Abstract
When new motor learning changes neurons and synapses in the spinal cord, it may affect previously learned behaviors that depend on the same spinal neurons and synapses. To explore these effects, we used operant conditioning to strengthen or weaken the right soleus H-reflex pathway in rats in which a right spinal cord contusion had impaired locomotion. When up-conditioning increased the H-reflex, locomotion improved. Steps became longer, and step-cycle asymmetry (i.e., limping) disappeared. In contrast, when down-conditioning decreased the H-reflex, locomotion did not worsen. Steps did not become shorter, and asymmetry did not increase. Electromyographic and kinematic analyses explained how H-reflex increase improved locomotion and why H-reflex decrease did not further impair it. Although the impact of up-conditioning or down-conditioning on the H-reflex pathway was still present during locomotion, only up-conditioning affected the soleus locomotor burst. Additionally, compensatory plasticity apparently prevented the weaker H-reflex pathway caused by down-conditioning from weakening the locomotor burst and further impairing locomotion. The results support the hypothesis that the state of the spinal cord is a “negotiated equilibrium” that serves all the behaviors that depend on it. When new learning changes the spinal cord, old behaviors undergo concurrent relearning that preserves or improves their key features. Thus, if an old behavior has been impaired by trauma or disease, spinal reflex conditioning, by changing a specific pathway and triggering a new negotiation, may enable recovery beyond that achieved simply by practicing the old behavior. Spinal reflex conditioning protocols might complement other neurorehabilitation methods and enhance recovery.
Keywords: H-reflex, operant conditioning, spinal cord plasticity, motor control, spinal cord injury
motor learning is usually ascribed to plasticity in the cortex or elsewhere in the brain, but not in the spinal cord, which has traditionally been assumed to be simply a hard-wired structure for producing learned behaviors that depend on plasticity in the brain (Dayan and Cohen 2011; Doyon et al. 2009; Penhune and Steele 2012; Wolpaw 2010). However, studies in animals and humans indicate that motor learning often changes the spinal cord (reviewed in Wolpaw 2010; Wolpaw and Tennissen 2001). For example, spinal stretch reflexes are markedly reduced in professional ballet dancers (Nielsen et al. 1993). Spinal reflexes also change during the acquisition of much simpler behaviors (e.g., Meyer-Lohmann et al. 1986; Schneider and Capaday 2003).
Because the spinal cord is the final common pathway for all motor behaviors, spinal cord plasticity that contributes to new learning can affect previously learned behaviors. Normally these effects are not harmful. Even though the reflex pathways that are weakened in ballet dancers are important for walking (Bennett et al. 1996; Grey et al. 2007; Nielsen and Sinkjaer 2002; Pearson 2004; Sinkjaer et al. 2000; Stein et al. 2000; Yang et al. 1991), the dancers continue to walk satisfactorily. Similarly, walking is still symmetrical in normal rats in which an important reflex pathway has been weakened or strengthened in one leg (Chen et al. 2005). Walking is nevertheless affected. Ballet dancers are easily recognized because they stand and walk differently from other people (e.g., Kilgannon 1996; PBS 2011; Royal Opera House 2012). In normal rats in which operant conditioning has changed the soleus reflex pathway, the kinematics of locomotion and the reflexes of other muscles are also changed, and plasticity occurs at multiple sites in the spinal cord and the brain (Wolpaw 2010; Wolpaw and Chen 2009 for review).
Thus, when new learning changes the spinal cord, it affects older behaviors, and it produces widespread plasticity. We propose that much of this plasticity is compensatory: it is induced when the plasticity responsible for the new behavior disturbs older behaviors, and it serves to preserve these older behaviors. As new learning modifies the spinal cord, older behaviors undergo concurrent relearning. The activity-dependent adaptive process that creates both the plasticity serving the new behavior and the plasticity preserving older behaviors is essentially a negotiation between the requirements of the new and old behaviors. The result is a new spinal cord equilibrium, a “negotiated equilibrium” (Wolpaw 2010) that accommodates both the new behavior and the old. Furthermore, we propose that the target of this negotiation is optimization of the key features of the behaviors, such as a symmetrical step cycle (i.e., walking without a limp), rather than preservation of their muscular and kinematic details.
This study tested the negotiated equilibrium hypothesis. In rats in which an incomplete spinal cord injury had impaired locomotion, an operant conditioning protocol either strengthened or weakened the soleus H-reflex pathway. Given the pathway's role in locomotion, a stronger pathway was expected to improve locomotion, while a weaker pathway was expected to worsen it. In contrast, the hypothesis predicted that a stronger pathway would improve locomotion, but that compensatory plasticity would prevent a weaker pathway from further impairing it. The study confirms this prediction. Furthermore, muscular and kinematic analyses provide insight into the responsible mechanisms. The results illuminate the important role of the spinal cord in motor learning, and they support recent studies indicating that motor learning protocols that change the spinal cord can improve locomotion after spinal cord injury in both animals and humans (Chen et al. 2006a; Thompson et al. 2013).
METHODS
Subjects were 16 young adult Sprague-Dawley rats (4 males and 12 females) weighing 210–286 g [mean 252 (±26 SD) g] at the beginning of the study. [H-reflex conditioning is comparable in male and female rats (Chen and Wolpaw 1995, 1996, 1997, 2002, 2005, Chen et al. 1996, 2005, 2006a, 2006b, 2006c).] All procedures satisfied the “Guide for the Care and Use of Laboratory Animals” (National Academies Press, Washington, DC, 2011) and had been reviewed and approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. The procedures for spinal cord injury, electrode implantation, treadmill locomotion, H-reflex conditioning, histological evaluation, and data analysis have been described in detail previously (Chen and Wolpaw 1995, 1997, 2002; Chen et al. 2005, 2006a) and are summarized here. Figure 1A shows the study protocol.
Fig. 1.
A: experimental schedule. After the rat learned to walk on the treadmill, the right side of the midthoracic (T9) spinal cord received a calibrated contusion injury. Twenty days later, the rat was implanted with soleus EMG electrodes and nerve-cuff stimulating electrodes. Beginning at least 30 days later (i.e., at least 50 days after the injury), H-reflex data were collected under the control mode for 20 days and then under the up-conditioning (HRup) or down-conditioning (HRdown) mode for 50 days. Locomotion was assessed during the control-mode exposure and at the end of up- or down-conditioning. The impact of conditioning on the protocol H-reflex was determined by comparing the average daily H-reflex for the last 10 days of control-mode exposure (i.e., the initial H-reflex) to that for the last 10 days of conditioning. (Thick x-axis lines indicate these two 10-day periods.) B: measurement of right hindlimb ankle, knee, and hip angles. (Hip angle is the angle between the thigh and a horizontal line.) C: transverse section of the spinal cord at T9 from a representative rat. The right lateral column (LC) lesion is apparent. The dorsal columns [including the dorsal ascending tracts (DA) and the corticospinal tracts (CST)], the ventral columns (VC), and the left LC are not damaged.
Lateral Column Contusion
Under general anesthesia, each animal received a calibrated contusion injury to the right lateral column (LC) of the spinal cord at T9 using an Infinite Horizon Spinal Cord Impactor (Precision Systems and Instrumentation). The animal was anesthetized with an intraperitoneal injection of ketamine HCl (80 mg/kg) and xylazine (10 mg/kg). A partial dorsal laminectomy was performed at T9 with minimal disturbance of the dural envelope. The animal was then mounted on the impactor with the nearby dorsal processes rigidly fixed. The injury was performed at a force setting of 200 kdyn with a 1-mm impactor tip positioned on the dorsal surface of the spinal cord halfway from the midline to the right lateral edge. After the injury, the site was rinsed with normal saline and covered with Durafilm to minimize connective tissue adhesions to the dura, and the muscle and skin were sutured in layers. Care in the days immediately after the contusion injury included analgesia, antibiotics, bladder expression, and high-calorie dietary supplementation as previously described in detail (Chen and Wolpaw 1997, 2002). Bladder function returned within 2–5 days. Open-field locomotion was assessed according to the rating scale of Basso et al. (1995) [i.e., the Basso, Beattie, and Bresnahan (BBB) test]. In all animals, the BBB score had returned to normal or nearly normal (i.e., ≥20) by 12 days. [The recovery of a normal BBB score does not necessarily mean that locomotion is entirely normal. Indeed, kinematic and EMG analyses detected persistent abnormalities (see below and Chen et al. 2006).]
Electrode Implantation
At least 20 days after the LC injury, each rat was implanted under the same general anesthesia with chronic stimulating and recording electrodes, as described previously (Chen and Wolpaw 1995, 1997, 2002; Chen et al. 2001, 2002, 2005, 2006a). To elicit the right soleus H-reflex, a silicone rubber nerve cuff containing a pair of fine-wire electrodes was placed around the posterior tibial (PT) nerve just above the triceps surae branches. To record EMG activity, a pair of fine-wire EMG electrodes with final 0.5-cm segments stripped and separated by 0.2–0.3 cm was placed in the right soleus muscle. In seven rats, a second pair of fine-wire electrodes was placed in the left soleus muscle. The wires from the nerve cuff and the muscles were led subcutaneously to a connector plug mounted on the skull. Finally, small (2-mm) dots were tattooed on the skin over the lateral aspects of the ankle, knee, and hip joints bilaterally to facilitate three-dimensional (3D) video reconstruction of locomotion.
After surgery, the rat was kept warm and given an analgesic (Demerol, 0.2 mg, intramuscular) and was returned to its cage and allowed to eat and drink freely. Care in the days immediately after the implantation surgery included analgesia, antibiotics, and high-calorie dietary supplementation, as previously described in detail (Chen and Wolpaw 1997, 2002; Chen et al. 1996, 1999, 2001, 2002, 2005, 2006a).
Soleus H-Reflex Monitoring and Conditioning
Data collection started at least 30 days after the implantation surgery (i.e., at least 50 days after the injury) and continued 24 h/day, 7 days/wk for at least 70 days (Fig. 1A). During this period, each rat lived in a standard rat cage with a 40-cm flexible cable attached to the skull plug. The cable, which allowed the animal to move freely in the cage, conveyed the wires from the electrodes to a commutator above the cage, and from there to EMG amplifiers and a stimulus isolation unit. The rat had free access to water and food, except that during H-reflex conditioning it received food mainly by performing the task described below. Animal well-being was carefully checked several times each day, and body weight was measured weekly. Laboratory lights were dimmed from 2100 to 0600 each day.
Stimulus delivery and data collection were under the control of a computer system, which monitored ongoing EMG activity (gain 1,000, band-pass 100–1,000 Hz, sample rate 5,000 Hz) continuously 24 h/day, 7 days/wk, for the entire period of data collection. Whenever the absolute value (equivalent to the full-wave-rectified value) of right soleus background (i.e., ongoing) EMG activity remained within a defined range for a randomly varying 2.3- to 2.7-s period, the computer initiated a trial. In each trial, the computer stored the most recent 50 ms of EMG activity from the muscle (i.e., the background EMG interval), delivered a monophasic stimulus pulse to the nerve cuff, and stored the EMG activity for another 100 ms. Stimulus pulse amplitude and duration were initially set to produce a maximum H-reflex (as well as an M response that was typically just above threshold). In each rat, pulse duration remained fixed (typically 0.5 ms) throughout study. Pulse amplitude was adjusted by the computer after each trial to maintain the right soleus M response [i.e., average absolute value of EMG activity in the M response interval (typically 1.5–4.0 ms after PT nerve stimulation)] unchanged at a target size. This ensured that the effective strength of the nerve stimulus was stable throughout the experiment despite any changes that occurred in nerve-cuff electrode impedances or in other factors (Wolpaw 1987; Chen and Wolpaw 1995). H-reflex size was calculated as average absolute value of EMG activity in the H-reflex interval (typically 5.5–10.0 ms after stimulation) minus average absolute value of background EMG activity at the time of stimulation, and was expressed in units of average absolute value of background EMG activity at the time of stimulation.
To determine the initial size of the H-reflex, data were collected for 2–3 wk under the control mode, in which the computer simply digitized and stored soleus EMG activity for 100 ms following the stimulus. Then, each rat was exposed to right soleus H-reflex up-conditioning (HRup rats) or down-conditioning (HRdown rats) for 50 days. Under the up- or down-conditioning mode, the computer gave a reward (i.e., a 20-mg food pellet) 200 ms after PT nerve stimulation, if the absolute value of right soleus EMG activity in the H-reflex interval was above (up-conditioning) or below (down-conditioning) a criterion value. The criterion value was set and adjusted as needed each day so that the rat received an amount of food that met its daily requirement [e.g., about 700 reward pellets (i.e., 14 g) per day for a 300-g rat].
Locomotor Data Collection
Prior to the contusion injury, each rat learned to walk quadripedally on a motor-driven treadmill at 9–12 m/min (Chen et al. 2005, 2006a). During the period of data collection, locomotor data were collected from each rat in two treadmill sessions (Fig. 1A): the first session at the end of the control-mode exposure before soleus HRup or HRdown began; and the second session at the end of conditioning. In each rat, treadmill speed was the same for both sessions. During locomotion, EMG was continuously recorded (bandpass 100–1,000 Hz) from both soleus muscles (7 rats) or from the right soleus muscle only (9 rats), digitized (4,000 Hz), and stored. In the rats in which only the right soleus was implanted with EMG electrodes, locomotor kinematics were assessed bilaterally with a 3D video data collection and analysis system (Vicon Motion Systems).
In each treadmill session, data were collected under two conditions. One condition was undisturbed locomotion. In the other condition, the right soleus H-reflex was elicited by stimulating the PT nerve just after the middle of the stance phase (i.e., the “locomotor H-reflex”) as described in Chen et al. (2005, 2006a).
The EMG activity recorded during undisturbed locomotion was rectified, low-pass filtered, and used to calculate soleus locomotor burst amplitudes (i.e., EMG area between burst onset and offset) (Chen et al. 2005, 2006a). In the rats not studied with the 3D video analysis system, the EMG activity during undisturbed locomotion was also used to calculate the following: step-cycle duration [i.e., time in seconds between one right (or left) soleus burst onset and the next right (or left) soleus burst onset]; step-cycle length [i.e., the distance covered by a complete step-cycle, calculated as treadmill speed (in m/s) multiplied by step-cycle duration (in s)]; and step-cycle symmetry, defined as the ratio of the time from right soleus burst onset (RBO) to left soleus burst onset (LBO) to the time from LBO to RBO (i.e., a ratio of 1.00 indicates that the right/left timing of the step cycle is symmetrical).
The 3D locomotor kinematic data were analyzed with the Vicon Motus software (Vicon Motion Systems) to determine the following: step-cycle duration (i.e., average time between right foot contacts); step-cycle length [i.e., the distance covered by a complete step-cycle, calculated as treadmill speed (in m/s) multiplied by step-cycle duration (in s)]; and step-cycle symmetry, defined as the ratio of the time from right foot contact (RFC) to left foot contact (LFC) to the time from LFC to RFC (i.e., a ratio of 1.00 indicates that the right/left timing of the step cycle is symmetrical). In addition, the kinematic data were used to determine the average right and left ankle, knee, and hip joint angles and hip heights over the step-cycle (Fig. 1B). (The moment-to-moment angle measurements could be affected by movements of the tattooed dots relative to the underlying joints as the joints flexed and extended over the step-cycle. To minimize the impact of such movements on the data, the values reported here are average values for the entire step-cycle.)
The locomotor H-reflexes were measured during the late stance phase of the step-cycle as described in Chen et al. (2005). Just as for the H-reflexes elicited in the conditioning protocol (i.e., “protocol H-reflexes”), locomotor H-reflex sizes were calculated as average absolute value of EMG activity in the H-reflex interval minus average absolute value of background EMG activity at the time of stimulation, and were expressed in units of average absolute value of background EMG activity at the time of stimulation. (H-reflex conditioning could affect locomotor burst amplitude, and thus the level of background EMG activity when the locomotor H-reflex was elicited. Nevertheless, the normally wide intertrial variation in burst amplitude allowed offline analysis to compare pre- and postconditioning locomotor H-reflexes elicited at the same average level of background EMG activity by comparing pre- and postconditioning trials for which background EMG activity fell within the same range.)
Statistical Analysis
The data consist of H-reflex sizes, locomotor EMG activity, and locomotor kinematics. They fall into five categories: 1) H-reflex sizes measured throughout the day whenever the rats satisfied the background EMG criteria (i.e., referred to as “protocol H-reflexes”); 2) H-reflex sizes measured during the late stance phase of locomotion (i.e., referred to as “locomotor H-reflexes”); 3) soleus locomotor EMG burst amplitudes; 4) ankle, knee, and hip joint angles and hip heights during locomotion; and 5) putative key locomotor features (i.e., step-cycle length and step-cycle right/left timing symmetry). The primary objective of this study was to determine the impact of HRup or HRdown conditioning on locomotion in rats in which locomotion was impaired due to spinal cord injury. To do this, we compared the data prior to conditioning to that at the end of HRup or HRdown conditioning by paired t-test.
Perfusion, Postmortem Examination, and Lesion Verification
At the end of data collection (or, for some rats, after a subsequent reexposure to the control mode for a concurrent study), each rat was anesthetized, the soleus muscle was injected with cholera toxin subunit B-conjugated Alexa Fluor 488 (for retrograde labeling of soleus motoneurons), and 3 days later the rat was perfused through the heart for postmortem examination and lesion verification [and for ongoing anatomical and immunohistochemical studies reported elsewhere (e.g., Wang et al. 2006, 2009)]. The nerve cuff, EMG electrodes, and tibial nerve were examined, and the soleus muscles of both sides were removed and weighed. The spinal cord was removed, and blocks encompassing the lesion were embedded in paraffin. Transverse 20-μm-thick serial sections from the paraffin-embedded blocks were processed and used to determine the location and size of the lesions as previously described (Chen and Wolpaw 1997, 2002; Chen et al. 2002).
RESULTS
Animals remained healthy and active throughout data collection. Body weight increased from 210–286 g [252 (±26 SD) g] at the time of injury, to 240–345 g [292 (±33) g] at the time of implantation surgery, to 264–528 g [382 (±81) g] at the time of perfusion. Right and left soleus muscle weights (measured as percentage of body weight), averaged 0.048 (±0.002 SE)% for the right and 0.047 (±0.002)% for the left. They did not differ significantly from each other (P = 0.39 by paired t-test) and did not differ from soleus muscle weights of normal rats (Chen and Wolpaw 1995, 1997, 2002; Chen et al. 1996, 1999, 2001, 2002, 2005, 2006a). Examination of the nerve cuffs revealed the expected connective tissue investment of the wires and apparent good preservation of the nerve inside the cuff.
Histology
Quantitative analysis indicated that the T9 contusion injury was confined largely or totally to the right LC. On average, 42 (±17 SD)% (range: 12–74%) of the right LC remained. Other white-matter tracts were completely or almost completely intact. Specifically, 100% of the left LC, the left dorsal column corticospinal tract, the left dorsal column ascending tract, and the right and left ventral columns, 96 (±8)% of the right corticospinal tract, and 94 (±18)% of the right dorsal column ascending tract remained intact. Figure 1C shows a T9 transverse section from a representative rat.
The New Learning: Larger or Smaller Protocol H-reflexes
As expected (Chen and Wolpaw 1995; Wolpaw and Chen 2009), exposure to the HRup or HRdown mode produced a gradual mode-appropriate change in protocol H-reflex size (i.e., the size of the H-reflex elicited in the conditioning protocol). By days 41–50, H-reflex conditioning was successful [i.e., protocol H-reflex size change ≥20% in the correct direction (Chen and Wolpaw 1995; Wolpaw et al. 1993)] in each of the 14 (9 up and 5 down) rats described here. [Data from two rats in which conditioning was not successful (i.e., the protocol H-reflex changed <20%) are not included here because they are not relevant to the goal of the study: to determine the impact of operantly conditioned soleus H-reflex increase or decrease on locomotion in spinal-cord-injured rats. In brief, for these two rats, locomotor measures did not differ before and after exposure to up-conditioning.]
In the HRup rats, protocol H-reflex size at the end of up-conditioning (i.e., average of last 10 days) averaged 239 (±47 SE)% of initial size (P < 0.02 vs. initial by paired t-test). In the HRdown rats, protocol H-reflex size at the end of down-conditioning averaged 50 (±10)% of initial size (P < 0.01 vs. initial). Figure 2A (left) summarizes these results.
Fig. 2.
Effects of reflex conditioning on H-reflexes and locomotor bursts. A: average (±SE) protocol H-reflexes, locomotor H-reflexes, and soleus locomotor bursts from HRup and HRdown rats at the end of conditioning. *P < 0.05, **P < 0.01 vs. initial (i.e., preconditioning) value. B: data from individual HRup and HRdown rats. Average poststimulus EMG elicited in the conditioning protocol (left) or during locomotion (middle), and average soleus locomotor bursts (right), in the control mode (solid lines) and at the end of conditioning (dashed lines) are shown. M responses and H-reflexes are indicated.
Figure 2B (left) shows average daily peristimulus soleus EMG from a representative HRup rat (top) and an HRdown rat (bottom) for 1 day before conditioning (i.e., initial, solid line) and 1 day at the end of conditioning (i.e., after conditioning, dashed line). The protocol H-reflex at the end of conditioning is larger than the initial protocol H-reflex in the HRup rat and smaller in the HRdown rat. Background EMG and M response amplitude do not change.
The Previously Learned Behavior: Locomotion
Our goal was to determine the impact of HRup or HRdown on locomotion, and, at the same time, to understand the muscular and kinematic basis of the impact. Thus we analyzed locomotion on four successively higher levels, from the function of the H-reflex pathway during locomotion to key features of the complete behavior. The measurements made at each level were those that previous studies indicated were affected or were likely to be affected by H-reflex conditioning, and thus were most likely to clarify the interactions between the new learning and the older behavior.
At the most reduced level and the level most directly connected to the new learning, we measured the locomotor H-reflex, that is, the H-reflex during the stance phase of locomotion. At the next level up, the impact of the change in the H-reflex pathway was assessed by measuring the amplitude of the soleus locomotor burst, to which the H-reflex pathway normally contributes (Bennett et al. 1996; Grey et al. 2007; Nielsen and Sinkjaer 2002; Pearson 2004; Sinkjaer et al. 2000; Stein et al. 2000; Yang et al. 1991). Previous studies in normal rats showed that change in this burst parallels conditioned change in the H-reflex (Chen et al. 2005, 2006a). At the next level up, the kinematic impact of conditioning was assessed by measuring ankle, knee, and hip angles and hip heights. Previous studies in normal rats indicated that H-reflex change (presumably through its effect on the soleus locomotor burst) changed ankle angle, and that opposite changes in hip angle prevented a corresponding change in hip height (Chen et al. 2011). Finally, at the highest level, locomotion itself was assessed in terms of two putative key features: step-cycle length and right/left step symmetry. Previous studies had shown that H-reflex conditioning did not affect symmetry in normal rats but could reduce an asymmetry produced by a spinal cord injury in both rats and humans (Chen et al. 2005, 2006a; Thompson et al. 2013). Together, these successively higher levels of analysis sought to explain the impact of H-reflex conditioning on the complete behavior.
Locomotor H-reflexes.
Figure 2, A and B (middle), summarizes and illustrates the impact of up-conditioning and down-conditioning on the H-reflexes elicited during the late stance phase of locomotion (i.e., the locomotor H-reflexes). These H-reflexes displayed changes similar to those of the H-reflexes elicited in the conditioning protocols [i.e., the protocol H-reflexes; Fig. 2, A and B (left)]. In HRup rats, the locomotor H-reflex averaged 324 (±65)% of initial size at the end of conditioning (P < 0.01 vs. initial). In HRdown rats, it averaged 38 (±9)% at the end of conditioning (P < 0.01). These changes were consistent with those previously found in normal rats subjected to HRup or HRdown conditioning (Chen et al. 2005, 2011). They indicate that the altered functioning of the H-reflex pathway produced by the conditioning protocol was also present during locomotion.
Locomotor EMG activity.
Soleus locomotor activity consisted of a burst that coincided with and contributed to the stance phase of the step cycle. Figure 2, A and B (right), summarize and illustrate the effect of up-conditioning or down-conditioning on burst amplitude.
Up-conditioning markedly increased the right soleus burst: in HRup rats at the end of conditioning the burst averaged 170 (±31)% of its initial size (P < 0.05 vs. initial). This effect is similar to that previously found in normal rats subjected to soleus HRup (Chen et al. 2005). It appears to reflect the increased excitation provided by the up-conditioned H-reflex pathway. In contrast, down-conditioning did not decrease the burst. In HRdown rats at the end of conditioning, the burst averaged 110 (±9)% of initial size (P = 0.34). This result differs from that in normal rats subjected to down-conditioning (Chen et al. 2005).
Thus, in these spinal cord-injured rats, the stronger H-reflex pathway produced by up-conditioning increased the soleus locomotor burst. However, the weaker H-reflex pathway produced by down-conditioning did not decrease the burst. The smaller locomotor H-reflexes show that the H-reflex pathway remained weak during locomotion; nevertheless, this weakening did not have the corresponding effect on the locomotor burst expected from basic physiology and previous H-reflex conditioning studies (Chen et al. 2005, 2006a). The possible origins of this discrepancy are considered in the discussion.
Locomotor kinematics.
Table 1 shows the average right and left ankle, knee, and hip angles and hip heights (in percent of initial values) in HRup and HRdown rats during locomotion before and after conditioning. As it indicates, up-conditioning markedly increased right ankle angle (P < 0.001) and also increased right knee angle (P < 0.01), but had no significant effect on hip angle (P = 0.08). The increase in ankle angle is somewhat greater than that previously found in normal rats (Chen et al. 2011) and is consistent with the increased soleus locomotor burst. The increases in ankle and knee angles, in the absence of decrease in hip angle, meant that the right leg was, on the average, straighter during locomotion, which is consistent with the significant increase in right hip height (P < 0.05).
Table 1.
Average (SE) kinematic measures (right ankle, knee, and hip angles and hip height) and locomotor features (step length and right/left symmetry) before and after right soleus H-reflex up-conditioning or down-conditioning
| HRup |
HRdown |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Kinematic measures | ||||
| Ankle angle, ° | 69 (2) | 91 (1)‡ | 82 (4) | 70 (5) |
| Knee angle, ° | 83 (6) | 92 (6)† | 80 (5) | 76 (6) |
| Hip angle, ° | 44 (7) | 41 (6) | 39 (4) | 43 (5)* |
| Hip height, mm | 55 (3) | 58 (2)* | 54 (3) | 53 (3) |
| Locomotor features | ||||
| Step length, mm | 84 (2) | 94 (2)* | 94 (3) | 96 (4) |
| R/L symmetry | 91 (3) | 101 (2)‡ | 95 (7) | 99 (9) |
Values are averages (SE). HRup and HRdown, H-reflex up- or down-conditioning, respectively. R/L symmetry, ratio of the time from right foot contact (RFC) to left foot contact (LFC) to the time from LFC to RFC (in %). Thus 100% indicates a symmetrical step cycle. Significant differences from the before values are indicated (*P < 0.05, †P < 0.01, ‡P < 0.001). Up-conditioning is associated with increases in ankle angle, knee angle, and hip height, and with improvements in step length and R/L symmetry. Down-conditioning is associated only with an increase in hip angle.
Down-conditioning had no significant effect on ankle or knee angles, increased right hip angle (P < 0.02), and did not affect hip heights. The right ankle angle result was consistent with the lack of effect on the soleus locomotor burst, and it contrasted with the result in normal rats, in which down-conditioning markedly decreased right ankle angle (Chen et al. 2011).
Locomotion.
Based on the assumption that the distance covered by a step-cycle and the right/left symmetry of the step-cycle are important features of normal locomotion (see discussion), and on previous animal and human evidence that symmetry is impaired by lateralized spinal cord lesions and can be restored by appropriate H-reflex conditioning (Chen et al. 2006a; Thompson et al. 2013), we assessed locomotion in terms of the following: step-cycle length (the distance covered by a complete step-cycle); and right/left step symmetry, defined as the ratio of time from RFC to LFC to the time from LFC to RFC [or, in the rats not studied with the Vicon system, the ratio of time from RBO to LBO to the time from LBO to RBO (Chen et al. 2005)]. If the ratio was 1.00, the step cycle was symmetrical; if it was not, the rat was limping.
As summarized in Table 1, up-conditioning significantly increased step length (P < 0.02), while down-conditioning had no significant effect (P = 0.47).
In these spinal cord-injured rats before H-reflex conditioning, the right/left timing of the step cycle was asymmetrical: the ratio of the time from RFC to LFC to the time from LFC to RFC was significantly less than the normal value of 1.0 (P < 0.02 by one-sample t-test). This asymmetry was similar to, although smaller than, that produced by transection (rather than contusion) of the right LC (Chen et al. 2006a). The right stance phase was shorter than the left, indicating that the rats were limping. Consistent with its impact on the right soleus H-reflex pathway, up-conditioning strengthened right stance and eliminated the asymmetry (P < 0.001; Table 1). The role of the increased right locomotor burst in producing this effect is supported by the significant correlation of right burst amplitude with the duration of right stance (i.e., time from RFC to LFC) in every up-conditioned rat [average r = +0.54 (range +0.19 to +0.71); P < 0.002 for each rat].
In contrast, down-conditioning had no significant effect on step-cycle symmetry (P = 0.34; Table 1). Despite its impact on the H-reflex pathway, it did not shorten right stance.
Figure 3A illustrates the impact of up-conditioning on step length and right/left symmetry. Each plot shows five complete step cycles. The solid and open circles mark RFCs and LFCs, respectively, and the dashed vertical lines mark midpoints between RFCs (i.e., the points when LFCs should occur). Prior to conditioning, the LFCs occurred before the midpoints, indicating that right stance was inadequately sustained and the rat was limping. After up-conditioning, the LFCs occurred close to the midpoints between the RFCs, indicating that walking had become symmetrical. Furthermore, up-conditioning increased the distance covered by the five step cycles shown (i.e., it increased step length).
Fig. 3.
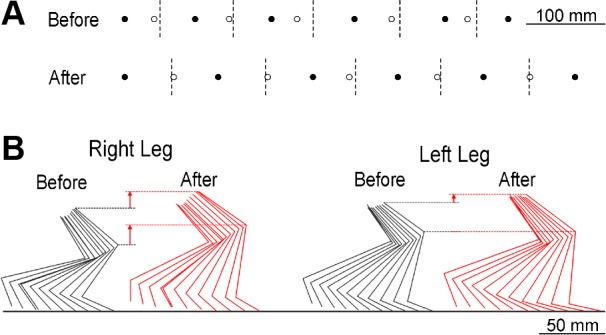
Impact of H-reflex up-conditioning on locomotion. A: step-cycle symmetry and length in a rat before and after up-conditioning. Five step-cycles are shown as the rat walks toward the right. Solid and open circles are the right and left foot contacts (RFCs and LFCs), respectively. Dashed vertical lines are halfway between RFCs, which is where LFCs should occur. Before conditioning, LFCs occur too soon, indicating that the rat is limping (due to weak right stance). After conditioning, LFCs occur in the correct place, indicating that the step-cycle is symmetrical, and, in addition, the steps are longer. B: stick figures indicating successive average right and left hindlimb positions (viewed from the right side) during the stance phase of the step cycle from a rat walking toward the right on the treadmill before and after up-conditioning. The dashed lines facilitate comparisons of the highest knee and hip heights. (Moment-to-moment variations in limb segment lengths reflect movements of the tattooed dots on the skin relative to the underlying joints. See methods.) By increasing right leg extension, up-conditioning markedly increases right knee and hip heights and increases step length. After up-conditioning, the right hip is no longer lower than the left.
Figure 3B further illustrates the impact of up-conditioning with stance-phase kinematic data from a representative HRup rat before and after up-conditioning. Through its effects on ankle and knee angles, up-conditioning markedly increased right hip height and leg extension, and thus increased the distance covered by the step cycle. The most striking aspects of this illustration are the marked elevations of the right knee and hip after up-conditioning.
For HRup rats, the increased step length and improved symmetry completed an explanatory connection from the increased protocol H-reflexes, to the increased locomotor H-reflexes, to the increased right soleus burst, to the increased right ankle angle, to improvement in key features of the complete behavior. While additional changes occurred in joint angles and the activity of other muscles in the right leg (and probably in the left leg as well), this connection accounted for the core of the locomotor impact, the strengthening of right stance, and was fully explicable both physiologically and kinematically. In contrast, for HRdown rats, the absence of change in step length or symmetry was consistent with the discrepancy between the decreased protocol H-reflex and the unchanged soleus locomotor burst. This discrepancy in turn accounted for the lack of change in ankle angle, step length, or step asymmetry. In summary, soleus HRup improved locomotion, while HRdown did not further impair it.
DISCUSSION
Spinal Cord Plasticity and Motor Learning
Recognition that the spinal cord undergoes plasticity comparable to that occurring elsewhere in the central nervous system (reviewed in Dobkin and Havton 2004; Mendell 1984; Wolpaw 2010; Wolpaw and Tennissen 2001) poses a critical new question for efforts to understand how important behaviors are acquired and maintained, as well as how they are, or might be, restored after injury or disease. The spinal cord (together with analogous brain stem areas) is the final common pathway for all the behaviors in an organism's repertoire. It is a multiuser resource that serves many different masters (i.e., many different behaviors). Its neuronal and synaptic properties are influenced by the brain, and they are normally maintained in a state that ensures the satisfactory performance of each behavior. When the acquisition of a new behavior changes the spinal cord, the continued performance of older behaviors may depend on additional changes; it may require that these older behaviors be, to some extent, relearned. How does this relearning come about, and how does it ensure that old behaviors are not disrupted?
Studies of the impact of H-reflex conditioning on locomotion (Chen et al. 2005, 2011) suggest that, in normal animals, this relearning preserves key features of older behaviors, the features that define satisfactory performance, such as step length, which directly affects speed, and step-cycle symmetry, which avoids limping. These features appear to be preserved by changes in muscular and kinematic details, such as the locomotor contributions of different leg muscles or the within-limb relationships among ankle, knee, and hip angles over the step-cycle (e.g., Chen et al. 2011). [Other key features may include parameters such as muscle efficiency, total energy consumption, or whole limb kinematics (e.g., Chang et al. 2009; Latash et al. 2007; Massaad et al. 2007).]
Based on these and related findings, Wolpaw (2010) proposed that the process through which the spinal cord accommodates a new behavior while preserving old behaviors can be understood as a negotiation among the behaviors. Prior to the new learning, spinal cord neurons and synapses are in a state of equilibrium that serves the behaviors in the current repertoire. When the learning of a new behavior changes the spinal cord, it disturbs this equilibrium and may thereby impair old behaviors. Henceforth, as each old behavior uses the newly modified spinal cord, any deficits in its key features induce compensatory plasticity that tends to restore these features. This plasticity may in turn affect the new behavior, as well as other old behaviors, and lead to further plasticity. The result is an ongoing iterative process, or negotiation, in which each behavior repeatedly induces plasticity that preserves its key features, despite the plasticity induced by other behaviors. Normally, the result is a new spinal cord equilibrium that satisfies the new and the old behaviors. Furthermore, when an old behavior has been impaired by trauma or disease, the new equilibrium may actually improve the old behavior (Chen et al. 2006a; Thompson et al. 2013).
The Present Study
This study tested the negotiated equilibrium hypothesis by comparing the impact on an impaired behavior of new learning designed to improve the behavior to that of new learning designed to further impair it. Based simply on the impairment (i.e., weak right stance), right HRup should have improved locomotion by increasing the contribution of the H-reflex pathway to the soleus locomotor burst, while down-conditioning should have further impaired it by decreasing the contribution. In contrast, the negotiated equilibrium hypothesis predicted that, while up-conditioning would improve locomotion, compensatory plasticity would prevent down-conditioning from further impairing it.
Up-conditioning did improve locomotion. Steps got longer, and step-cycle asymmetry (i.e., limping) disappeared. The improvement was not attributable to postinjury recovery, since the injury had occurred >50 days earlier, and further improvement does not occur after this time in similarly injured rats who are not up-conditioned (Chen et al. 2006a). In contrast, down-conditioning had no detectable effect on locomotion: steps did not get shorter or become more asymmetrical. These results are consistent with the negotiated equilibrium hypothesis.
How the New Learning Affected the Old Behavior
By following the connection from the altered H-reflex pathway to locomotion, the EMG and kinematic data show how up-conditioning and down-conditioning produced their locomotor effect, or lack of effect. Table 2 shows the impact of up- or down-conditioning at each level of this connection. The change in the strength of the H-reflex pathway was still present during locomotion: the locomotor H-reflex, like the protocol H-reflex, was much larger than its initial value in HRup rats and much smaller in HRdown rats (Fig. 2). This is consistent with previous studies (Chen et al. 2005, 2011) and with extensive evidence that H-reflex conditioning changes the spinal cord (reviewed in Wolpaw 2010; Wolpaw and Chen 2009).
Table 2.
The impact of HRup orHRdown at successively higher levels of analysis, from the H-reflex elicited in the conditioning protocol to key features of locomotion (i.e., step-length and step-cycle symmetry)
| Level of Analysis | HRup | HRdown |
|---|---|---|
| Protocol H-reflex | ↑ | ↓ |
| Locomotor H-reflex | ↑ | ↓ |
| Locomotor burst | ↑ | No change |
| Ankle angle | ↑ | No change |
| Step length and symmetry | ↑ | No change |
As Fig. 2 and Table 2 show, the stronger H-reflex pathway after up-conditioning was associated with a larger locomotor burst. This agrees with results in normal rats (Chen et al. 2005) and with the known role of Group I (and II) afferent input in producing the burst (Bennett et al. 1996; Grey et al. 2007; Nielsen and Sinkjaer 2002; Pearson 2004; Sinkjaer et al. 2000; Stein et al. 2000; Yang et al. 1991). The larger burst explains the increased ankle angle. This increase and the increased knee angle increased right leg extension and accounted for the longer steps and improved symmetry (e.g., Fig. 3).
In contrast, the weaker H-reflex pathway produced by down-conditioning was not associated with a smaller locomotor burst [Fig. 2B (bottom right) and Table 2]. Indeed, the burst was slightly, though not significantly, larger after down-conditioning. This contrasts with the result in normal rats (Chen et al. 2005) and is not consistent with the normal role of the H-reflex pathway in producing the burst. The unchanged burst accounts for the lack of significant change in ankle angle and, at the next level up, in step length and symmetry (Tables 1 and 2). Thus it is unnecessary to propose [as was necessary for normal rats (Chen et al. 2005)] that changes in the locomotor behavior of other muscles accounted for the lack of effect of down-conditioning on the step cycle. The discrepancy between the smaller locomotor H-reflex and the unchanged burst implies that soleus motoneurons received additional excitatory input (and/or decreased inhibitory input) that compensated for the decreased excitation from the H-reflex pathway and preserved the burst. The modified input may have reflected plasticity in spinal interneurons conveying descending input or other sensory input to the motoneurons, and/or supraspinal plasticity that changed descending input. This compensatory plasticity, which prevented down-conditioning from further impairing locomotion, was presumably part of the relearning of locomotion triggered by the weakened H-reflex pathway. The fact that it differed from the compensatory plasticity that prevented down-conditioning from impairing locomotion in normal rats (Chen et al. 2005) presumably reflected differences between normal and spinal cord-injured rats in the starting position for the responsible adaptive process and/or in their capacities for adaptation.
These results raise a further question. Why did the improvement in locomotion caused by HRup not occur during postinjury recovery; that is, how did up-conditioning produce an improvement beyond that which had already occurred in the normal course of recovery?
Activity-Dependent Plasticity and Recovery of Locomotion after a Spinal Cord Injury
Normal locomotion involves many neuronal populations in the spinal cord and brain and the synaptic connections among them. Many (perhaps all) of these neurons and synapses are capable of activity-dependent plasticity (reviewed in Dobkin and Havton 2004; Mendell 1984; Wolpaw 2010; Wolpaw and Tennissen 2001). Thus, when a spinal cord injury or other lesion impairs locomotion, the sites and kinds of plasticity that might improve locomotion are many, and their possible combinations are far more numerous. Spontaneous postinjury activity and current rehabilitation methods [which typically involve the practice of locomotion in some form (e.g., Dobkin et al. 2006; Rossignol and Frigon 2011; Wernig et al. 2000)] are likely to induce plasticity at many sites through various mechanisms and at different rates. Although the overall impact is generally positive, there is no certainty that the optimal changes will occur and that recovery will be maximized. Indeed, plasticity in the H-reflex pathway, which typically develops gradually (reviewed in Wolpaw 2010), may not even be engaged, or engaged beneficially, during normal postinjury recovery. Furthermore, a spinal cord injury may impair the brain-spinal cord interactions that guide and maintain adaptive plasticity in the spinal cord. For example, damage to ascending pathways may prevent the sensory input that reflects a locomotor asymmetry from reaching the supraspinal sites that might induce appropriate corrective plasticity (Chen et al. 2013).
In contrast to spontaneous recovery and standard rehabilitation, which may induce plasticity in many places, but not necessarily in the H-reflex pathway, the conditioning protocol targets plasticity to the H-reflex pathway; the H-reflex itself is the behavior that is the focus of this new learning. Thus, if this plasticity is in the correct direction for improving locomotion, it may improve locomotion beyond the level previously achieved because it produces a beneficial change that would not have occurred otherwise. This appears to be what happened in the up-conditioned rats of the present study.
At the same time, the negotiated equilibrium hypothesis and recent human data (Thompson et al. 2013) suggest that H-reflex conditioning, by adding a new behavior to the repertoire supported by the spinal cord, can have a substantially wider impact on locomotion. Because this new learning changes the spinal cord, it disturbs the equilibrium that supports older behaviors. Thus it triggers a new negotiation to accommodate all of the behaviors in the expanded repertoire. By triggering this widespread adaptive process, it introduces the possibility that the new, renegotiated equilibrium will incorporate multiple changes that support better locomotion. This possibility becomes more likely if the plasticity that produces the new behavior (e.g., a larger H-reflex) itself improves locomotion. The ability of appropriate H-reflex conditioning to trigger widespread beneficial plasticity is strongly supported by a recent study in people with chronic spinal cord injuries (Thompson et al. 2013). In addition to the benefit directly attributable to the modified H-reflex pathway (e.g., improved step symmetry), conditioning of the soleus H-reflex in one leg increased locomotor modulation of EMG activity in calf and thigh muscles of both legs. These widespread effects could not be explained by plasticity in the soleus H-reflex pathway; they indicated the presence of more extensive plasticity. It appeared that the new negotiation triggered by appropriate H-reflex conditioning produced a new negotiated equilibrium superior to that previously achieved (i.e., at least in regard to locomotion).
Possible Therapeutic Use of Spinal Reflex Conditioning
Because the sites and mechanisms of the lesions that impair motor function differ across individuals and disorders, the plasticity needed to restore a behavior (e.g., locomotion) is also likely to differ. Thus new methods (e.g., H-reflex conditioning) that can strengthen or weaken a specific pathway according to the nature of the functional impairment could complement other rehabilitation methods (e.g., locomotor training) and enhance functional recovery. Animal and human data support this possibility. In rats in which a spinal cord injury had impaired locomotion by weakening right stance, up-conditioning of the right soleus H-reflex strengthened stance and improved locomotion (Chen et al. 2006a). Conversely, in people in whom a spinal cord injury had impaired locomotion by causing spasticity, down-conditioning of the soleus H-reflex ameliorated the spasticity (e.g., reduced clonus) and improved locomotion (Thompson et al. 2013).
The ability of reflex conditioning protocols to target specific pathways, and thus specific deficits (e.g., weak stance, clonus), could be particularly important when spinal cord regeneration becomes possible (e.g., Becker and McDonald 2012; Houle and Côté 2009; Ibarra and Martiñón 2009; Tohda and Kuboyama 2011; Yoon and Tuszynski 2012). A regenerated spinal cord will probably not function well immediately. Methods for inducing and guiding plasticity will be needed to restore useful function. H-reflex conditioning and comparable conditioning of other reflexes (e.g., Chen et al. 2006b) could help to modify spinal cord pathways so as to maximize recovery. Furthermore, reflex conditioning protocols might enhance recovery after other kinds of trauma, such as peripheral nerve injury and regeneration (Chen et al. 2010).
Conclusions
In rats with impaired locomotion due to a spinal cord injury, up-conditioning of the soleus H-reflex improved locomotion, while down-conditioning did not further impair it. The results support the predictions of the negotiated equilibrium hypothesis: 1) when acquisition of a new behavior changes the spinal cord, old behaviors undergo relearning that preserves their key features (although it may change their muscular and kinematic details); 2) when an old behavior has been impaired by trauma or disease, the acquisition of a new behavior that changes the spinal cord may improve key features of the old behavior but will not further impair them. Reflex conditioning protocols could help to improve locomotor and other functional abnormalities associated with spinal cord injuries or other disorders.
GRANTS
This work was supported by the National Institutes of Health [HD-36020 (X. Y. Chen), NS-061823 (X. Y. Chen and J. R. Wolpaw), NS-22189 (J. R. Wolpaw), and HD-32571 (A. W. English)], and the New York State Spinal Cord Injury Research Trust Fund (X. Y. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.C., Y.W., X.Y.C., and J.R.W. conception and design of research; Y.C., L.C., R.L., Y.W., and X.Y.C. performed experiments; Y.C., L.C., R.L., Y.W., X.Y.C., and J.R.W. analyzed data; Y.C., L.C., Y.W., X.Y.C., and J.R.W. interpreted results of experiments; Y.C., L.C., R.L., Y.W., and X.Y.C. prepared figures; Y.C., Y.W., X.Y.C., and J.R.W. drafted manuscript; Y.C., L.C., R.L., Y.W., X.Y.C., and J.R.W. approved final version of manuscript; X.Y.C. and J.R.W. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Drs. Jonathan S. Carp, Dennis J. McFarland, Aiko K. Thompson, and Elizabeth Winter Wolpaw for comments on the manuscript.
REFERENCES
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21, 1995 [DOI] [PubMed] [Google Scholar]
- Becker D, McDonald JW., 3rd Approaches to repairing the damaged spinal cord: overview. Handb Clin Neurol 109: 445–461, 2012 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB. Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. J Physiol 496: 837–850, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YH, Auyang AG, Scholz JP, Nichols TR. Whole limb kinematics are preferentially conserved over individual joint kinematics after peripheral nerve injury. J Exp Biol 212: 3511–3521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Chen L, Wolpaw JR. Sensorimotor cortex ablation prevents H-reflex up-conditioning and causes a paradoxical response to down-conditioning in rats. J Neurophysiol 96: 119–127, 2006c [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Chen Y, Wolpaw JR. Operant conditioning of reciprocal inhibition in rat soleus muscle. J Neurophysiol 96: 2144–2150, 2006b [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Wolpaw JR, Jakeman LB. Corticospinal tract transection reduces H-reflex circadian rhythm in rats. Brain Res 942: 101–108, 2002 [DOI] [PubMed] [Google Scholar]
- Chen XY, Feng-Chen KC, Chen L, Stark DM, Wolpaw JR. Short-term and medium-term effects of spinal cord tract transections on soleus H-reflex in freely moving rats. J Neurotrauma 18: 313–327, 2001 [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol 73: 411–415, 1995 [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Reversal of H-reflex operant conditioning in the rat. Exp Brain Res 112: 58–62, 1996 [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Dorsal column but not lateral column transection prevents down-conditioning of H-reflex in rats. J Neurophysiol 78: 1730–1734, 1997 [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Probable corticospinal tract control of spinal cord plasticity in the rat. J Neurophysiol 87: 645–652, 2002 [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Ablation of cerebellar nuclei prevents H-reflex down-conditioning in rats. Learn Mem 12: 248–254, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR, Jakeman LB, Stokes BT. Operant conditioning of H-reflex in spinal-cord injured rats. J Neurotrauma 13: 755–766, 1996 [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR, Jakeman LB, Stokes BT. Operant conditioning of H-reflex increase in spinal-cord injured rats. J Neurotrauma 16: 175–186, 1999 [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Schalk G, Stokes BT, Wolpaw JR. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J Neurosci 25: 6898–6906, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 26: 12537–12543, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Liu RL, Wang Y, Wolpaw JR, Chen XY. Locomotor effects of H-reflex conditioning in rats with transection of the dorsal column ascending tract (Abstract). Program No. 645.04 2013 Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience, 2013 [Google Scholar]
- Chen Y, Chen L, Wang Y, Wolpaw JR, Chen XY. Operant conditioning of rat soleus H-reflex oppositely affects another H-reflex and changes locomotor kinematics. J Neurosci 31: 11370–11375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Chen L, Sun C, English AW, Wolpaw JR, Chen XY. H-reflex up-conditioning encourages recovery of EMG activity and H-reflexes after sciatic nerve transection and repair in rats. J Neurosci 30: 16128–16136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron 72: 443–454, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH, Havton LA. Basic advances and new avenues in therapy of spinal cord injury. Annu Rev Med 55: 255–282, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, DeForge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M, Scott M, Spinal Cord Injury Locomotor Trial (SCILT) Group Weight-supported treadmill vs. overground training for walking after acute incomplete SCI. Neurology 66: 484–493, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199: 61–75, 2009 [DOI] [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N, Sinkjaer T. Positive force feedback in human walking. J Physiol. 581.1: 99–105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle JD, Côté MP. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann N Y Acad Sci 1279: 154–163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra A, Martiñón S. Pharmacological approaches to induce neuroregeneration in spinal cord injury: an overview. Curr Drug Discov Technol 6: 82–90, 2009 [DOI] [PubMed] [Google Scholar]
- Kilgannon C. By Their Walk Shall You Know Them (Online). New York Times: http://www.nytimes.com/1996/11/10/nyregion/by-their-walk-shall-you-know-them.html [November 10, 1996]. [Google Scholar]
- Latash ML, Scholz JP, Schöner G. Toward a new theory of motor synergies. Motor Control 11: 276–308, 2007 [DOI] [PubMed] [Google Scholar]
- Massaad F, Lejeune TM, Detrembleur C. The up and down bobbing of human walking: a compromise between muscle work and efficiency. J Physiol 582.2: 789–799, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM. Modifiability of spinal synapses. Physiol Rev 64: 260–324, 1984 [DOI] [PubMed] [Google Scholar]
- Meyer-Lohmann J, Christakos CN, Wolf H. Dominance of the short-latency component in perturbation induced electromyographic responses of long-trained monkeys. Exp Brain Res 64: 393–399, 1986 [DOI] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from the Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol 66: 116–121, 1993 [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Sinkjaer T. Reflex excitation of muscles during human walking. Adv Exp Med Biol 508: 369–375, 2002 [PubMed] [Google Scholar]
- PBS SUNDAYARTS: Sara Mearns, Principal Dancer, New York City Ballet (Online). http://video.pbs.org/video/2135346634/ (see time 13:25–13:55) [Sept. 18, 2011].
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004 [DOI] [PubMed] [Google Scholar]
- Penhune V, Steele C. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res 226: 579–591, 2012 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci 34: 413–440, 2011 [DOI] [PubMed] [Google Scholar]
- Royal Opera House Royal Ballet Daily Class (complete video) Royal Ballet LIVE (Online). http://www.youtube.com/watch?v=5EVMjnHFg-w&feature=related (see time 27:40–28:00 et passim) [April 12, 2012].
- Schneider S, Capaday C. Progressive adaptation of the soleus H-reflex with daily training at walking backward. J Neurophysiol 89: 648–656, 2003 [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Anderson JB, Ladoucerur M, Christensen LOD, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523.3: 817–827, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J Physiol 525: 781–791, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Pomerantz F, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33: 2365–2375, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohda C, Kuboyama T. Current and future therapeutic strategies for functional repair of spinal cord injury. Pharmacol Ther 132: 57–71, 2011 [DOI] [PubMed] [Google Scholar]
- Wang Y, Pillai S, Wolpaw JR, Chen XY. Motor learning changes GABAergic terminals on spinal motoneurons in normal rats. Eur J Neurosci 23: 141–150, 2006 [DOI] [PubMed] [Google Scholar]
- Wang Y, Pillai S, Wolpaw JR, Chen XY. H-reflex down-conditioning greatly increases the number of identifiable GABAergic interneurons in rat ventral horn. Neurosci Lett 452: 124–129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A, Nanassy A, Muller S. Laufband (LB) therapy in spinal cord lesioned persons. Prog Brain Res 128: 89–97, 2000 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol 57: 443–459, 1987 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist 16: 532–549, 2010 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. Operant conditioning of reflexes. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford, UK: Academic, 2009, vol. 7, p. 225–233 [Google Scholar]
- Wolpaw JR, Herchenroder PA, Carp JS. Operant conditioning of the primate H-reflex: factors affecting the magnitude of change. Exp Brain Res 97: 31–39, 1993 [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci 4: 807–843, 2001 [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus H-reflex in walking in humans. Exp Brain Res 87: 679–687, 1991 [DOI] [PubMed] [Google Scholar]
- Yoon C, Tuszynski MH. Frontiers of spinal cord and spine repair: experimental approaches for repair of spinal cord injury. Adv Exp Med Biol 760: 1–15, 2012 [DOI] [PubMed] [Google Scholar]




