Abstract
General anesthetics inhibit neurotransmitter release from both neurons and secretory cells. If inhibition of neurotransmitter release is part of an anesthetic mechanism of action, then drugs that facilitate neurotransmitter release may aid in reversing general anesthesia. Drugs that elevate intracellular cAMP levels are known to facilitate neurotransmitter release. Three cAMP elevating drugs (forskolin, theophylline, and caffeine) were tested; all three drugs reversed the inhibition of neurotransmitter release produced by isoflurane in PC12 cells in vitro. The drugs were tested in isoflurane-anesthetized rats. Animals were injected with either saline or saline containing drug. All three drugs dramatically accelerated recovery from isoflurane anesthesia, but caffeine was most effective. None of the drugs, at the concentrations tested, had significant effects on breathing rates, O2 saturation, heart rate, or blood pressure in anesthetized animals. Caffeine alone was tested on propofol-anesthetized rats where it dramatically accelerated recovery from anesthesia. The ability of caffeine to accelerate recovery from anesthesia for different chemical classes of anesthetics, isoflurane and propofol, opens the possibility that it will do so for all commonly used general anesthetics, although additional studies will be required to determine whether this is in fact the case. Because anesthesia in rodents is thought to be similar to that in humans, these results suggest that caffeine might allow for rapid and uniform emergence from general anesthesia in human patients.
Keywords: emergence from anesthesia, isoflurane, propofol, caffeine, cAMP elevating drugs
there are no drugs available to the clinician or scientist that reverses the coma-like state induced by general anesthetics (Solt et al. 2011). Such drugs might be useful in a clinical or laboratory setting. In several recent articles Solt and colleagues demonstrated that intravenous administration of methylphenidate could shorten the time it took adult rats to emerge from anesthesia (Chemali et al. 2012; Solt et al. 2011; Taylor et al. 2013). Methylphenidate is known to inhibit dopamine transport; Solt and colleagues suggested that inhibition of dopamine transport results in elevated extracellular dopamine levels that accelerate recovery from anesthesia (Taylor et al. 2013) by dopamine receptor activation.
Anesthetics are thought to produce anesthesia, in part, by potentiating GABAA receptor activity (Concas et al. 1990; Reynolds et al. 2003; Solt and Forman 2007). Additionally, there is increasing evidence for a presynaptic locus for the action of anesthetics (Hemmings 2009; Hemmings et al. 2005; Westphalen et al. 2009, 2013). Our own preliminary data as well as those from other laboratories suggest that anesthetics directly inhibit the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) neurotransmitter release machinery (Crowder et al. 2005; Herring et al. 2009, 2011; Saifee et al. 2011; van Swinderen et al. 1999; Winegar and MacIver 2006), and this too may be important for anesthesia. Looked at from this latter perspective, drugs that reverse the inhibitory effects of anesthetics on the release machinery might have the capacity to accelerate recovery from anesthesia. cAMP signaling modulates neurotransmitter release; elevating intracellular cAMP levels augment neurotransmitter release in neurons and in secretory cells (Burgoyne and Morgan 2003; Byrne and Kandel 1996; Fujita-Yoshigaki 1998; Fujita-Yoshigaki et al. 1998; Hilfiker et al. 2001; Kasai et al. 2012; Kuromi and Kidokoro 2000, 2003; Lonart et al. 1998, 2003; Machado et al. 2001; Sakaba and Neher 2001, 2003; Seino and Shibasaki 2005; Takahashi et al. 1999; Trudeau et al. 1996, 1998, 1999). cAMP signaling has also been shown to play an important role in forms of augmented synaptic plasticity, like long-term potentiation (Bliss and Collingridge 1993; Huang and Kandel 1994; Huang et al. 1994; Weisskopf et al. 1994). In Caenorhabditis elegans, elevated cAMP levels result in worms that are significantly less sensitive to isoflurane anesthesia (Saifee et al. 2011). From these observations we hypothesize that drugs that elevate intracellular cAMP concentration ([cAMP]i) would help reverse anesthesia. Forskolin, theophylline, and caffeine are all drugs that elevate cAMP by different mechanisms. Theophylline and caffeine also inhibit adenosine receptors (Lazarus et al. 2011; Ribeiro et al. 2002). In this study, all three drugs were tested for their ability to accelerate emergence from anesthesia.
MATERIALS AND METHODS
PC12 Cell Culture
PC12 cells were grown on collagen-coated 10 cm Petri dishes in culture medium that consisted of RPMI-1640, 10% heat-inactivated horse serum, 5% fetal bovine serum, 2 mM glutamine, and 10 μg/ml gentamicin in a humidified 7% CO2 incubator at 37°C. Culture medium was replaced every other day, and cells were passaged once per week. Cells were replated on poly-lysine-coated glass coverslips 24 hours before recording.
Amperometric Measurement of Catecholamine Release
Carbon fiber electrodes were fabricated and used as previously described by Grabner et al. (2005). The detection threshold for amperometric events was set at five times the baseline root mean squared noise, and the spikes were automatically detected. Amperometric spike features, quantal size, and kinetic parameters were analyzed with a series of macros written in Igor Pro (Wavemetrics) and kindly supplied to us by Dr. Eugene Mosharov.
There can be some variation between experiments. Although there is significant variation week-to-week and even day-to-day, we observed modest cell-to-cell variation in experiments done on the same day using the same cultures. Thus, for each recording for an experimental group, a control cell was added on the same day at about the same time. Without a matching control the experiment was not used. A Student's t-test was used to assess differences between populations of cells.
PC12 Cell Permeabilization and Stimulation
An amperometric electrode was placed gently against a cell. Following 2 min in a Ca2+-free solution (step 1), the cell was permeabilized with 20 μM digitonin (Ca2+-free) for 25 s (step 2) and then stimulated for 2–3 min with a solution containing 100 μM Ca2+ (step 3). The cell was allowed to recover for 2 min in Ca2+-free media (step 4), and the cycle began again at step 2. Cells were stimulated four to five times in this way. The recording solutions had standard compositions previously described in (Grabner et al. 2005).
Measurement of the Isoflurane Concentration
Isoflurane solutions were prepared and measured as previously described (Jones et al. 1992; Jones and Harrison 1993). Isoflurane was prepared in sealed plastic intravenous bags. We have previously found that isoflurane concentrations in the bags and in the bath are remarkably constant for up to 1.5 h when measured in representative experiments using gas chromatography (Xie et al. 2006). All isoflurane concentrations in this article are provided in millimolars. The minimum alveolar concentration required for immobility in response to a noxious stimulus in 50% of trials (Eger et al. 1965) equivalents of isoflurane have been reported to be in the range of ∼0.3 mM (Franks and Lieb 1996) to ∼0.5 mM (Franks and Lieb 1996; Jones and Harrison 1993) at 25°C.
Anesthetizing Adult Rats
All studies on rats were approved by the University of Chicago Animal Use Committee.
Isoflurane.
Adult Sprague-Dawley rats, weighing 420–510 g, were placed in a gas-tight anesthesia box where they were exposed to 3% anesthesia (in 3 l/min O2) for 8 min. During this time the rats became unconscious and were insensitive to tail pinch. Isoflurane was dialed back to 2% (in 2 l/min O2). Rats were removed from the gas tight box, and an anesthesia nose cone was put in place, which delivered 2% isoflurane (in 2 l/min O2). At this point an intravenous line was inserted into a tail vein. The rats were then put back into the gas tight anesthesia box for an additional period such that they were exposed to 45 min of isoflurane in total. Five minutes before termination of the anesthesia the rats were injected with either saline or saline containing a drug (forskolin or caffeine). Anesthesia was terminated and rats were placed on their backs in the middle of a large table. Recovery time from anesthesia was the time from when the animals were removed from the anesthesia chamber to when they stood with four paws on the table. Theophylline was less effective when administered 5 min before anesthesia was terminated; for this reason theophylline was injected into the rats 30 min before anesthesia was terminated. The same groups of rats were reused multiple times for different experiments with at least 3 days between subsequent rounds of anesthesia. The same animals were used to test drugs and to serve as their own controls.
Using a calibrated isoflurane detection probe (Philips G-5 gas analyzed and Philips MX800 monitor) placed in the sealed anesthesia box or in the nose cone, we determined that the anesthesia levels provided by the anesthesia machine were accurate.
Propofol.
Adult rats were anesthetized with 3% (3 l/min O2) isoflurane for 8 min at which time an intravenous line was inserted. The animals were allowed to wake and then a bolus of propofol (4 mg/kg) was injected along with either saline or with 25 mg/kg caffeine in saline. The animals were allowed to wake. The same animals were used as controls and to test caffeine (n = 16 per group).
Measuring vital signs in rats.
Rats were induced with 3% isoflurane (in O2), for ∼8 min in a gas tight anesthesia chamber. The rats were then removed from the anesthesia box, and a nose cone, delivering 2% isoflurane, was attached. Five minutes after the nose cone, heart rate, blood pressure, and respiration were measured with an IITC Life Science Multi Channel Blood Pressure System, using a tail cuff. Approximately 5 min later, vital signs were measured again. Predrug measurement lasted ∼10 min. At this point an intravenous line was inserted into the rat tail, while the nose cone and 2% isoflurane (2 l/min O2) were maintained. Drugs were applied as a bolus in saline, and the intravenous line was then removed. Five minutes after the drugs were injected vital signs were again acquired. Five minutes later they were measured again. This second measurement also lasted ∼10 min. After the measurements were complete, anesthesia was discontinued and the rats were returned to their cages. The data from both measurements before drug administration were averaged as was the data from the two measurements after drug administration. Pulse oximetry was also measured during the experiments but not in all animals. Pulse oximetry was consistently at 100% with an occasional reading of 99%.
An unpaired t-test was used to test for statistical significance.
RESULTS
Isoflurane Blocks Neurotransmitter Release in PC12 Cells: Forskolin, Theophylline, and Caffeine Reverse This Block
Anesthetics modulate the activity of various channels and receptors, thereby altering neurotransmitter release. For the studies outlined in this article, PC12 cells were permeabilized with digitonin, and then cells were stimulated by exposing them to Ca2+. Cytoplasmic constituents stay intact in digitonin permeabilized cells (Holz et al. 1994; Li et al. 2003). Permeabilization disrupts the cell resting potential and equilibrates the intracellular and extracellular Ca2+ levels. Under these conditions, modulation of channels or receptors should have no effect on neurotransmitter release. In earlier studies, PC12 cells were also dialyzed with known Ca2+ concentrations, via a patch pipette from a constant holding potential of −65 mV; these studies validated the digitonin methodology (Herring et al. 2009, 2011). Exocytosis was elicited in the presence and absence of isoflurane (0.5 mM). Basal (Ca2+-independent) neurotransmitter release is virtually nonexistent in digitonin permeabilized PC12 cells in Ca2+-free conditions, but robust release is observed upon exposing cells to Ca2+-containing solutions (Graham et al. 2002; Jankowski et al. 1992). Physiologically, release is evoked by the activation of voltage-gated Ca2+ channels. The proximity of Ca2+ channels to synaptic release sites suggests that intracellular Ca2+ concentration may rise to levels >100 μM at the vesicle (Llinas et al. 1992). To mimic these levels in our experiments, evoked neurotransmitter release was elicited by exposing digitonin-permeabilized cells to 100 μM Ca2+, for 2 min, in the absence or presence of isoflurane.
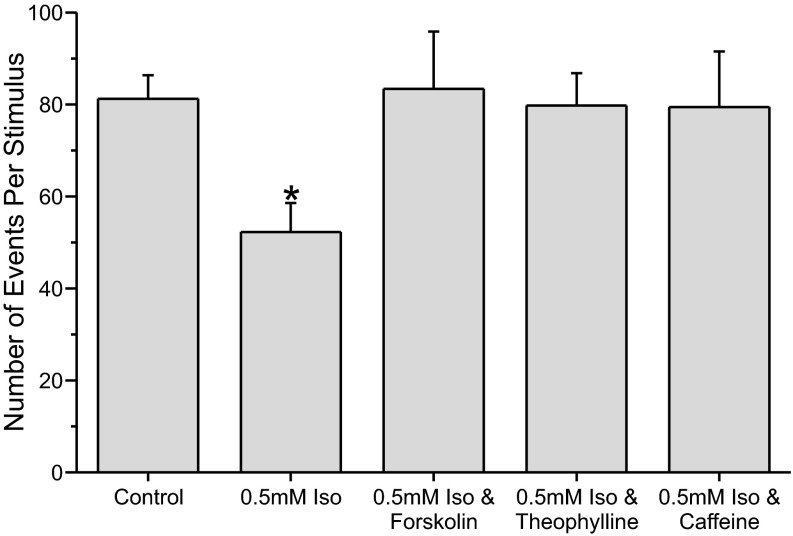
In previous studies we have shown that 0.5 mM (∼1.5 minimum alveolar concentration) isoflurane inhibits neurotransmitter release by ∼39% (Herring et al. 2009, 2011). Figure 1 shows that isoflurane (0.5 mM) significantly inhibited neurotransmitter release by ∼36% (P < 0.05) in this study. Three drugs that elevate intracellular cAMP were then tested for their ability to reverse the inhibition produced by isoflurane. Figure 1 shows that all three drugs, forskolin (5 μM), theophylline (50 μM), or caffeine (50 μM), reversed the isoflurane-mediated inhibition of neurotransmitter release.
Fig. 1.
Forskolin, theophylline, and caffeine reversed the inhibition of neurotransmitter release produced by isoflurane (Iso). PC12 cells were permeabilized with digitonin (20 μM) and then stimulated by exposing cells to a solution containing 100 μM Ca2+. Neurotransmitter release was monitored with an amperometric electrode that gently touched the cell. Under these conditions, isoflurane (0.5 mM) significantly inhibited neurotransmitter release by ∼36% (P < 0.05, relative to control and all other conditions). The number of events recorded from control PC12 cells were about the same as those from PC12 cells exposed to isoflurane + forskolin (5 μM) or isoflurane and theophylline (50 μM) or isoflurane and caffeine [50 μM; control 81.28 ± 5.1 (n = 36), isoflurane 52.29 ± 6.31 (n = 17), isoflurane + forskolin 83.4 ± 6.1 (n = 17), isoflurane + theophylline 79.75 ± 7.07 (n = 12), and isoflurane + caffeine 79.43 ± 12.12 (n = 7) events per stimulation]. *Neurotransmitter release significantly different from control.
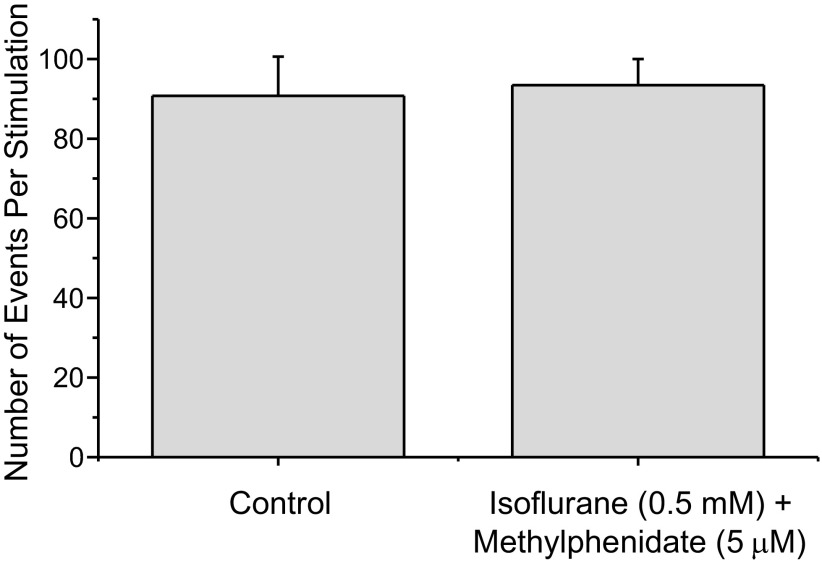
Interestingly, methylphenidate also appears to reverse the inhibition of neurotransmitter release produced by isoflurane (Fig. 2), perhaps by elevating [cAMP]i (Pascoli et al. 2005).
Fig. 2.
Methylphenidate reversed the inhibition of neurotransmitter release produced by isoflurane. Isoflurane (0.5 mM) normally inhibits neurotransmitter release by ∼40%. Control PC12 cells produced about the same number of events when stimulated as did PC12 cells exposed to isoflurane + 5 μM methyphenidate (control 86.6 ± 9.6, iso + meth 91.6 ± 6.1 events per stimulation, n = 17).
Recovery from Isoflurane Anesthesia in Behaving Rats
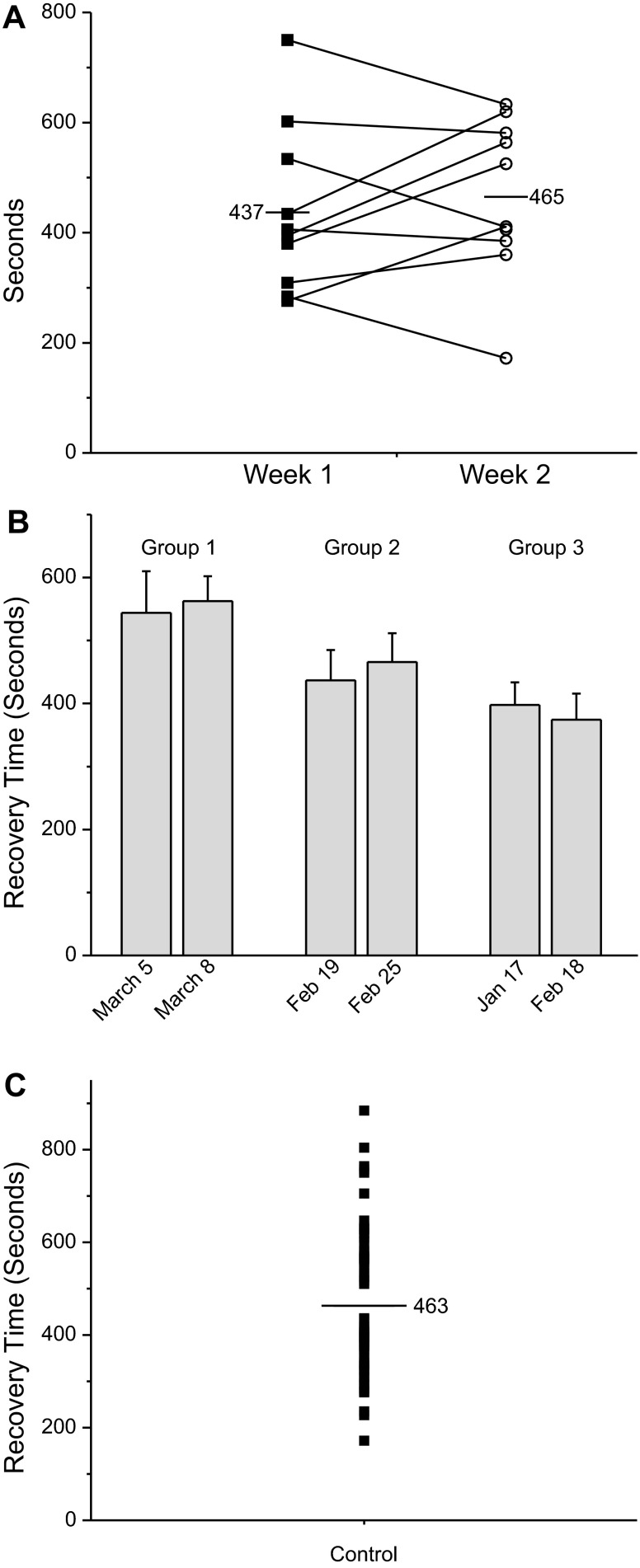
These results suggest that elevating intracellular cAMP reverses the inhibition of neurotransmitter release produced by isoflurane in vitro. Does elevating cAMP alter recovery from anesthesia in animals? To address this question, we examined emergence from anesthesia in rats. First, we assessed waking from anesthesia in the absence of any other drug to assess population variability. Rats were placed in an anesthetizing apparatus where they were exposed to 3% isoflurane (in 3 l/min O2) for 8 min and then to 2% isoflurane for an additional 45 min. Recovery time from anesthesia for rats is defined as the time from when the animals are removed from the anesthetizing chamber to when they stand upright with four paws on the table. Figure 3A shows data from 10 animals subjected to this protocol (filled square). A week later the experiment was repeated in an identical manner in the same group of rats (open circle). Each filled square or open circle in the graph represents data from a single trial. Note that although there is some variability in recovery time from week 1 exposure to the week 2 exposure, average recovery times for week 1 (437 s) and week 2 (465 s) are similar. Figure 3B plots averaged data from 3 groups of 10 control animals. Each group was exposed to isoflurane anesthesia twice. The average recovery times between groups were significantly different, but within each group there was no significant difference. These data suggest that the optimal method for studying alteration in waking times is to use the same animals as controls and test subjects, which is how data were gathered for this study. The same cohort of animals was used for each of the drugs studied. Figure 3C plots the distribution of recovery times for all 30 animals. Although additional studies are required for a definitive statement, the data in Fig. 3C may not be normally distributed.
Fig. 3.
Variability in recovery times from isoflurane anesthesia. Rats were exposed to isoflurane and then exposed to isoflurane again a week later. Animals, 420–510 g, were placed in an airtight anesthesia box and exposed to 3% isoflurane (in 3 l/min O2) for 8 min. The rats were then kept in the same chamber while isoflurane was reduced to 2% (2 l/min O2) for another 45 min. The animals were removed from the chamber and allowed to recover on a table top breathing room air. They were placed on their backs and the time to recover from isoflurane anesthesia was the time from terminating the 2% anesthetic exposure until the animals had all four paws on the table. A: recovery time from a single group of 10 rats exposed twice to isoflurane. The first exposure is plotted as ■ and the second exposure as (○). Lines connect the 2 exposures from the same rat. Although there was some variability in recovery times, even in the same animal, the average recovery time was remarkably constant. B: plot data obtained from 3 different groups of rats. Each group was exposed to isoflurane twice. Note that the average recovery time for each group stayed relatively constant between exposures. C: distribution of all recovery times for all 30 rats tested in this study.
[There are risks associated with multiple applications of anesthesia, especially in the very young, the very old, and the critically ill. In the elderly there is postoperative cognitive decline following anesthesia and there is a possible link between anesthetic exposure and Alzheimer's disease; in vitro studies suggest anesthetics promote apoptosis and decreased neurogenesis (Bittner et al. 2011; Hudson and Hemmings 2011; Run et al. 2010). Children younger than 2 yr who undergo multiple surgeries requiring general anesthesia may be up to three times more likely than other children to develop speech and language problems (Flick et al. 2011; Sun 2010). The literature on multiple rounds of anesthesia in healthy adults is sparse. Our studies in rats found no obvious changes in rats exposed to multiple rounds of anesthesia nor were there any changes in the response to anesthesia.]
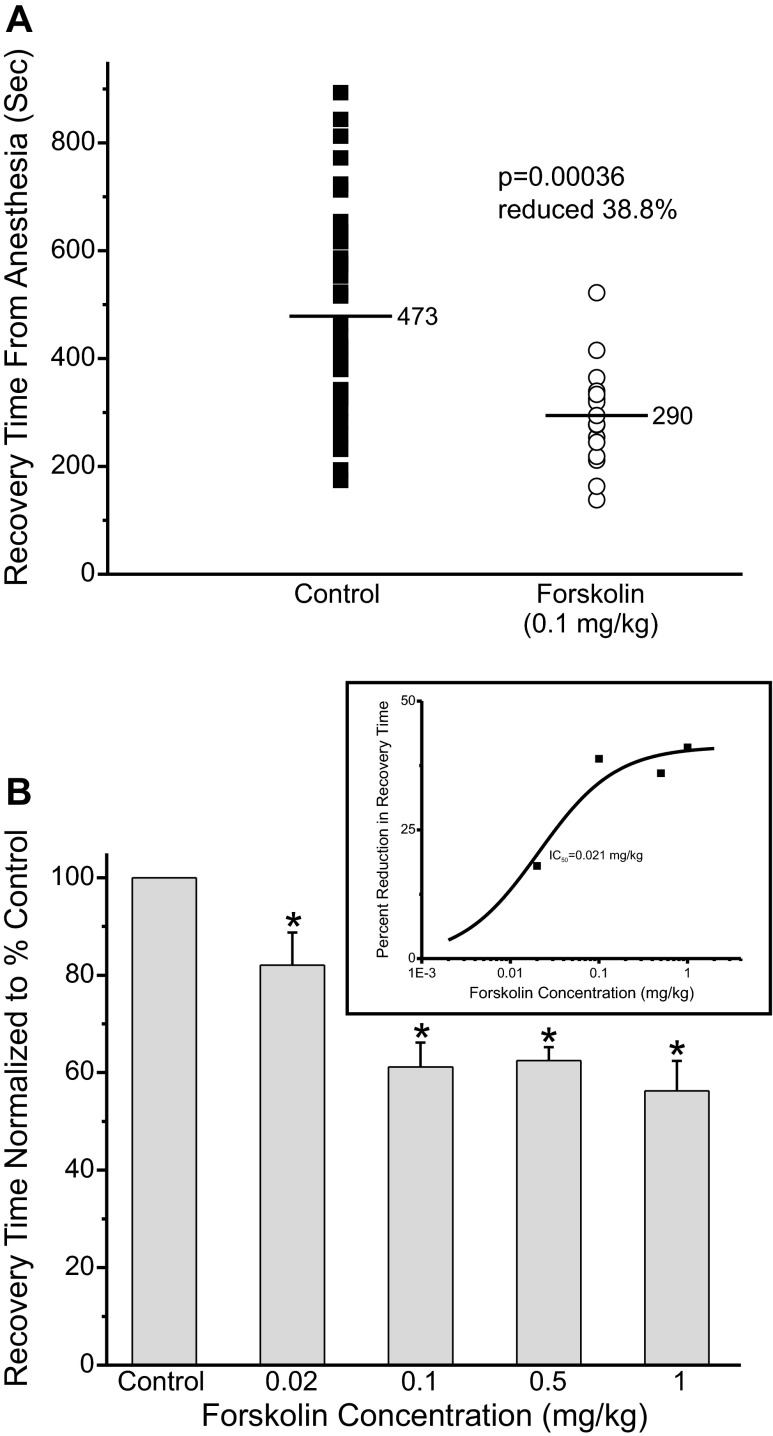
Forskolin Accelerates Recovery from Isoflurane Anesthesia
Forskolin (5 μM), a drug that elevates cAMP by stimulating adenylate cyclase activity, was tested to determine whether elevating cAMP could alter recovery from anesthesia. Adult rats were anesthetized as above. An intravenous line was inserted into a tail vein. For the forskolin studies the animals received an intravenous injection of either saline (control, closed square) or saline with drug (open circle) 5 min before discontinuing the anesthetic. The anesthetic was then terminated, and the animals were allowed to wake and right themselves, breathing room air. On average, recovery time was reduced significantly by ∼39% in animals injected with forskolin (open circles), 0.1 mg/kg, compared with control (Fig. 4A). Figure 4B shows the average recovery time at different concentrations of forskolin normalized to the control value. Figure 4B, inset, shows the dose-response curve fit to the averaged recovery data showing that the midpoint of the response occurs at ∼0.021 mg/kg forskolin.
Fig. 4.
Forskolin accelerated recovery from isoflurane anesthesia. A: adult rats were anesthetized with 3% isoflurane (3 l/min O2) for 8 min and were then exposed to 2% isoflurane (2 l/min O2) for 45 min. During the anesthesia an intravenous line was inserted into a tail vein, while anesthesia was maintained with a nose cone. Five minutes before discontinuing the anesthetic the animals received an intravenous injection of either saline (control, ■) or saline with 0.1 mg/kg forskolin (○). The anesthetic was then terminated and the animals were allowed to wake up. Every symbol represents a single trial. The same animals were used as controls and to test forskolin. B: average waking time normalized to the control value, set to 100%, at different forskolin concentrations. *Waking times that were significantly different than control. (n = 14; control, n = 16; 0.02 mg/kg, n = 16; 0.1 mg/kg, n = 20; 0.5 mg/kg, n = 9; 1 mg/kg). Inset: percent reduction in waking time as a function of the forskolin concentration. The data are fit with a standard dose-response function, with a midpoint at 0.021 mg/kg forskolin and a maximal reduction of waking time of ∼41.2%.
Theophylline Accelerates Recovery from Isoflurane Anesthesia
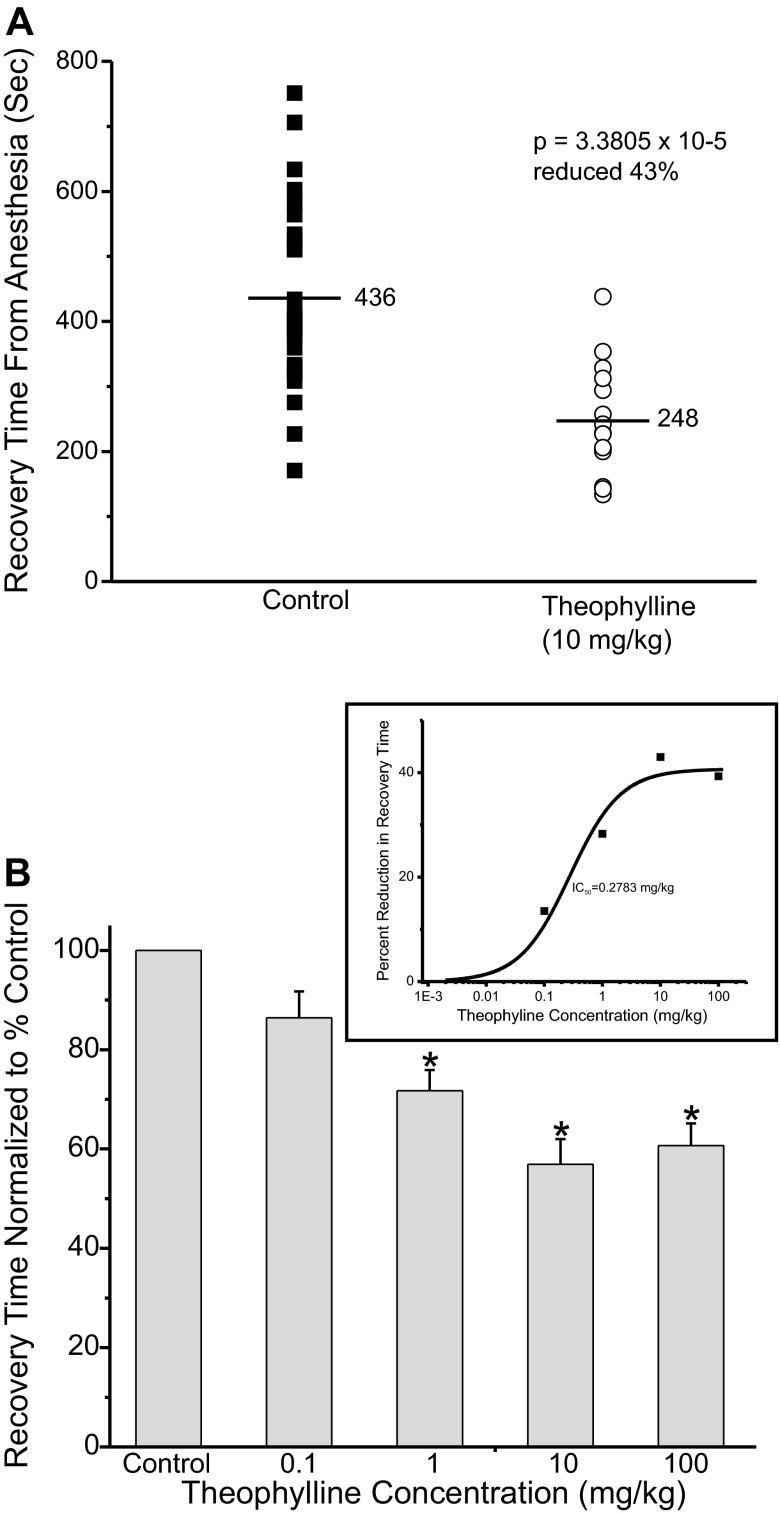
Theophylline was tested to determine whether it too could alter recovery from anesthesia. Theophylline elevates intracellular cAMP by inhibiting phosphodiesterase, the enzyme that normally degrades cAMP. Thus theophylline elevates cAMP levels by a mechanism distinct from that of forskolin. Figure 5A shows a plot of recovery from anesthesia times from control rats (closed squares) or from animals injected with theophylline (open circle; 10 mg/kg). Theophylline was injected 30 min before discontinuing the isoflurane. On average recovery time was reduced by a significant ∼43%. Figure 5B shows a plot of the average normalized recovery time at different concentrations of theophylline. Figure 5B, inset, shows the dose-response curve fit to the data showing that the midpoint occurs at ∼0.28 mg/kg theophylline.
Fig. 5.
Theophylline accelerated recovery from isoflurane anesthesia. A: adult rats were anesthetized as described in Fig. 3. Thirty minutes before discontinuing the anesthetic the animals received an intravenous injection of either saline (control, ■) or saline with 10 mg/kg theophylline (○). The anesthetic was then terminated and the animals were allowed to recover. Each symbol represents a single trial of 1 rat. B: average waking time normalized to control value, set to 100%, at different theophylline concentrations. (n = 12; control, n = 21; 0.1 mg/kg, n = 11; 1 mg/kg, n = 15; 10 mg/kg, n = 12; 100 mg/kg). Inset: plots of percent reduction in waking time as a function of the theophylline concentration. The midpoint was ∼0.28 mg/kg forskolin, max reduction in recovery time ∼41%. *Waking times significantly different from control.
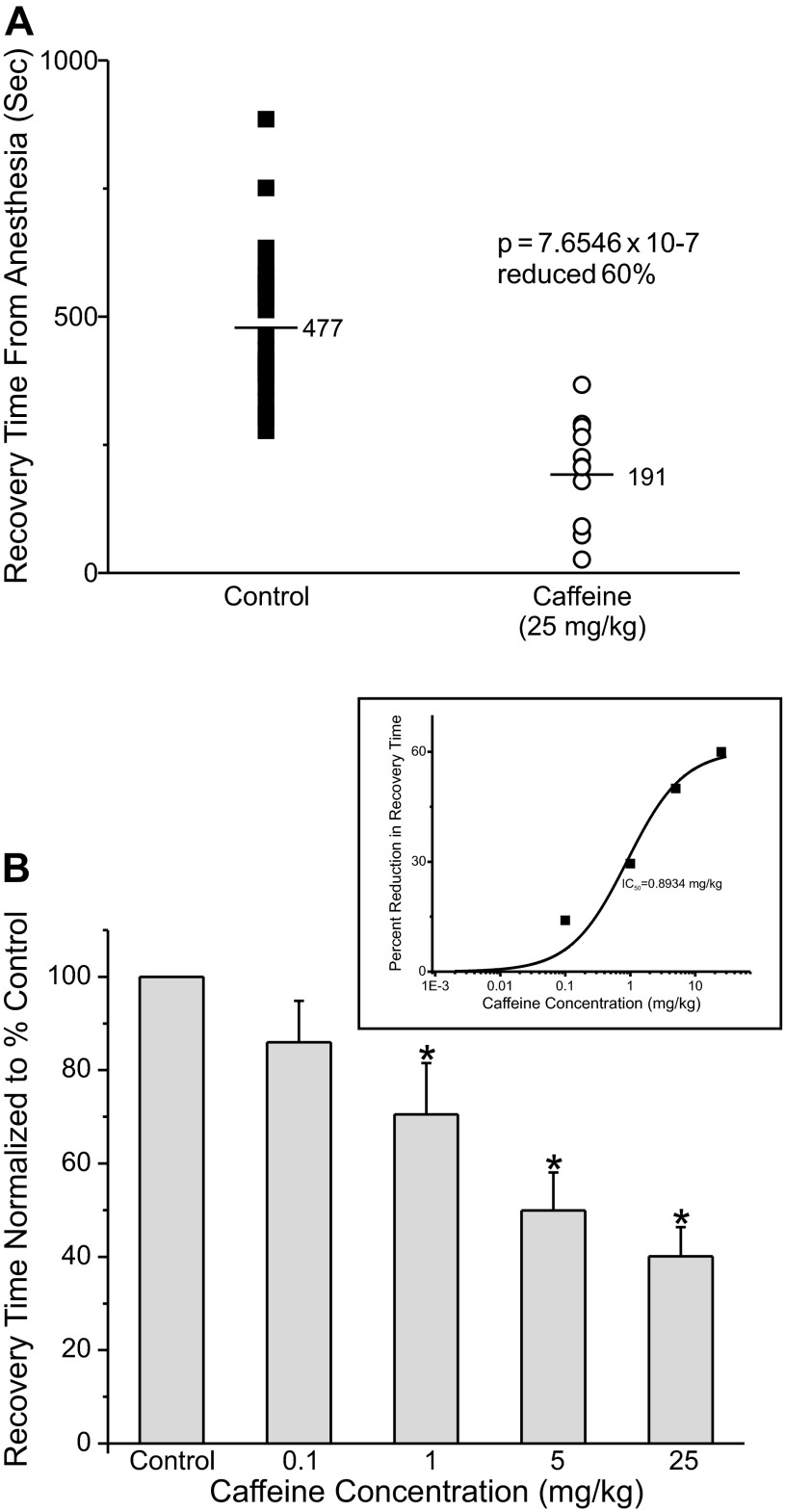
Caffeine Accelerates Recovery from Isoflurane Anesthesia
Caffeine elevates intracellular cAMP by inhibiting phosphodiesterase. Caffeine is of particular practical interest as it is such a widely used and largely innocuous drug. Figure 6A shows a graph of recovery times from isoflurane anesthesia for control rats (closed squares) or for animals injected with caffeine (open circles; 25 mg/kg), 5 min before discontinuing the isoflurane. Caffeine dramatically sped recovery from anesthesia by ∼60%. Figure 6B shows the average recovery time at different concentrations of caffeine. Figure 6B, inset, shows a plot of the dose-response curve fit to the data showing that the midpoint occurs at ∼0.9 mg/kg. Of note, an average person consumes >0.9 mg/kg of caffeine in a cup of coffee.
Fig. 6.
Caffeine accelerated recovery from isoflurane anesthesia. A: adult rats were anesthetized as described in Fig. 3. Five minutes before discontinuing the isoflurane the animals received an intravenous injection of either saline (control, ■) or saline with 25 mg/kg caffeine (○). The isoflurane was terminated and the animals were allowed to recover. B: average waking time normalized to the control value, set to 100%, at different caffeine concentrations (n = 13; control, n = 8; 0.1 mg/kg, n = 13; 1 mg/kg, n = 12; 5 mg/kg, n = 12; 25 mg/kg). Inset: plot of percent reduction in waking time as a function of the caffeine concentration. The midpoint was at ∼0.9 mg/kg caffeine and the maximal reduction of waking time was ∼60.5%. *Waking times significantly different from control.
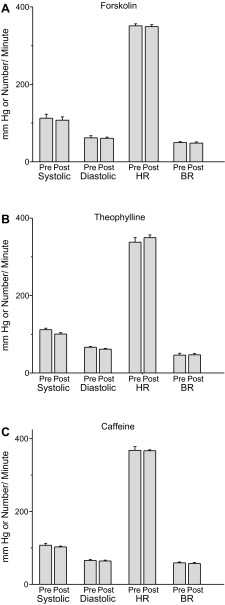
Caffeine, Forskolin, and Theophylline Do not Alter Heart Rate, Blood Pressure, or Breathing Rate
Figure 7 shows a plot of blood pressure, heart rate, and breathing rate immediately before or 10 min after injection of forskolin, theophylline, or caffeine, in isoflurane-anesthetized rats. No significant changes were observed as a result of forskolin (0.1 mg/kg) or theophylline (10 mg/kg) or caffeine administration (25 mg/kg). In all cases O2 blood saturation was maintained between 99 and 100% (see materials and methods).
Fig. 7.
There was no significant change in blood pressure, heart rate or respiratory rate in rats exposed to forskolin, theophylline, or caffeine. Rats were anesthetized as described in Fig. 3. A: forskolin (0.1 mg/kg) was injected into each animal. Just before forskolin injection blood pressure, heart rate and respiratory rate was measured. Ten minutes after forskolin, the parameters were remeasured. B: theophylline (10 mg/kg) was injected into each animal. Just before theophylline injection blood pressure, heart rate and respiratory rate were measured. Ten minutes after theophylline, the parameters were remeasured. C: caffeine (25 mg/kg) was injected into each animal. Just before caffeine injection blood pressure, heart rate and respiratory rate was measured. 10 min after caffeine, the parameters were remeasured. h, Heart rate; BR, breathing rate; pre, predrug application; post, 10 min after drug application.
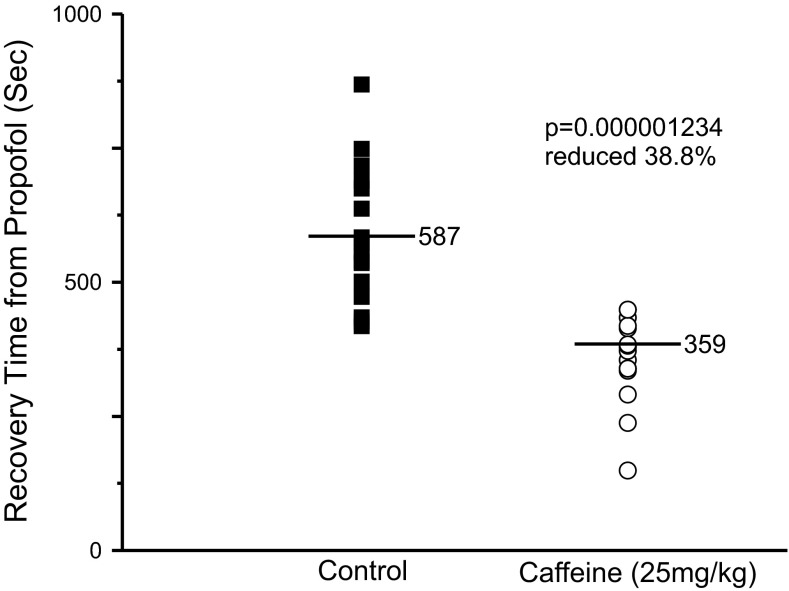
Caffeine Accelerates Recovery from Propofol Anesthesia
Propofol is an intravenous anesthetic that is structurally different than isoflurane and is thought to produce anesthesia primarily through enhancing activation of GABAA receptors in response to GABA (Concas et al. 1990; Solt and Forman 2007). We tested the ability of caffeine to accelerate recovery from propofol anesthesia to determine whether caffeine might accelerate recovery from anesthesia for different classes of anesthetics. Figure 8 shows a plot of recovery times from propofol anesthesia from control rats (closed square) or from rats injected with 25 mg/kg caffeine (open circle). Adult rats were placed in the anesthetizing chamber where they were exposed to 3% isoflurane for 8 min. Intravenous lines were inserted into a tail vein and the rats were allowed to wake. The rats then received a single bolus injection of propofol (4 mg/kg) and either saline (control) or caffeine in saline (25 mg/kg). All rats became unconscious within 5 s of propofol injection. The rats were placed on their backs on a table and allowed to wake and right in room air. As before, waking was defined as the time it took for the rats to stand with four paws on the table. Caffeine accelerated recovery from propofol anesthesia, as it did for isoflurane. These results suggest that caffeine can accelerate the recovery process for a variety of general anesthetics. Furthermore, because breathing rates were unaltered following caffeine and because caffeine accelerated emergence from both isoflurane and propofol anesthesia, our results suggest that accelerating emergence from isoflurane anesthesia was probably not due to altered gas exchange.
Fig. 8.
Caffeine accelerated recovery from propofol anesthesia. Adult rats were anesthetized with 3% isoflurane for 8 min at which time an intravenous line was inserted. The animals were allowed to wake and then a bolus of propofol (4 mg/kg) was injected along with either saline (control, ■) or with 25 mg/kg caffeine in saline (○). All rats became unconscious again within 5 s of propofol injection. The animals were allowed to wake. The same animals were used as controls and to test caffeine (n = 16 per group).
DISCUSSION
Anesthetics have been used successfully for over a century and a half, but until recently there have been no methods to accelerate emergence from anesthesia. In several recent studies, Solt and colleagues showed that the methylphenidate, used to treat attention deficit hyperactivity disorder and other disorders, could accelerate the emergence from isoflurane and propofol anesthesia (Chemali et al. 2012; Solt et al. 2011). Methylphenidate is known to inhibit the reuptake of dopamine and norepinephrine (Heal et al. 2009). Solt and colleagues proposed that inhibition of dopamine transport and the subsequent elevation of extracellular dopamine and activation of dopamine receptors were key to the ability of methylphenidate to accelerate the emergence from anesthesia (Chemali et al. 2012; Solt et al. 2011; Taylor et al. 2013). Methylphenidate also appears to reverse the inhibition of neurotransmitter release produced by isoflurane (Fig. 2), perhaps by elevating [cAMP]i (Pascoli et al. 2005). This ability of methylphenidate to reverse the inhibition of neurotransmitter release may play a role in accelerating recovery from anesthesia.
In the past, other groups have attempted to accelerate recovery from anesthesia or to reverse anesthesia. Alkire et al. (2009) infused via a cannula, a K+ channel blocking antibody into rats; the antibody restored consciousness while animals were still receiving anesthesia. Unfortunately, about a third of animals treated in this manner exhibited seizures. Activation of histamine receptors also altered anesthetic action; injection of histaminergic agonists into nucleus basalis magnocellularis dramatically accelerated emergence from anesthesia (Luo and Leung 2009). Cholinergic activation has also emerged in the literature as a potential means of reversing anesthesia (Leung et al. 2011; Tai et al. 2013). Hudetz et al. (2003) infused either a cholinesterase inhibitor or a muscarinic agonist intracerebroventricularly to decrease anesthesia. In a different study, Alkire et al. (2007) microinjected nicotine, via a cannula, into the thalamus of rats and showed that these animals were aroused from anesthesia. Although compelling, these studies are of limited clinical utility as they involve injecting drugs directly into the brain. Several human trials have explored cholinergic activation as a means to reverse anesthesia. These studies have shown that physostigmine, a cholinesterase inhibitor, reversed postoperative somnolence (Hill et al. 1977) and that physostigmine could reverse propofol anesthesia (Meuret et al. 2000). Unfortunately, physostigmine was less reliable when used with the popular volatile anesthetic sevoflurane (Plourde et al. 2003). Physostigmine usefulness would also be limited by significant peripheral autonomic side-effects.
Comparing the effectiveness of reversing anesthesia in these earlier studies to the data shown in this article is complicated since the methodologies are so diverse. For instance, Alkire et al. (2009) injected an antibody to the Kv1.2 channel into the thalamus of anesthetized rats. Only 17% of the rats woke during the injection. In that study the authors did not terminate anesthesia and determine whether animals emerged more rapidly from anesthesia. In a second study by Alkier et al. (2007) nicotine was injected into thalamus in an effort to reverse the effects of anesthesia. The therapeutic window was small. Too little nicotine had no effect and too much induced seizures. Even the optimal dose produced either no effect or seizures at higher rates than it did arousal. In that study the authors did not terminate anesthesia and determine whether animals emerged more rapidly from anesthesia. In the study by Hudetz et al. (2003) arousal from anesthesia was never measured. In that study brain wave activity was monitored. Similarly, Hill et al. (1977) employed a cholinesterase inhibitor to alter postoperative somnolence, not emergence time. Our work is most comparable the studies of methylphenidate applied intravenously (Chemali et al. 2012; Solt et al. 2011) and of histamine agonists directly infused into nucleus basalis (Luo and Leung 2009); both paradigms dramatically accelerated emergence from anesthesia. In the methylphenidate studies, the rats were more lightly anesthetized than in our study. That makes the comparison between our work and these prior studies somewhat difficult. On the other hand, we ourselves studied methylphenidate, using conditions identical to those illustrated in this manuscript for caffeine (data not shown). In our hands, methylphenidate dramatically accelerated emergence from anesthesia following termination of anesthesia, as previously reported (Chemali et al. 2012; Solt et al. 2011). Interestingly, methylphenidate produced a slightly smaller response than did caffeine, but this effect was not statistically significant nor were the studies carried out in the same cohort of animals. Additional studies carried in the same cohort of animals will be required to determine whether caffeine or methylphenidate is the more effective drug or whether they have equal efficacy. Interestingly, the study by Luo and Leung (2009) used a 60-min exposure to 2.1% isoflurane, conditions very similar to our own; in this study injection of histamine agonists accelerated emergence from isoflurane anesthesia by over 50%, a result quite similar to our own.
Data from a variety of laboratories has provided evidence for a presynaptic locus for the action of anesthetics (Hemmings 2009; Hemmings et al. 2005; Westphalen et al. 2009, 2013). Our own earlier studies, as well as studies from other laboratories, suggest that inhibition of neurotransmitter release might play an important role in anesthesia (Crowder et al. 2005; Herring et al. 2009, 2011; Saifee et al. 2011; van Swinderen et al. 1999; Winegar and MacIver 2006). It appears that drugs that reverse the inhibitory effects of anesthetics on the release machinery affect anesthesia. It is well known that cAMP signaling modulates neurotransmitter release; elevating intracellular cAMP levels augments neurotransmitter release in neurons and in secretory cells (Burgoyne and Morgan 2003; Byrne and Kandel 1996; Fujita-Yoshigaki 1998; Fujita-Yoshigaki et al. 1998; Hilfiker et al. 2001; Kasai et al. 2012; Kuromi and Kidokoro 2000, 2003; Lonart et al. 1998, 2003; Machado et al. 2001; Sakaba and Neher 2003, 2001; Seino and Shibasaki 2005; Takahashi et al. 1999; Trudeau et al. 1996, 1998, 1999). cAMP signaling has also been shown to play an important role in forms of augmented synaptic plasticity, like long-term potentiation (Bliss and Collingridge 1993; Huang and Kandel 1994; Huang et al. 1994; Weisskopf et al. 1994). Furthermore, a C. elegans mutant that results in elevated levels of intracellular cAMP levels is resistant to isoflurane anesthesia (Saifee et al. 2011). Although it is tempting to assign a direct role for inhibition of neurotransmitter release in anesthesia, at present the link is purely circumstantial. In a similar manner, relief from anesthetic block of neurotransmitter by elevation of cAMP has not yet been causally linked to accelerated recovery from anesthesia. Additionally, elevated cAMP has important postsynaptic effects (Lee and Messing 2008); we do not yet know whether such effects play a role in accelerating emergence from anesthesia.
Forskolin is known to potently stimulate adenylyl cyclase (Laurenza et al. 1989; Simonds 1999), thereby elevating intracellular cAMP. Forskolin has been used since ancient times to treat heart disorders. More recently it has been used to treat congestive heart failure, asthma, and glaucoma. When tested in vitro, forskolin completely reversed isoflurane-mediated inhibition of the neurotransmitter release machinery. Forskolin dramatically accelerated recovery from anesthesia in rats.
Theophylline, a methylxanthine drug used in the treatment of asthma (Kips et al. 1999), infant apnea, and chronic obstructive airway disease (Jenne 1987), is structurally similar to caffeine. Theophylline relaxes bronchial smooth muscle and increases cardiac contractility. Theophylline inhibits phosphodiesterase, thereby elevating intracellular cAMP (Essayan 2001). Theophylline also blocks adenosine receptors (Ribeiro et al. 2002). In our studies theophylline completely reversed isoflurane-mediated inhibition of the neurotransmitter release machinery and it dramatically accelerated recovery from anesthesia.
The drug with the highest efficacy was caffeine. Caffeine is a stimulant drug that has a variety of effects, but the two most noteworthy are its ability to block adenosine receptors (Lazarus et al. 2011) and to elevate cytosolic cAMP levels (Rang et al. 2007) by inhibiting phosphodiesterase. Caffeine is the most commonly used psychoactive drug (Nehlig et al. 1992) and in the United States >90% of adults use it daily. Clinically, caffeine is primarily used to treat neonatal apnea and certain types of headache. Caffeine binds with similar affinity to A1 and A2a receptors and antagonizes both (Lazarus et al. 2011) at the concentrations shown in Fig. 5 (Chen et al. 2013). Adenosine regulates sleep and waking (Huang et al. 2011; Lazarus et al. 2011). Caffeine, like theophylline, blocks adenosine receptors nonselectively, but it appears that A2A receptors mediate the arousal effects of caffeine since knocking out this receptor or blocking it pharmacologically suppresses caffeine-mediated arousal (El Yacoubi et al. 2000; Huang et al. 2005; Lazarus et al. 2011; Svenningsson et al. 1997). Although some of the accelerated recovery from anesthesia produced by caffeine may be mediated by adenosine receptors, the robust response observed to forskolin suggests that elevation of cAMP dramatically accelerates recovery from anesthesia. Nonetheless, future studies should explore the involvement of adenosine receptors in the ability of caffeine to accelerate recovery from anesthesia.
Optimal delivery times were not investigated in this study. Caffeine and forskolin were injected as a bolus 5 min before isoflurane administration was terminated. It may be that a different time, other than 5 min, would have produced a more robust result. At first theophylline was injected into rats 5 min before isoflurane anesthesia was terminated, but this combination produced a smaller effect than did injection of theophylline 30 min before discontinuing anesthesia. Our data suggest that theophylline may take somewhat longer to reach its target or that it may act more slowly or that both are true. We do not know whether 30 min provides the largest possible response to theophylline. Similarly, in the experiment where caffeine accelerated recovery from propofol anesthesia, both drugs were injected at the same time. Future studies will be required to determine whether this is the most efficacious timing possible and to determine optimal dosages, since only one concentration was tested.
Responses to forskolin and theophylline saturated at higher concentrations. However, the response to caffeine did not saturate. Larger concentrations, which may have produced even larger responses, were not tested to avoid potential side effects in the rats.
The data in Fig. 7 show no significant change in blood pressure or heart rate in rats injected with forskolin, theophylline or caffeine. Elevating intracellular cAMP can cause vasodilation of arteries (Rang et al. 2007), which should lower blood pressure, an undesirable side-effect for anesthesia. We were surprised when no significant change in blood pressure was observed after cAMP was elevated. Although fortuitous, this result is somewhat puzzling. It is possible that elevating cAMP levels also stimulated cardiac muscle, which increased contractile force thereby maintaining blood pressure. It may be that at the concentrations of drugs tested the two effects cancelled and there was no resulting change in vital signs. Alternatively, since vital signs were measured after anesthesia, blood vessels may have already been dilated. Similarly, emergence from anesthesia may also involve multiple targets, all sensitive to elevated cAMP. Both pre- and postsynaptic loci may be targeted.
It will be interesting to know whether caffeine accelerates recovery from anesthesia in human patients in a manner similar to that observed in rat. Because caffeine is so widely used in the general population and at doses similar to those outlined in this study and because the FDA categorizes caffeine as a “generally recognized as safe” drug, it might provide a relatively innocuous and inexpensive way to accelerate recovery from anesthesia in patients who are slow to “wake.” More important will be to identify drugs that reverse the cognitive problems associated with anesthesia. Even after emerging from anesthesia, patients can still exhibit cognitive impairment that lasts for hours. For instance 30 min after sevoflurane anesthesia patients provided 56% correct answers in a Digital Substitution Test (DSST); test subjects with a 0.1% blood alcohol level had a similar accuracy (Larsen et al. 2000). In a different study, 26% of patients were unable to complete a DSST, 2 h following termination of either sevoflurane or desflurane anesthesia, even though average eye-opening and extubation occurred within 10 min of discontinuing anesthesia (Nathanson et al. 1995). The cognitive abilities of elderly patients were impaired 3 h after anesthesia was terminated (Chen et al. 2001). Even subanesthetic doses of drugs like sevoflurane cause significant cognitive impairment (Galinkin et al. 1997; Janiszewski et al. 1999). Drugs that accelerate cognitive recovery in addition to accelerating “waking” times would be extremely useful as they would allow patients to be released more rapidly leading to better outcomes and lower costs. Future studies will be required to determine whether caffeine can reverse some of the cognitive deficits associated with anesthesia in addition to shortening waking times.
GRANTS
This study was funded by a National Institute of General Medical Sciences Grant GM-081809 (to A. P. Fox and J. Xie) as well as a Brain Research Foundation grant (to J. Xie).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Q.W., R.F., P.M., and Z.X. performed experiments; Q.W., R.F., P.M., A.P.F., and Z.X. analyzed data; Q.W., R.F., P.M., A.P.F., and Z.X. interpreted results of experiments; Q.W. and A.P.F. prepared figures; Q.W., R.F., P.M., and Z.X. edited and revised manuscript; R.F., A.P.F., and Z.X. conception and design of research; A.P.F. and Z.X. approved final version of manuscript.
REFERENCES
- Alkire MT, Asher CD, Franciscus AM, Hahn EL. Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology 110: 766–773, 2009 [DOI] [PubMed] [Google Scholar]
- Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology 107: 264–272, 2007 [DOI] [PubMed] [Google Scholar]
- Bittner EA, Yue Y, Xie Z. Brief review: anesthetic neurotoxicity in the elderly, cognitive dysfunction and Alzheimer's disease. Can J Anaesth 58: 216–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev 83: 581–632, 2003 [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci 16: 425–435, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology 116: 998–1005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets–what are the challenges? Nat Rev Drug Discov 12: 265–286, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhao M, White PF, Li S, Tang J, Wender RH, Sloninsky A, Naruse R, Kariger R, Webb T, Norel E. The recovery of cognitive function after general anesthesia in elderly patients: a comparison of desflurane and sevoflurane. Anesth Analg 93: 1489–1494, 2001 [DOI] [PubMed] [Google Scholar]
- Concas A, Santoro G, Mascia MP, Serra M, Sanna E, Biggio G. The general anesthetic propofol enhances the function of gamma-aminobutyric acid-coupled chloride channel in the rat cerebral cortex. J Neurochem 55: 2135–2138, 1990 [DOI] [PubMed] [Google Scholar]
- Crowder CM, Metz LB, Dasgupta N. UNC-13 is necessary for isoflurane sensitivity in C. elegans. Neuroscience Meeting Planner (Online) Washington, DC: Society for Neuroscience, 2005 [Google Scholar]
- Eger EI, 2nd, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology 26: 756–763, 1965 [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Menard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol 129: 1465–1473, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol 108: 671–680, 2001 [DOI] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128: e1053–1061, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Temperature dependence of the potency of volatile general anesthetics: implications for in vitro experiments. Anesthesiology 84: 716–720, 1996 [DOI] [PubMed] [Google Scholar]
- Fujita-Yoshigaki J. Divergence and convergence in regulated exocytosis: the characteristics of cAMP-dependent enzyme secretion of parotid salivary acinar cells. Cell Signal 10: 371–375, 1998 [DOI] [PubMed] [Google Scholar]
- Fujita-Yoshigaki J, Dohke Y, Hara-Yokoyama M, Furuyama S, Sugiya H. Snare proteins essential for cyclic AMP-regulated exocytosis in salivary glands. Eur J Morphol 36, Suppl: 46–49, 1998 [PubMed] [Google Scholar]
- Galinkin JL, Janiszewski D, Young CJ, Klafta JM, Klock PA, Coalson DW, Apfelbaum JL, Zacny JP. Subjective, psychomotor, cognitive, and analgesic effects of subanesthetic concentrations of sevoflurane and nitrous oxide. Anesthesiology 87: 1082–1088, 1997 [DOI] [PubMed] [Google Scholar]
- Grabner CP, Price SD, Lysakowski A, Fox AP. Mouse chromaffin cells have two populations of dense core vesicles. J Neurophysiol 94: 2093–2104, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ME, O'Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc Natl Acad Sci USA 99: 7124–7129, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology 57: 608–618, 2009 [DOI] [PubMed] [Google Scholar]
- Hemmings HC., Jr Sodium channels and the synaptic mechanisms of inhaled anaesthetics. Br J Anaesth 103: 61–69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Yan W, Westphalen RI, Ryan TA. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol 67: 1591–1599, 2005 [DOI] [PubMed] [Google Scholar]
- Herring BE, McMillan K, Pike CM, Marks J, Fox AP, Xie Z. Etomidate and propofol inhibit the neurotransmitter release machinery at different sites. J Physiol 589: 1103–1115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, Xie Z, Marks J, Fox AP. Isoflurane inhibits the neurotransmitter release machinery. J Neurophysiol 102: 1265–1273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Czernik AJ, Greengard P, Augustine GJ. Tonically active protein kinase A regulates neurotransmitter release at the squid giant synapse. J Physiol 531: 141–146, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GE, Stanley TH, Sentker CR. Physostigmine reversal of postoperative somnolence. Can Anaesth Soc J 24: 707–711, 1977 [DOI] [PubMed] [Google Scholar]
- Holz RW, Brondyk WH, Senter RA, Kuizon L, Macara IG. Evidence for the involvement of Rab3A in Ca(2+)-dependent exocytosis from adrenal chromaffin cells. J Biol Chem 269: 10229–10234, 1994 [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem 1: 74–82, 1994 [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell 79: 69–79, 1994 [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci 8: 858–859, 2005 [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem 11: 1047–1057, 2011 [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Wood JD, Kampine JP. Cholinergic reversal of isoflurane anesthesia in rats as measured by cross-approximate entropy of the electroencephalogram. Anesthesiology 99: 1125–1131, 2003 [DOI] [PubMed] [Google Scholar]
- Hudson AE, Hemmings HC., Jr Are anaesthetics toxic to the brain? Br J Anaesth 107: 30–37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiszewski DJ, Galinkin JL, Klock PA, Coalson DW, Pardo H, Zacny JP. The effects of subanesthetic concentrations of sevoflurane and nitrous oxide, alone and in combination, on analgesia, mood, and psychomotor performance in healthy volunteers. Anesth Analg 88: 1149–1154, 1999 [DOI] [PubMed] [Google Scholar]
- Jankowski JA, Schroeder TJ, Holz RW, Wightman RM. Quantal secretion of catecholamines measured from individual bovine adrenal medullary cells permeabilized with digitonin. J Biochem 267: 18329–18335, 1992 [DOI] [PubMed] [Google Scholar]
- Jenne JW. Theophylline as a bronchodilator in COPD and its combination with inhaled beta-adrenergic drugs. Chest 92: 7S–14S, 1987 [PubMed] [Google Scholar]
- Jones MV, Brooks PA, Harrison NL. Enhancement of gamma-aminobutyric acid-activated Cl− currents in cultured rat hippocampal neurones by three volatile anaesthetics. J Physiol 449: 279–293, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Harrison NL. Effects of volatile anesthetics on the kinetics of inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurophysiol 70: 1339–1349, 1993 [DOI] [PubMed] [Google Scholar]
- Kasai H, Takahashi N, Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev 92: 1915–1964, 2012 [DOI] [PubMed] [Google Scholar]
- Kips JC, Peleman RA, Pauwels RA. The role of theophylline in asthma management. Curr Opin Pulm Med 5: 88–92, 1999 [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron 27: 133–143, 2000 [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two synaptic vesicle pools, vesicle recruitment and replenishment of pools at the Drosophila neuromuscular junction. J Neurocytol 32: 551–565, 2003 [DOI] [PubMed] [Google Scholar]
- Larsen B, Seitz A, Larsen R. Recovery of cognitive function after remifentanil-propofol anesthesia: a comparison with desflurane and sevoflurane anesthesia. Anesth Analg 90: 168–174, 2000 [DOI] [PubMed] [Google Scholar]
- Laurenza A, Sutkowski EM, Seamon KB. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci 10: 442–447, 1989 [DOI] [PubMed] [Google Scholar]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci 31: 10067–10075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Messing RO. Protein kinases and addiction. Ann NY Acad Sci 1141: 22–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Petropoulos S, Shen B, Luo T, Herrick I, Rajakumar N, Ma J. Lesion of cholinergic neurons in nucleus basalis enhances response to general anesthetics. Exp Neurol 228: 259–269, 2011 [DOI] [PubMed] [Google Scholar]
- Li Q, Ho CS, Marinescu V, Bhatti H, Bokoch GM, Ernst SA, Holz RW, Stuenkel EL. Facilitation of Ca(2+)-dependent exocytosis by Rac1-GTPase in bovine chromaffin cells. J Physiol 550: 431–445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science 256: 677–679, 1992 [DOI] [PubMed] [Google Scholar]
- Lonart G, Janz R, Johnson KM, Sudhof TC. Mechanism of action of rab3A in mossy fiber LTP. Neuron 21: 1141–1150, 1998 [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Sudhof TC, Linden DJ. Phosphorylation of RIM1alpha by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell 115: 49–60, 2003 [DOI] [PubMed] [Google Scholar]
- Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology 111: 725–733, 2009 [DOI] [PubMed] [Google Scholar]
- Machado JD, Morales A, Gomez JF, Borges R. cAMP modulates exocytotic kinetics and increases quantal size in chromaffin cells. Mol Pharmacol 60: 514–520, 2001 [PubMed] [Google Scholar]
- Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology 93: 708–717, 2000 [DOI] [PubMed] [Google Scholar]
- Nathanson MH, Fredman B, Smith I, White PF. Sevoflurane versus desflurane for outpatient anesthesia: a comparison of maintenance and recovery profiles. Anesth Analg 81: 1186–1190, 1995 [DOI] [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 17: 139–170, 1992 [DOI] [PubMed] [Google Scholar]
- Pascoli V, Valjent E, Corbille AG, Corvol JC, Tassin JP, Girault JA, Herve D. cAMP and extracellular signal-regulated kinase signaling in response to d-amphetamine and methylphenidate in the prefrontal cortex in vivo: role of beta 1-adrenoceptors. Mol Pharmacol 68: 421–429, 2005 [DOI] [PubMed] [Google Scholar]
- Plourde G, Chartrand D, Fiset P, Font S, Backman SB. Antagonism of sevoflurane anaesthesia by physostigmine: effects on the auditory steady-state response and bispectral index. Br J Anaesth 91: 583–586, 2003 [DOI] [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM, Flower RJ. Rang and Dale's Pharmacology (6th ed). New York: Elsevier Health Sciences, 2007 [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. J Neurosci 23: 8608–8617, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastiao AM, de Mendonca A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 68: 377–392, 2002 [DOI] [PubMed] [Google Scholar]
- Run X, Liang Z, Gong CX. Anesthetics and tau protein: animal model studies. J Alzheimers Dis 22, Suppl 3: 49–55, 2010 [DOI] [PubMed] [Google Scholar]
- Saifee O, Metz LB, Nonet ML, Crowder CM. A gain-of-function mutation in adenylate cyclase confers isoflurane resistance in Caenorhabditis elegans. Anesthesiology 115: 1162–1171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature 424: 775–778, 2003 [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Preferential potentiation of fast-releasing synaptic vesicles by cAMP at the calyx of Held. Proc Natl Acad Sci USA 98: 331–336, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 85: 1303–1342, 2005 [DOI] [PubMed] [Google Scholar]
- Simonds WF. G protein regulation of adenylate cyclase. Trends Pharmacol Sci 20: 66–73, 1999 [DOI] [PubMed] [Google Scholar]
- Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology 115: 791–803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt K, Forman SA. Correlating the clinical actions and molecular mechanisms of general anesthetics. Curr Opin Anaesthesiol 20: 300–306, 2007 [DOI] [PubMed] [Google Scholar]
- Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth 105, Suppl 1: i61–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Ongini E, Fredholm BB. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience 79: 753–764, 1997 [DOI] [PubMed] [Google Scholar]
- Tai SK, Ma J, Leung LS. Medial septal cholinergic neurons modulate isoflurane anesthesia. Anesthesiology 2013. August 21 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kadowaki T, Yazaki Y, Ellis-Davies GC, Miyashita Y, Kasai H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic beta cells. Proc Natl Acad Sci USA 96: 760–765, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology 118: 30–39, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Emery DG, Haydon PG. Direct modulation of the secretory machinery underlies PKA-dependent synaptic facilitation in hippocampal neurons. Neuron 17: 789–797, 1996 [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Fang Y, Haydon PG. Modulation of an early step in the secretory machinery in hippocampal nerve terminals. Proc Natl Acad Sci USA 95: 7163–7168, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Parpura V, Haydon PG. Activation of neurotransmitter release in hippocampal nerve terminals during recovery from intracellular acidification. J Neurophysiol 81: 2627–2635, 1999 [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Saifee O, Shebester L, Roberson R, Nonet ML, Crowder CM. A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc Natl Acad Sci USA 96: 2479–2484, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 265: 1878–1882, 1994 [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Desai KM, Hemmings HC., Jr Presynaptic inhibition of the release of multiple major central nervous system neurotransmitter types by the inhaled anaesthetic isoflurane. Br J Anaesth 110: 592–599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Gomez RS, Hemmings HC., Jr Nicotinic receptor-evoked hippocampal norepinephrine release is highly sensitive to inhibition by isoflurane. Br J Anaesth 102: 355–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegar BD, MacIver MB. Isoflurane depresses hippocampal CA1 glutamate nerve terminals without inhibiting fiber volleys. BMC Neurosci 7: 5, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Herring BE, Fox AP. Excitatory and inhibitory actions of isoflurane in bovine chromaffin cells. J Neurophysiol 96: 3042–3050, 2006 [DOI] [PubMed] [Google Scholar]