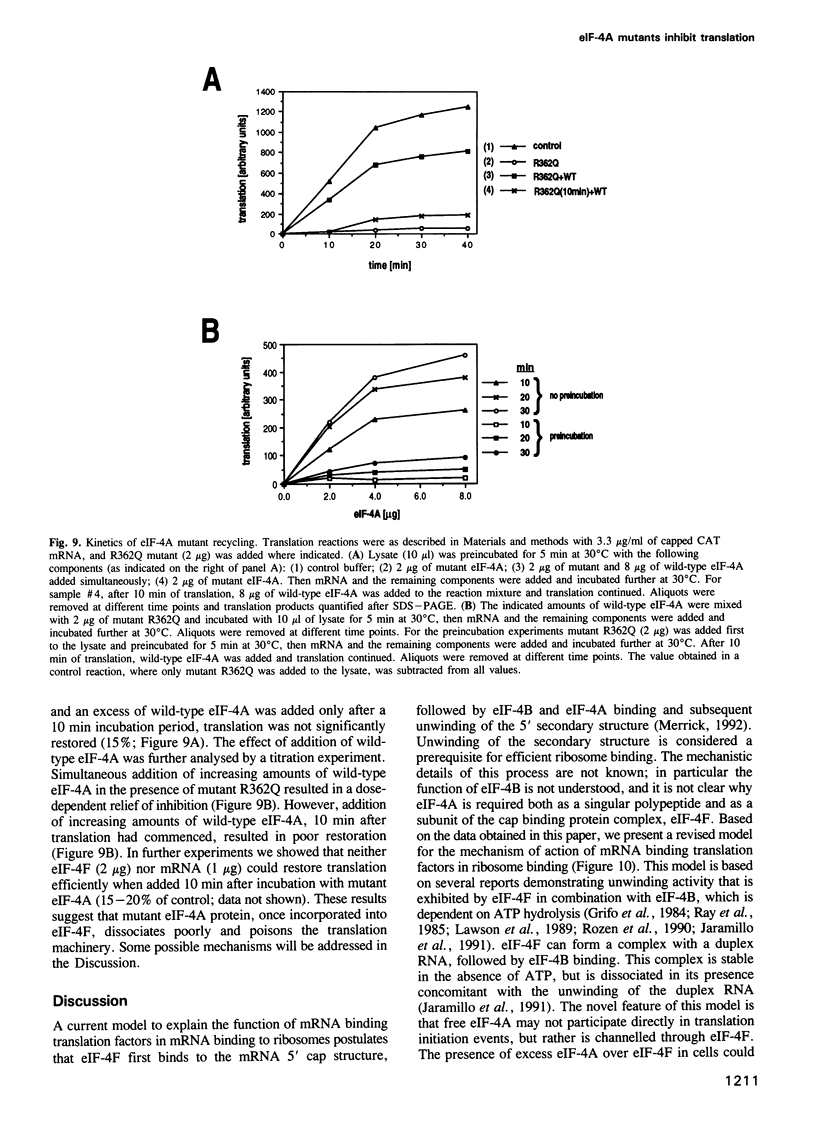
Abstract
Eukaryotic translation initiation factor-4A (eIF-4A) plays a critical role in binding of eukaryotic mRNAs to ribosomes. It has been biochemically characterized as an RNA-dependent ATPase and RNA helicase and is a prototype for a growing family of putative RNA helicases termed the DEAD box family. It is required for mRNA-ribosome binding both in its free form and as a subunit of the cap binding protein complex, eIF-4F. To gain further understanding into the mechanism of action of eIF-4A in mRNA-ribosome binding, defective eIF-4A mutants were tested for their abilities to function in a dominant negative manner in a rabbit reticulocyte translation system. Several mutants were demonstrated to be potent inhibitors of translation. Addition of mutant eIF-4A to a rabbit reticulocyte translation system strongly inhibited translation of all mRNAs studied including those translated by a cap-independent internal initiation mechanism. Addition of eIF-4A or eIF-4F relieved inhibition of translation, but eIF-4F was six times more effective than eIF-4A, whereas eIF-4B or other translation factors failed to relieve the inhibition. Kinetic experiments demonstrated that mutant eIF-4A is defective in recycling through eIF-4F, thus explaining the dramatic inhibition of translation. Mutant eIF-4A proteins also inhibited eIF-4F-dependent, but not eIF-4A-dependent RNA helicase activity. Taken together these results suggest that eIF-4A functions primarily as a subunit of eIF-4F, and that singular eIF-4A is required to recycle through the complex during translation. Surprisingly, eIF-4F, which binds to the cap structure, appears to be also required for the translation of naturally uncapped mRNAs.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann M., Blum S., Pelletier J., Sonenberg N., Wilson T. M., Trachsel H. Translation initiation factor-dependent extracts from Saccharomyces cerevisiae. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):155–159. doi: 10.1016/0167-4781(90)90158-x. [DOI] [PubMed] [Google Scholar]
- Altmann M., Blum S., Wilson T. M., Trachsel H. The 5'-leader sequence of tobacco mosaic virus RNA mediates initiation-factor-4E-independent, but still initiation-factor-4A-dependent translation in yeast extracts. Gene. 1990 Jul 2;91(1):127–129. doi: 10.1016/0378-1119(90)90173-o. [DOI] [PubMed] [Google Scholar]
- Altmann M., Müller P. P., Wittmer B., Ruchti F., Lanker S., Trachsel H. A Saccharomyces cerevisiae homologue of mammalian translation initiation factor 4B contributes to RNA helicase activity. EMBO J. 1993 Oct;12(10):3997–4003. doi: 10.1002/j.1460-2075.1993.tb06077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony D. D., Merrick W. C. Analysis of 40 S and 80 S complexes with mRNA as measured by sucrose density gradients and primer extension inhibition. J Biol Chem. 1992 Jan 25;267(3):1554–1562. [PubMed] [Google Scholar]
- Anthony D. D., Merrick W. C. Eukaryotic initiation factor (eIF)-4F. Implications for a role in internal initiation of translation. J Biol Chem. 1991 Jun 5;266(16):10218–10226. [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Blum S., Mueller M., Schmid S. R., Linder P., Trachsel H. Translation in Saccharomyces cerevisiae: initiation factor 4A-dependent cell-free system. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6043–6046. doi: 10.1073/pnas.86.16.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S., Schmid S. R., Pause A., Buser P., Linder P., Sonenberg N., Trachsel H. ATP hydrolysis by initiation factor 4A is required for translation initiation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7664–7668. doi: 10.1073/pnas.89.16.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolecchia R., Buser P., Stotz A., Linder P. A new yeast translation initiation factor suppresses a mutation in the eIF-4A RNA helicase. EMBO J. 1993 Oct;12(10):4005–4011. doi: 10.1002/j.1460-2075.1993.tb06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Etchison D., Milburn S. Separation of protein synthesis initiation factor eIF4A from a p220-associated cap binding complex activity. Mol Cell Biochem. 1987 Jul;76(1):15–25. doi: 10.1007/BF00219394. [DOI] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Gehrke L., Auron P. E., Quigley G. J., Rich A., Sonenberg N. 5'-Conformation of capped alfalfa mosaic virus ribonucleic acid 4 may reflect its independence of the cap structure or of cap-binding protein for efficient translation. Biochemistry. 1983 Oct 25;22(22):5157–5164. doi: 10.1021/bi00291a015. [DOI] [PubMed] [Google Scholar]
- Goyer C., Altmann M., Lee H. S., Blanc A., Deshmukh M., Woolford J. L., Jr, Trachsel H., Sonenberg N. TIF4631 and TIF4632: two yeast genes encoding the high-molecular-weight subunits of the cap-binding protein complex (eukaryotic initiation factor 4F) contain an RNA recognition motif-like sequence and carry out an essential function. Mol Cell Biol. 1993 Aug;13(8):4860–4874. doi: 10.1128/mcb.13.8.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifo J. A., Abramson R. D., Satler C. A., Merrick W. C. RNA-stimulated ATPase activity of eukaryotic initiation factors. J Biol Chem. 1984 Jul 10;259(13):8648–8654. [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Hansen J. L., Etchison D. O., Hershey J. W., Ehrenfeld E. Localization of cap-binding protein in subcellular fractions of HeLa cells. Mol Cell Biol. 1982 Dec;2(12):1639–1643. doi: 10.1128/mcb.2.12.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo M., Dever T. E., Merrick W. C., Sonenberg N. RNA unwinding in translation: assembly of helicase complex intermediates comprising eukaryotic initiation factors eIF-4F and eIF-4B. Mol Cell Biol. 1991 Dec;11(12):5992–5997. doi: 10.1128/mcb.11.12.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1986 May;83(9):2850–2854. doi: 10.1073/pnas.83.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. G., Lee K. A., Maimone M. M., Abramson R. D., Dever T. E., Merrick W. C., Thach R. E. Dissociation of double-stranded polynucleotide helical structures by eukaryotic initiation factors, as revealed by a novel assay. Biochemistry. 1989 May 30;28(11):4729–4734. doi: 10.1021/bi00437a033. [DOI] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Linder P., Lasko P. F., Ashburner M., Leroy P., Nielsen P. J., Nishi K., Schnier J., Slonimski P. P. Birth of the D-E-A-D box. Nature. 1989 Jan 12;337(6203):121–122. doi: 10.1038/337121a0. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y. V., Lee H. S., Lejbkowicz F., Kenan D. J., Chan E. K., Agol V. I., Keene J. D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993 Jul;67(7):3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W. C. Purification of protein synthesis initiation factors from rabbit reticulocytes. Methods Enzymol. 1979;60:101–108. doi: 10.1016/s0076-6879(79)60010-1. [DOI] [PubMed] [Google Scholar]
- Morgan M. A., Shatkin A. J. Initiation of reovirus transcription by inosine 5'-triphosphate and properties of 7-methylinosine-capped, inosine-substituted messenger ribonucleic acids. Biochemistry. 1980 Dec 23;19(26):5960–5966. doi: 10.1021/bi00567a003. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., Trachsel H. The mouse protein synthesis initiation factor 4A gene family includes two related functional genes which are differentially expressed. EMBO J. 1988 Jul;7(7):2097–2105. doi: 10.1002/j.1460-2075.1988.tb03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A., Méthot N., Sonenberg N. The HRIGRXXR region of the DEAD box RNA helicase eukaryotic translation initiation factor 4A is required for RNA binding and ATP hydrolysis. Mol Cell Biol. 1993 Nov;13(11):6789–6798. doi: 10.1128/mcb.13.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A., Sonenberg N. Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. EMBO J. 1992 Jul;11(7):2643–2654. doi: 10.1002/j.1460-2075.1992.tb05330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Ray B. K., Lawson T. G., Kramer J. C., Cladaras M. H., Grifo J. A., Abramson R. D., Merrick W. C., Thach R. E. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J Biol Chem. 1985 Jun 25;260(12):7651–7658. [PubMed] [Google Scholar]
- Rozen F., Edery I., Meerovitch K., Dever T. E., Merrick W. C., Sonenberg N. Bidirectional RNA helicase activity of eucaryotic translation initiation factors 4A and 4F. Mol Cell Biol. 1990 Mar;10(3):1134–1144. doi: 10.1128/mcb.10.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper G. C., Voorma H. O., Thomas A. A. Eukaryotic initiation factors-4E and -4F stimulate 5' cap-dependent as well as internal initiation of protein synthesis. J Biol Chem. 1992 Apr 15;267(11):7269–7274. [PubMed] [Google Scholar]
- Smith M. R., Jaramillo M., Liu Y. L., Dever T. E., Merrick W. C., Kung H. F., Sonenberg N. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 1990 Jul;2(7):648–654. [PubMed] [Google Scholar]
- Sonenberg N., Guertin D., Cleveland D., Trachsel H. Probing the function of the eucaryotic 5' cap structure by using a monoclonal antibody directed against cap-binding proteins. Cell. 1981 Dec;27(3 Pt 2):563–572. doi: 10.1016/0092-8674(81)90398-6. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Poliovirus translation. Curr Top Microbiol Immunol. 1990;161:23–47. doi: 10.1007/978-3-642-75602-3_2. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V., Agol V. I. Complete translation of encephalomyocarditis virus RNA and faithful cleavage of virus-specific proteins in a cell-free system from Krebs-2 cells. FEBS Lett. 1978 Mar 1;87(1):7–11. doi: 10.1016/0014-5793(78)80121-5. [DOI] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Thach R. E. Cap recap: the involvement of eIF-4F in regulating gene expression. Cell. 1992 Jan 24;68(2):177–180. doi: 10.1016/0092-8674(92)90461-k. [DOI] [PubMed] [Google Scholar]
- Timmer R. T., Benkowski L. A., Schodin D., Lax S. R., Metz A. M., Ravel J. M., Browning K. S. The 5' and 3' untranslated regions of satellite tobacco necrosis virus RNA affect translational efficiency and dependence on a 5' cap structure. J Biol Chem. 1993 May 5;268(13):9504–9510. [PubMed] [Google Scholar]
- Yan R., Rychlik W., Etchison D., Rhoads R. E. Amino acid sequence of the human protein synthesis initiation factor eIF-4 gamma. J Biol Chem. 1992 Nov 15;267(32):23226–23231. [PubMed] [Google Scholar]
- Yoder-Hill J., Pause A., Sonenberg N., Merrick W. C. The p46 subunit of eukaryotic initiation factor (eIF)-4F exchanges with eIF-4A. J Biol Chem. 1993 Mar 15;268(8):5566–5573. [PubMed] [Google Scholar]










