Abstract
Recent stimulus history, or adaptation, can alter neuronal response properties. Adaptation effects have been characterized in a number of visually responsive structures, from the retina to higher visual cortex. However, it remains unclear whether adaptation effects across stages of the visual system take a similar form in response to a particular sensory event. This is because studies typically probe a single structure or cortical area, using a stimulus ensemble chosen to provide potent drive to the cells of interest. Here we adopt an alternative approach and compare adaptation effects in primary visual cortex (V1) and area MT using identical stimulus ensembles. Previous work has suggested these areas adjust to recent stimulus drive in distinct ways. We show that this is not the case: adaptation effects in V1 and MT can involve weak or strong loss of responsivity and shifts in neuronal preference toward or away from the adapter, depending on stimulus size and adaptation duration. For a particular stimulus size and adaptation duration, however, effects are similar in nature and magnitude in V1 and MT. We also show that adaptation effects in MT of awake animals depend strongly on stimulus size. Our results suggest that the strategies for adjusting to recent stimulus history depend more strongly on adaptation duration and stimulus size than on the cortical area. Moreover, they indicate that different levels of the visual system adapt similarly to recent sensory experience.
Keywords: surround suppression, adaptation duration, motion processing, plasticity, V1
adaptation, the stimulus history of the preceding hundreds of milliseconds to minutes, can profoundly change neuronal response properties. Adaptation effects have been characterized in the retina (e.g., Baccus and Meister 2002; Brown and Maslund 2001; Chander and Chichilnisky 2001; Rieke 2001; Zaghloul et al. 2005), lateral geniculate nucleus (LGN; e.g., Camp et al. 2009; McLelland et al. 2009; Solomon et al. 2004), primary visual cortex (e.g., Carandini et al. 1997; Crowder et al. 2006; Dragoi et al. 2000; Felsen et al. 2002; Ghisovan et al. 2009; Movshon and Lennie 1989; Muller et al. 1999; Ohzawa et al. 1985; Wissig and Kohn 2012), area MT (e.g., Kohn and Movshon 2004; Krekelberg et al. 2006b; Van Wezel and Britten 2002; Yang and Lisberger 2009), and inferotemporal cortex (e.g., Liu et al. 2009; Sawamura et al. 2006), among many others (for recent reviews see Kohn 2007; Webster 2011).
These studies have provided a rich description of how cortical circuits adjust to recent visual input, but they have left unclear how a particular sensory event alters the distributed representation of information in the visual system. This is because studies, understandably, have focused on measuring effects with stimuli that are most appropriate for driving the structure of interest: for instance, flickering checkerboards in the retina or images of objects in inferotemporal cortex. As a result, it remains unclear whether different networks have similar strategies for adjusting to a particular recent input.
Several studies have attempted to discern whether changes in sensitivity measured in one network could be explained by effects inherited from earlier areas or structures (Kohn and Movshon 2003; Movshon and Lennie 1979; Nelson 1991a, 1991b; Ohzawa et al. 1985; Priebe et al. 2002). However, these have focused on changes in responsivity (or contrast sensitivity) to a single stimulus and have not probed whether tuning is altered in a similar way at successive stages of processing. Changes in tuning are critical because they indicate how neuronal resources are allocated to represent the external environment. Furthermore, even when adaptation effects are thought to be inherited from earlier areas, this does not dictate that the altered representation in the recipient area mirrors that in the source area. This is because adaptation-induced changes in the feedforward input to an area can alter the interactions among neurons there so that tuning is affected in distinct ways in the source and target area (Compte and Wang 2006; Kohn and Movshon 2004).
In this report we provide a straightforward comparison between adaptation effects on orientation and direction tuning in primary visual cortex (V1) and area MT, using identical stimulus ensembles in the two areas. V1 and MT are attractive targets for comparison because they are part of a well-studied motion processing pathway (Born and Bradley 2005). In addition, previous work has suggested that neurons in these two areas adapt differently. In V1, adaptation effects involve stimulus-specific suppression. As a result, an adapter that falls on the flank of a neuron's tuning curve will cause its preference to shift away from the adapter (Dragoi et al. 2000; Felsen et al. 2005; Muller et al. 1999). In MT, on the other hand, responsivity is reduced most strongly for stimuli that differ from the adapter. As a result, tuning shifts toward the adapter (Kohn and Movshon 2004; Krekelberg et al. 2006b; Schlack et al. 2007).
The observation that V1 and MT tuning are altered in a qualitatively different manner suggests distinct strategies for adjusting to recent stimulus history in these two networks. However, recent work offers an alternative interpretation. Wissig and Kohn (2012) showed that adaptation could cause either stimulus-specific suppression of V1 responses, when the adapter and test stimuli were small, or “MT-like” effects, when stimuli were large. The proposed mechanism is a stimulus-specific reduction in surround suppression following adaptation with a large stimulus. This reduces the suppressive influence recruited by test stimuli that extend beyond the classical receptive field (Angelucci and Bressloff 2006; Cavanaugh et al. 2002). The reduction of surround suppression is a form of disinhibition and thus can enhance responsivity and cause tuning to shift toward the adapter. Patterson et al. (2013) showed that the size dependence of adaptation effects was evident after prolonged (40 s) but not brief (0.4 s) adaptation. As a result, V1 shows a broad range of adaptation effects, depending on stimulus size and adaptation duration.
Building on these observations, we sought to test whether MT effects mirror those in V1, for brief and prolonged adaptation with small and large grating stimuli. We have found that, for each choice of stimulus parameters, effects in MT are similar in nature and magnitude to those in V1. We also show that adaptation effects in MT of an awake monkey are strongly modulated by stimulus size. Our results suggest that tuning in V1 and MT are affected similarly by recent visual experience.
MATERIALS AND METHODS
All procedures were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine at Yeshiva University (anesthetized animals) or the Rutgers University Animal Care and Use Committee (awake animal) and were in compliance with the guidelines set forth in the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals.
Surgical preparation (anesthetized).
Monkeys (Macaca fascicularis) were given 0.05 mg/kg atropine and 1.5 mg/kg diazepam before surgery. Ketamine (10 mg/kg) was used to induce anesthesia. Monkeys were then intubated and started on 1.0–2.5% isoflurane in a 98% O2-2% CO2 mixture. Intravenous catheters were inserted into the saphenous vein of each leg, and the animals were positioned in a stereotaxic device. A craniotomy and durotomy were performed to expose the underlying cortex. Once electrodes had been inserted, the opening was covered with agar to prevent desiccation. During recordings, isoflurane was discontinued and sufentanil citrate (6–24 μg·kg−1·h−1) was administered intravenously to maintain anesthesia; the paralytic vecuronium bromide (0.15 mg·kg−1·h−1) was infused to prevent eye movements. Vital signs including ECG, EEG, blood pressure, pulse oxygen arterial saturation (SpO2), end-tidal CO2, airway pressure, and temperature were monitored continuously. The corneas were protected with permeable contact lenses, and topical atropine was applied to dilate the pupils. Corrective lenses were used to focus the visual image.
Surgical preparation (awake).
Recordings were performed in one macaque monkey (Macaca mulatta). Headposts and recording chambers were implanted under full isoflurane anesthesia in an aseptic environment. A recording chamber (high-density polyethylene, 25.3-mm diameter) was positioned over the left parietal cortex, oriented normal to the skull.
Recording (anesthetized).
In V1, recordings were performed with an ∼4 × 4-mm 96-electrode Utah array, inserted roughly 600 μm into cortex (1-mm electrode length, 400-μm spacing). MT recordings were performed with an array of movable electrodes and tetrodes (Thomas Recording), inserted at an angle of 20 deg from horizontal and positioned roughly 16 mm lateral to the midline and 8 mm posterior to the lunate sulcus. V1 and MT recordings were performed in separate animals. Neuronal responses that exceeded a user-defined voltage threshold were digitized at 30 or 40 kHz. Waveforms were classified using Plexon Offline Sorter into single and multiple units.
Recording (awake).
Before each recording session, a guide tube was inserted through the dura mater to gain access to the cortex. Glass or parylene-C-coated tungsten electrodes (FHC) were lowered through the guide tube with an electronic micropositioner (NAN). MT was identified online on the basis of functional criteria (high fraction of direction-selective cells with receptive fields that are small compared with the neighboring area MST) and its expected depth relative to the dura mater. Recording locations were confirmed using structural magnetic resonance imaging. Signals were digitized at 25 kHz and stored for offline spike sorting (using KlustaKwik). Eye position was recorded using an Eyelink II infrared video system (500 Hz).
Stimulus presentation (anesthetized).
Custom software based on OpenGL (EXPO) was used to generate all stimuli, which were displayed on a calibrated CRT monitor (1,024 × 768 pixels, refresh rate of 100 Hz, ∼40 cd/m2 mean luminance) placed 80 (MT) or 110 cm (V1) from the animal. In V1, spatial receptive fields were estimated using small drifting gratings (0.5-deg diameter, 4 orientations, 1 cycle/deg, drift rate of 6.25 Hz, 250-ms presentation) presented at a range of locations spanning a 3 × 3-deg region of visual space. In MT, we used a similar approach but with 1.3-deg-diameter gratings presented in a 20 × 15-deg area. These measurements were used to center the stimuli over the aggregate spatial receptive field and, when considering responses to small gratings, to select units whose receptive fields were at least 50% covered by the stimulus for further analysis.
Stimulus presentation and behavioral control (awake).
We used in-house OpenGL-based software (neurostim.sf.net) for behavioral control and stimulus presentation on a Sony Artisan monitor (GDM-520; 150 Hz, 1,024 × 768 pixels at 57 cm). The animal was rewarded with a drop of juice for fixating a small red dot for the duration of a trial. Trials in which fixation was not maintained within an invisible 2 × 2-deg box were discarded. The receptive field was mapped using automated methods (Hartmann et al. 2011; Krekelberg and Albright 2005) and used to center the stimulus on the receptive field. We first mapped the direction tuning of the cells with the small and large gratings moving in 1 of 16 equally spaced directions, presented for 1 s. This provided us with the preadaptation tuning curve and was used to select the adapter direction.
Paradigms and visual stimuli (anesthetized and awake).
We measured adaptation effects using full-contrast sinusoidal gratings with a spatial frequency of 1 cycle/deg and a drift rate of 6.25 Hz. Gratings were either small (1.3-deg diameter) or large (7.4-deg diameter), with the adapter and test stimuli always matched in size.
In anesthetized animals, we measured direction tuning curves before and after adaptation with 16 equally spaced directions (22.5-deg increments) in V1, and 12 (30-deg increments) in MT. In the brief (0.4 s) adaptation paradigm, control (preadaptation) and postadaptation conditions were interleaved. Preadaptation trials consisted of a 0.4-s presentation of a gray screen followed by a test stimulus presented for 0.4 s. Adaptation trials consisted of a 0.4-s presentation of an adapter (one of the stimuli in the test ensemble), followed immediately by a 0.4-s presentation of a test stimulus. Trials were separated by 1.2 s of gray screen to allow recovery (Patterson et al. 2013). In the prolonged adaptation paradigm, we used an adapt–test–top-up design. The initial adaptation interval was 40 s, followed by a sequence of 1-s test stimuli and 5-s top-up adapters. Preadaptation test stimuli were separated by 5-s intervals of gray screen presentation to maintain the same temporal structure as in the postadaptation epochs. Preadaptation measurements always preceded postadaptation.
In the awake animal, preadaptation measurements were made first, using gratings in 16 equally spaced directions (22.5-deg increments). On adaptation trials, the adapter was presented for 4 s and immediately followed by a 1-s test grating whose direction of motion was varied around the preferred direction of the neuron in 9 equally spaced steps of 22.5 deg. We chose this reduced paradigm to maximize useful data collection (given the need to maintain fixation) while still allowing a comparison of pre- and postadaptation tuning curves.
Data analysis.
We analyzed all units that had a preadaptation maximum stimulus driven firing rate greater than 1 SD above the mean spontaneous rate (78% of cells recorded in V1 and 56% in MT). For V1 responses, the F1 component of the response and the mean firing rate were calculated, and the greater of the two was used for further analysis. The spontaneous firing rate (i.e., measured during the presentation of a gray screen) was subtracted from the raw response to provide a measure of evoked activity.
Since there are both direction-selective (DS) and orientation-selective (OS) neurons in V1, we first quantified tuning for each unit with an orientation selectivity index (OSI) and direction selectivity index (DSI). DSI was calculated as
where Rn is the response to a stimulus drifting in direction θn. OSI was calculated similarly, but with i2θn replacing iθn. Neurons whose DSI was greater than its OSI were categorized as DS cells, and as OS otherwise. For OS cells, the tuning curve peak closest to the adapter was used, encompassing 180 deg of stimulus orientation. We did not average across directions that shared the same orientation because we wanted to compare V1 effects with DS units in MT. Although both peaks of OS tuning functions were affected by adaptation, there was a slightly stronger effect on the peak closest to the adapter. Thus, although the anesthetized V1 data presented in this report are the same as those used in Patterson et al. (2013), our present use of only one peak of the tuning curve gave rise to small quantitative differences compared with that study.
We then fit tuning curves with a von Mises function:
where rp is the predicted response, m is the baseline offset, a defines the response amplitude, b determines the tuning width, θpref is the location of the peak of the tuning curve, and θ is the direction of the test stimulus (spanning either 180 or 360 deg). Fits were determined by maximizing the log likelihood of the data given the model predictions, based on the assumption of Poisson spiking statistics (El-Shamayleh and Movshon 2011). The fit quality was calculated as a normalized log likelihood, where the lower bound (a value of 0) consisted of the likelihood of a model with predicted responses equal to the average response across all conditions, and the upper bound (a value of 1) was calculated by using the data as the model (El-Shamayleh and Movshon 2011; Stocker and Simoncelli 2006). We used the fits to measure changes in peak response, shifts in preferred direction, and changes in bandwidth.
Units were discarded from further analysis on the basis of the following criteria: 1) units with a pre- or postadaptation fit quality of <0.5 (46% of cells in V1, fit quality of remaining cells was 0.80; 51% of cells in anesthetized MT, remaining fit quality 0.77; 65% of cells in awake MT, remaining fit quality 0.89), which indicated poor tuning; 2) units with a bandwidth (width of the tuning curve halfway between the minimum and maximum) less than 22.5 deg for V1 (8% of remaining cells) or awake MT (0%) or 30 deg for anesthetized MT (0.5%), because we could not accurately measure the tuning curve of these units with our test ensemble; and 3) units whose shift in preference was greater than 1 bandwidth unit (4% of remaining V1 cells; 10% of remaining anesthetized MT cells; 0% of remaining awake MT cells), because such large shifts likely indicate that the pre- and postadaptation data arose from two different units (Dragoi et al. 2000; Kohn and Movshon 2004). For the awake MT data, we applied these criteria in two distinct ways. First, we applied them to both the pre- and postadaptation tuning for responses to both small and large gratings. Of 43 MT cells recorded for both sizes, 15 passed the criteria for all conditions. Although this resulted in a reduction of the population size, it allowed a within-cell comparison of the tuning properties across multiple conditions. Second, we applied the selection criteria separately to the responses to small and large gratings. This yielded a larger population of cells, since it allowed us to include cells whose isolation was lost before we collected data for both size conditions. The use of less stringent criteria for the data from awake or anesthetized animals did not change the results in any notable way.
Unless otherwise indicated, error bars reflect 95% confidence intervals based on bootstrap analysis. Unless otherwise indicated, t-tests were used to determine statistical significance. Ratios were log-transformed before statistical evaluation.
RESULTS
We recorded in V1 and MT of 13 anesthetized monkeys. Neurons in V1 had spatial receptive fields at eccentricities of 2–3 deg in the lower visual field. The receptive fields in MT had eccentricities ranging from 4 to 8 deg. The recordings consisted of both well-isolated single units and small multiunit clusters. We found no difference in adaptation effects for these types of recordings, so we pooled our results (see Wissig and Kohn 2012 for a detailed comparison). We also recorded from well-isolated single units and small multiunit clusters in area MT of one awake macaque monkey, with receptive fields at eccentricities ranging from 0.5 to 5 deg.
Characterization of V1 and MT responses.
We first determined basic response properties of V1 and MT neurons based on the large anesthetized data set so that we could best compare adaptation effects in the two areas. We measured response latency, defined by the first 10-ms time bin in which the unit fired 1.5 SD above its mean spontaneous rate, when that bin was followed by at least 4 successive bins that exceeded the same threshold. Latency differed only slightly between V1 (median of 70 ms) and MT (median of 80 ms, P = 0.08 for comparison with V1), consistent with previous estimates (Schmolesky et al. 1998). For our analysis we therefore used a common response window beginning 50 ms after stimulus onset and extending to its offset, unless otherwise noted.
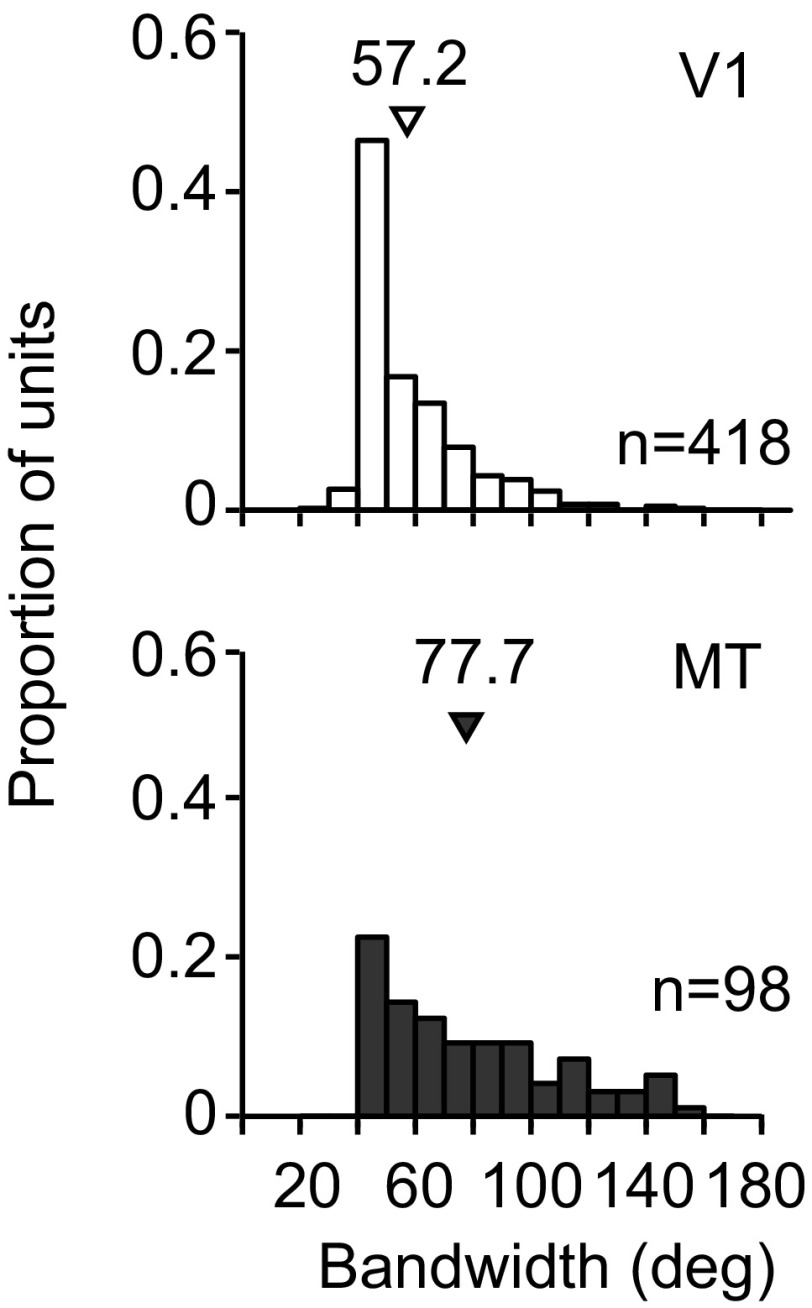
We next compared the bandwidth of V1 and MT tuning, defined as the width of the tuning curve midway between the maximal and minimal response. V1 neurons (n = 418) had narrower tuning (57.2 ± 0.9 deg, mean ± SE) than MT (77.7 ± 3.1 deg, P < 0.0001 for comparison, n = 98) when measured with large gratings (7.4-deg diameter; Fig. 1), consistent with previous work (Albright, 1984). Mean bandwidth was also narrower in V1 (69.6 ± 1.9 deg, P < 0.0001 for comparison) than MT (98.4 ± 5.1 deg), when measured with small gratings (1.3-deg diameter). The tuning measured with small gratings was substantially broader than that measured with large gratings in both V1 (P < 0.0001) and MT (P < 0.0001). This is presumably a result of the surround suppression recruited by large stimuli, which previous studies have shown can lead to a sharpening of tuning in V1 (Chen et al. 2005; Ringach et al. 1997). Because of differences in bandwidth across areas and stimulus sizes, we normalized all relevant measurements by the bandwidth of each cell to ensure a fair comparison.
Fig. 1.
Characteristics of V1 and MT neuronal responses. Histograms show the tuning bandwidths for the V1 (top; open bars) and MT units (bottom, shaded bars). Arrowheads indicate mean bandwidth.
Effects of prolonged adaptation are similar in V1 and MT.
We have recently shown that the effects of prolonged adaptation in V1 are dependent on stimulus size (Patterson et al. 2013; Wissig and Kohn 2012). Prolonged adaptation (40 s) with small gratings causes a strong, stimulus-specific suppression of responsivity, resulting in repulsive shifts in preference for neurons whose preference is slightly offset from the adapter; adaptation with large gratings has a weaker effect on responsivity and often causes tuning to shift toward the adapter.
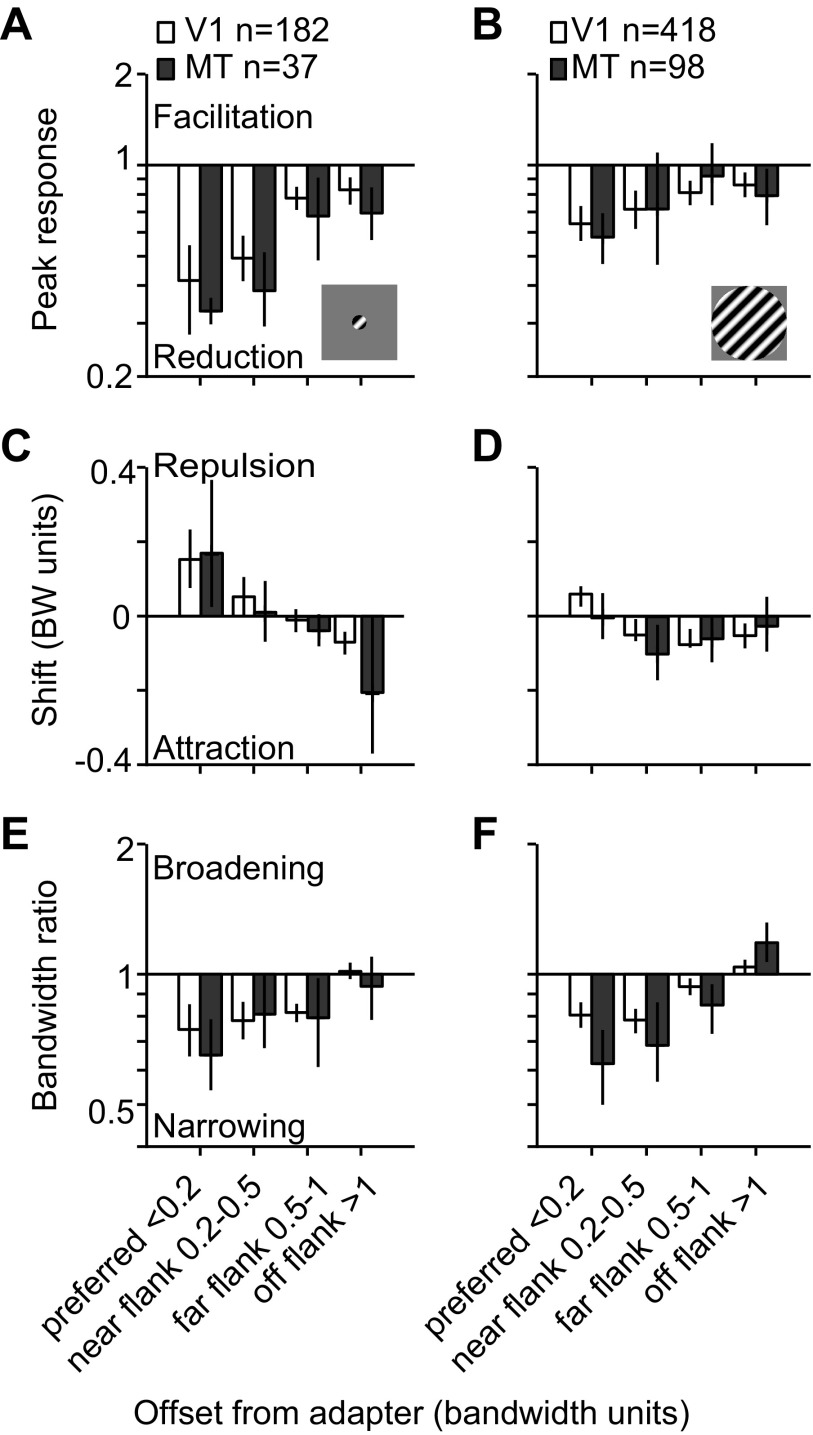
We therefore first determined whether the effects of prolonged adaptation in MT displayed the same dependence on stimulus size as those in V1. Figure 2A compares the peak response ratio (after adaptation compared with before) for V1 (open bars) and MT units (filled bars) when measured with small gratings; Fig. 2B shows the corresponding data for large gratings. The effects are binned as a function of the neuron's offset from the adapter, since it is well established that this offset strongly influences the observed effects (Dragoi et al. 2000; Kohn and Movshon 2004; Muller et al. 1999). Neurons within <0.2 bandwidth units of the adapter were designated as preferred adapted cells, 0.2–0.5 bandwidth units away as near-flank adapted, 0.5–1 bandwidth units away as far-flank adapted, and those >1 bandwidth unit away as off-flank adapted.
Fig. 2.
Comparison of the effects of prolonged adaptation effects in V1 and MT for small and large gratings. A and B: peak response ratio plotted as a function of the units' preference relative to the adapter (in bandwidth units) for responses to small (A) and large gratings (B). C and D: shifts in preference plotted as a function of preference for small (C) and large gratings (D). E and F: bandwidth ratio plotted as a function of preference for small (E) and large gratings (F). Error bars indicate 95% confidence interval (CI).
A three-factor ANOVA showed that the response ratio depended on stimulus size (F = 24.87, P < 0.0001) and the neuron's offset from the adapter (F = 17.07, P < 0.0001) but was not significantly different in V1 and MT (F = 3.30, P = 0.07). In both areas, adaptation with small gratings caused a stronger reduction in peak response, with a geometric mean response ratio for preferred adapted neurons of 0.42 in V1 and 0.33 in MT. There was no difference between effects for preferred adapted neurons in V1 and MT (P = 0.4) or for those at any other offset (P > 0.1). For large gratings, the mean response ratio for preferred adapted neurons in both V1 (0.64, P = 0.007) and MT (0.58, P = 0.0008) was greater than after adaptation with small gratings, indicating a weaker adaptation effect. There was no difference between effects measured in V1 and MT with large stimuli at any offset (P > 0.3).
It is notable that the strongest reduction in both V1 and MT responsivity was observed after adaptation with small stimuli. The small gratings we used are approximately the size of a parafoveal V1 receptive field (Cavanaugh et al. 2002) and thus elicited a robust response in V1 neurons (mean peak firing rate of 31.8 ± 1.9 spikes/s). Large gratings encroach on the suppressive surround and thus led to weaker V1 responses (17.8 ± 0.7 spikes/s, P < 0.0001 for difference from responses to small gratings). In MT we observed more robust responses to large gratings than small ones (17.8 ± 1.9 vs. 10.9 ± 2.1 spikes/s, P = 0.055). This is because the small gratings were much smaller than the typical MT receptive field size at the eccentricity of our recordings (3–7 deg), whereas the large stimulus filled the MT RF. Thus small adapters caused a stronger loss of responsivity in MT, despite driving neurons roughly half as well as large gratings.
As for the response ratio, shifts in preference depended on stimulus size (F = 6.33, P = 0.01) and the neurons' offset from the adapter (F = 12.43, P < 0.0001), but we observed no difference between effects in V1 and MT (F = 1.19, P = 0.3). Adaptation with small gratings caused repulsive shifts in tuning in preferred adapted neurons in V1 (0.16 bandwidth units) and MT (0.17 bandwidth units, P = 0.9 for difference). Effects were weak for near- and far-flank adapted cells with no difference between areas (P > 0.4). The preference of off-flank adapted units shifted toward the adapter in both V1 (−0.07 bandwidth units) and MT (−0.2 bandwidth units, P = 0.02 for comparison with V1).
Whereas the most prominent effect of adapting with small gratings were repulsive shifts in preference, adaptation with large stimuli caused attractive shifts in preference in both areas (Fig. 2D). V1 neurons whose preferences fell within 1 bandwidth unit of the adapter (preferred and flank adapted cells) shifted −0.04 bandwidth units on average (P = 0.0009 for difference from 0) and MT neurons shifted −0.05 bandwidth units (P = 0.02 for difference with 0). Shifts in preference were significantly more attractive in both V1 (P < 0.0001) and MT (P = 0.01) for large compared with small gratings. There was no difference in the average shift in preference between V1 and MT for any offset when large stimuli were used (P > 0.1).
Finally, we observed clear bandwidth narrowing in both V1 and MT for both small and large gratings (Fig. 2, E and F). Bandwidth narrowing depended on the neurons' offset from the adapter (F = 41.33, P < 0.0001) but not on stimulus size (F = 1.40, P = 0.2; see also Wissig and Kohn 2012; Patterson et al. 2013) or cortical area (F = 3.63, P = 0.06). For large stimuli, however, MT did show a slightly greater bandwidth narrowing for preferred adapted cells (0.80 in V1, 0.62 in MT, P = 0.004) and greater broadening for off-flank adapted cells (1.04 in V1, 1.18 in MT, P = 0.005).
In summary, the nature and magnitude of effects induced by prolonged adaptation depended strongly on stimulus size but not on whether the recordings were in V1 or MT. In both areas, adaptation with small gratings caused a strong response reduction and repulsive shifts in preference for neurons slightly offset from the adapter. Adaptation with large gratings resulted in a weaker response reduction and more attractive shifts in both areas. This similarity occurred despite V1 neurons being driven more strongly by the small stimulus whereas MT neurons were driven more strongly by the large stimulus.
Size-dependent adaptation effects in MT of awake monkeys.
Our anesthetized data demonstrate that adaptation effects are strongly modulated by stimulus size. To determine whether effects in awake animals also depend on stimulus size, we measured direction tuning in MT of awake monkeys before and after adaptation. Given the need for maintained fixation during the entire trial, the adaptation duration was limited to 4 s. This duration is sufficient in anesthetized animals to reveal the size dependence of adaptation effects (Patterson et al. 2013). We measured responses from 100 to 400 ms after test stimulus onset to avoid the onset transient, for which adaptation effects are not size dependent (Patterson et al. 2013).
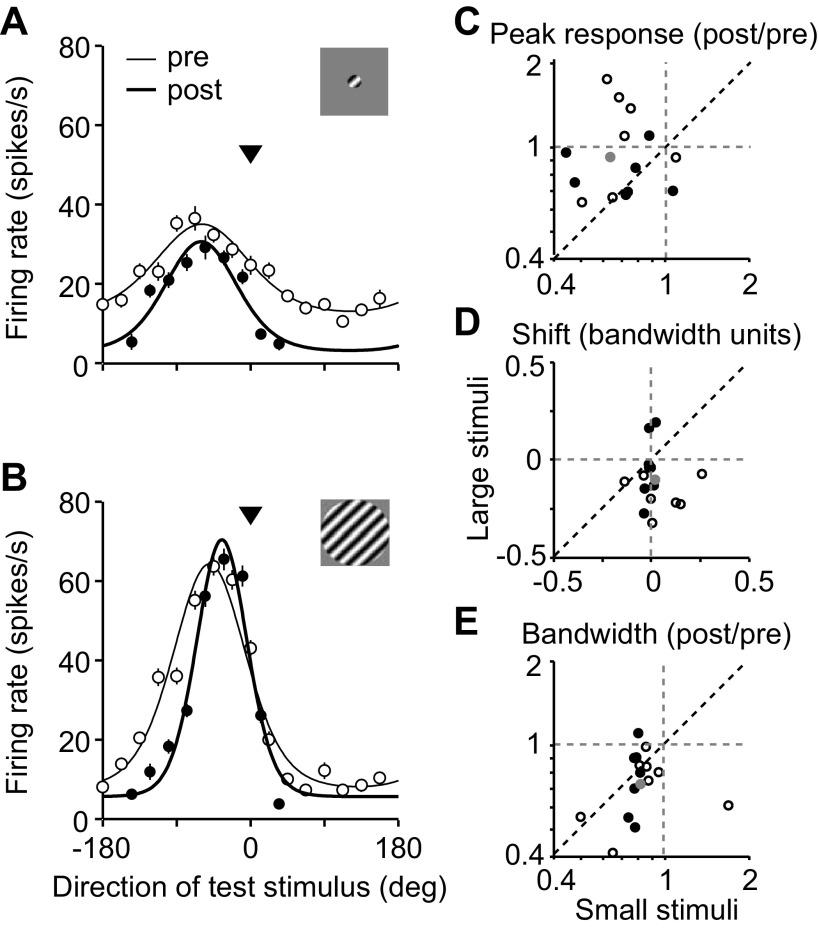
The direction tuning for one MT neuron is shown in Fig. 3, measured with small (Fig. 3A) and large (Fig. 3B) gratings. Adaptation with small stimuli caused a reduction in peak response (filled compared with open symbols) and a weak repulsive shift in preference (0.02 bandwidth units). With large gratings, responsivity increased after adaptation (response ratio of 1.09), although the neuron was driven much more strongly than with small gratings. Tuning also shifted toward the adapter (−0.13 bandwidth units).
Fig. 3.
Adaptation effects in MT of awake macaque monkeys. A and B: direction tuning of an example MT unit before (open circles) and after (filled circles) 4-s adaptation with small (A) or large gratings (B). Data points are mean measured responses; error bars indicate SE. Solid lines are von Mises fits to the data. Arrowhead indicates the direction of the adapter. C: a comparison of the peak response ratio for responses to small and large gratings. Each open and filled circle represents data from an individual MT unit; gray circle indicates mean values. Filled circles indicate cells whose preference was within 1 bandwidth unit of the adapter. D: same as C for shifts in preference. E: same as C for bandwidth ratio.
A similar size dependence was apparent in the effects measured in the full population, for which we obtained good measurements of tuning before and after adaptation for both stimulus sizes (n = 15 neurons passed the tuning selection criteria for both large and small adapters; see materials and methods). Figure 3, C–E, compares changes in responsivity, preference, and bandwidth for all cells for responses to small and large gratings. Filled symbols represent cases when the adapter fell within 1 bandwidth unit of the unit's preference; open symbols represent cases where the adapter was more offset. Peak responsivity (Fig. 3C) was reduced when small stimuli were used (mean ratio of 0.63 across all offsets, P = 0.001 for difference from 1) but not when large stimuli were used (0.92, P = 0.3). The response ratio was significantly smaller for small stimuli (P = 0.02). Tuning shifted toward the adapter when large stimuli were used (−0.11 bandwidth units, P = 0.01 for difference from 0) but not when small stimuli were used (0.02 bandwidth units, P = 0.4), and there was a significant difference between the two conditions (Fig. 3D; P = 0.02). A significant bandwidth narrowing was observed with both small (0.83, P = 0.01) and large gratings (0.72, P < 0.001), which was similar in magnitude for the two conditions (Fig. 3E; P = 0.13).
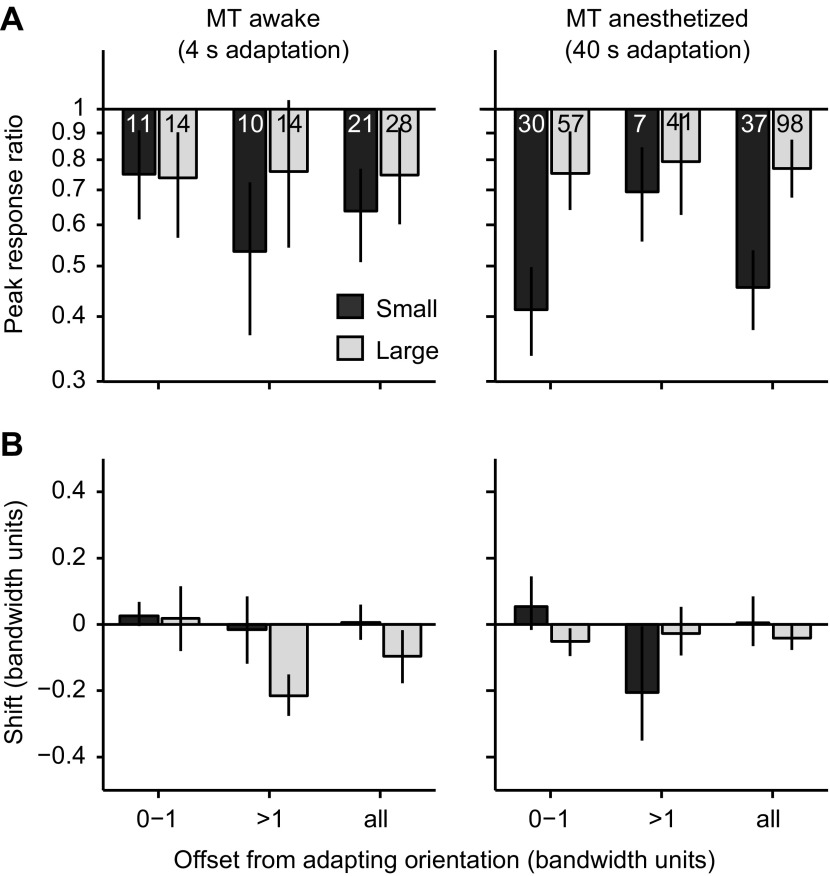
We performed additional analysis using units that met our selection criteria for responses to either small or large gratings. This yielded 21/51 (41%) recorded units for small stimuli and 28/58 (48%) for large stimuli. The effects in these cells are shown in Fig. 4 as a function of the offset of neuronal preference from the adapter; the data from anesthetized animals (Fig. 2) are replotted here to allow a more direct comparison. We compared effects in these units from awake animals with those measured in MT of anesthetized animals, using a three-way ANOVA with size (small vs. large), state (awake vs. anesthetized), and offset of neuronal preference as factors. For response ratios, this revealed an effect of stimulus size (P = 0.002) but not offset (P = 0.4) or state (P = 0.1). For shifts in preference, there was a significant influence of stimulus size (P = 0.01) and offset (P = 0.0003) but not state (P = 0.7). Bandwidth ratios were not significantly influenced by any factor (P > 0.08). Thus effects were clearly modulated by stimulus size and adapter offset, but there was no significant difference between data from anesthetized and awake MT.
Fig. 4.
Comparison of adaptation effects in MT of awake and anesthetized monkeys, as a function of offset of neuronal preference from the adapter. A: comparison of response ratios in awake (left) and anesthetized monkeys (right) for offsets of 0–1, >1, and all offsets. Dark shaded bars indicate responses with small gratings; light shaded bars data for large gratings. Numbers indicate numbers of cells. B: same as A for shifts in neuronal preferences.
We conclude that the influence of stimulus size on adaptation effects is robust to anesthesia. We note that although the differences between anesthetized and awake data sets did not reach statistical significance, our data do not allow us to determine whether adaptation effects are the same in these two experimental preparations. This is both because of the limited power afforded by our awake data and because of the difference in adaptation duration (4 s in the awake vs. 40 s in the anesthetized).
In summary, we found that adaptation effects in awake monkeys were size dependent, as they were in anesthetized animals. Adaptation with small gratings caused a significant response reduction, whereas large gratings did not reduce peak responsivity and caused tuning to shift more strongly toward the adapter.
Adaptation effects in direction-selective and orientation-selective V1 neurons.
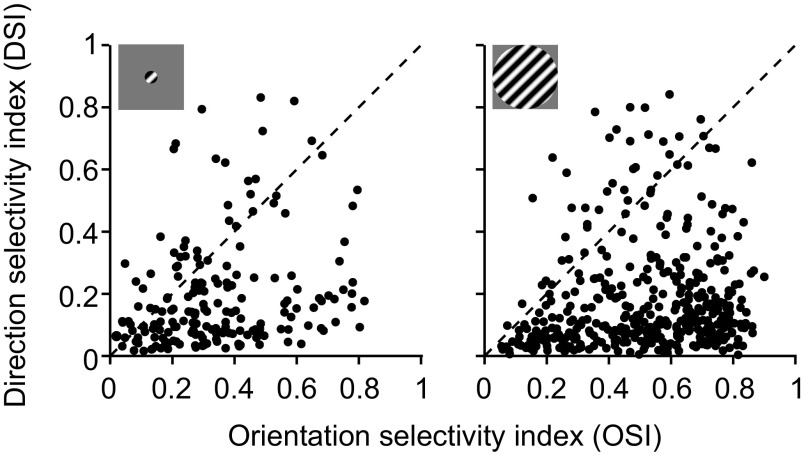
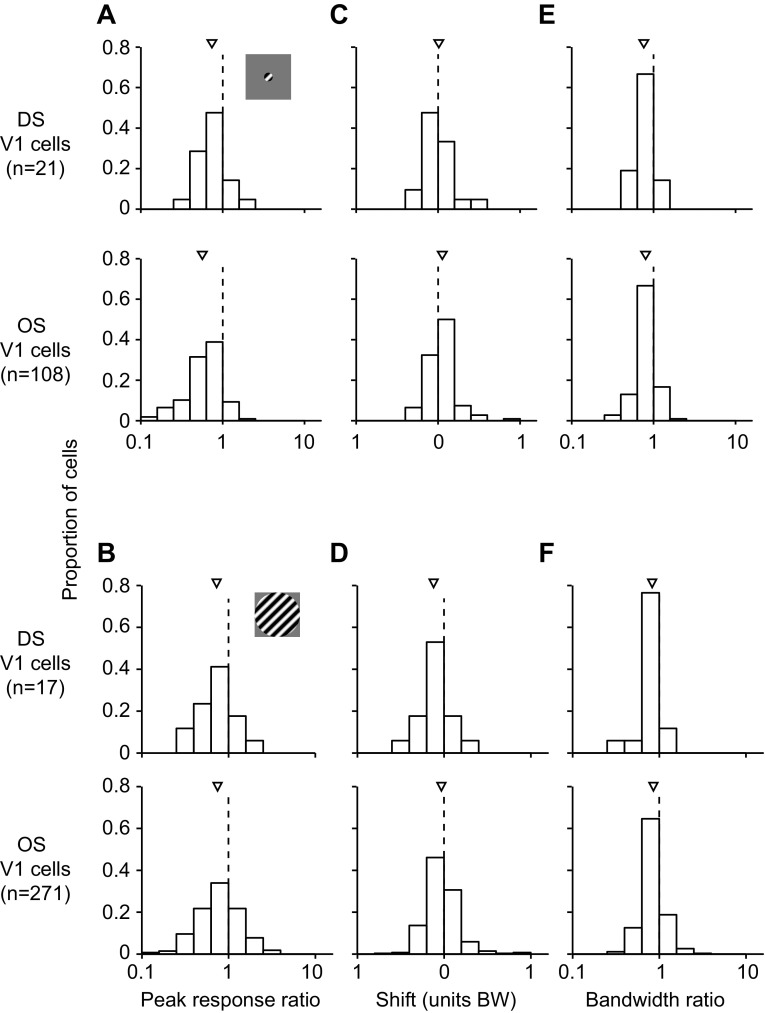
MT receives direct input from direction-selective (DS) V1 cells (Movshon and Newsome 1996). The majority of our sampled units were orientation selective (OS), and we wondered whether DS cells in V1 might adapt differently. Previous reports suggest that there is no difference between shifts in tuning preference in DS and OS V1 neurons (Kohn and Movshon 2004), but that study used stimuli that were optimized for each individual unit and did not investigate the size dependence of the induced effects. To determine whether DS and OS cells adapt similarly, we compared the effects of 40-s adaptation with small and large stimuli for these cell types.
We defined DS cells as those which had a direction selectivity index that was greater than the orientation selectivity index (see materials and methods). By this lax definition, 14% (85/600) of the recorded V1 units were DS (Fig. 5). We focused on a subset of units (n = 38) whose preference was within 1 bandwidth unit of the adapter, because effects were strongest for these neurons. Figure 6A shows the peak response ratios for the DS (top) and OS cells (bottom) for response to small gratings. There was no significant difference in these ratios between DS and OS cells (0.74 for DS vs. 0.56 for OS, P = 0.07). This was also the case for responses measured with large gratings (Fig. 6B; 0.73 for DS vs. 0.74 for OS, P = 0.9). Shifts in preference were repulsive and similar in magnitude for both cell types when small stimuli were used (Fig. 6C; 0.01 bandwidth units for DS, 0.05 bandwidth units for OS, P = 0.3). When large stimuli were used, shifts were attractive and did not differ significantly between cell classes (Fig. 6D; −0.12 bandwidth units for DS, −0.03 bandwidth units for OS, P = 0.053), although DS cells tended to shift more toward the adapter than OS cells. There was also no difference between the change in tuning bandwidth for DS and OS cells for responses to small (Fig. 6E; 0.76 for DS, 0.80 for OS, P = 0.5) or large gratings (Fig. 6F; 0.83 for DS, 0.86 for OS, P = 0.7).
Fig. 5.
Direction and orientation selectivity in V1 neurons. Orientation selectivity index (OSI) is plotted against direction selectivity index (DSI) for small (left) and large gratings (right). Each symbol represents data from a unit. We defined as direction selective those units falling above the diagonal (DSI > OSI).
Fig. 6.
Comparison of prolonged adaptation (40 s) effects for V1 direction-selective (DS) and orientation-selective (OS) units whose preference was within 1 bandwidth unit of the adapter. A and B: peak response ratio measured with small (A) and large gratings (B). Data from DS units are shown at top and those from OS units at bottom. Arrowheads indicate mean values. C and D: shifts in preference for responses measured with small (C) and large gratings (D). E and F: bandwidth ratio measured with small (E) and large gratings (F).
Adaptation effects were thus similar for V1 OS and DS cells. This suggests that the cells more likely to project directly to MT do not adapt differently from the rest of the population.
Effects of brief adaptation are similar in V1 and MT.
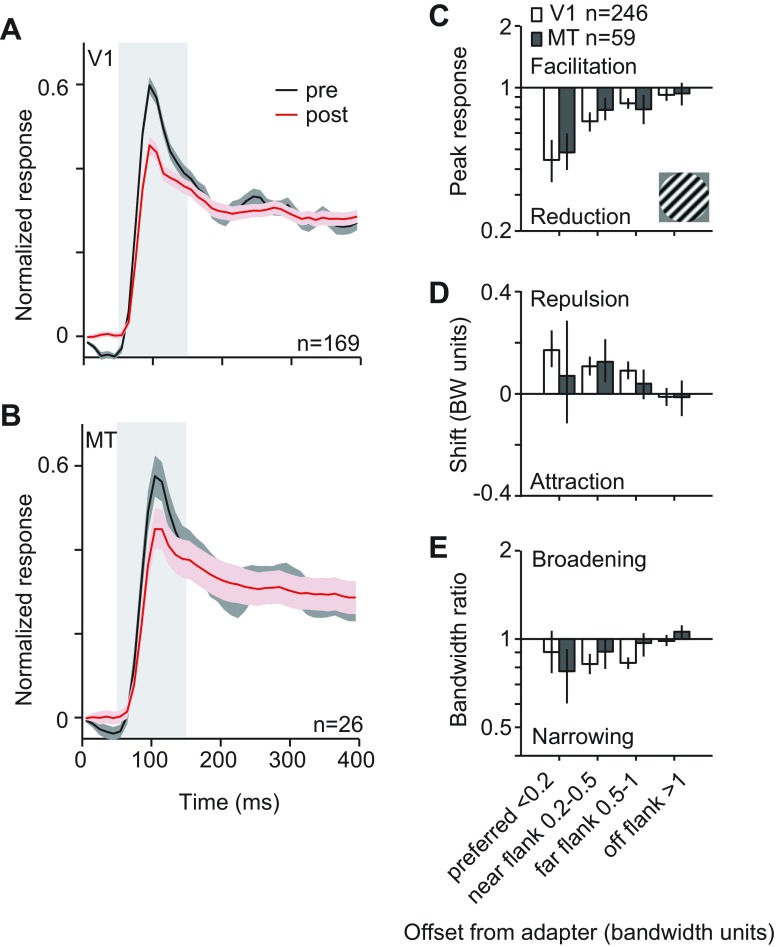
Brief (0.4 s) adaptation affects primarily the early response epoch, or onset transient, in V1 (Patterson et al. 2013). During this epoch, surround suppression is weak. As a result, the effects of brief adaptation with large grating stimuli are similar to those seen with small gratings: both cause a stimulus-specific loss of responsivity, leading to repulsive shifts in preference for neurons whose preference is slightly offset from the adapter (Patterson et al. 2013). We next sought to determine whether MT showed a similar behavior to V1 following brief adaptation with large stimuli.
We first compared response dynamics in V1 and MT, using each neuron's preferred stimulus. Figure 7, A and B, shows population peristimulus time histograms for V1 and MT, respectively, for neurons whose preference was within 1 bandwidth unit of the adapter. In both areas, adaptation reduced responsivity primarily in the initial response epoch (red after adaptation compared with black before), with little or no effect on the late response epoch. For this reason, we compared effects on tuning during the first 100 ms of the response (50 to 150 ms; gray highlighted regions in Fig. 7, A and B, slightly longer than the 50- to 100-ms epoch used in Patterson et al. 2013).
Fig. 7.
Comparison of brief adaptation effects in V1 and MT for large stimuli. A and B: peristimulus time histogram (PSTH) for the preferred stimulus for all units whose preference was within 1 bandwidth of the adapter. Data for before adaptation are shown in black and those for adaptation in red for units in V1 (A) and MT (B). Gray highlighted area indicates the time window used to measure tuning. Shading on curve indicates SE. We normalized the PSTH for each neuron by its peak amplitude and then averaged across units. C: peak response ratios, as a function of units' offset from adapter in bandwidth units, for V1 and MT units. D: shifts in preference for V1 and MT units. E: bandwidth ratios for V1 and MT units. Error bars indicate 95% CI.
Response ratios were similar in V1 and MT (Fig. 7C), with the effects depending on the preference of the neuron (2-way ANOVA; F = 11.46, P < 0.0001) but not area (F = 0.33, P = 0.6). Response ratios were smallest for preferred adapted cells (0.44 in V1 and 0.48 in MT). There was no difference between response ratios for V1 and MT neurons for any offset (P > 0.3). Similarly, shifts in preference were repulsive in both V1 and MT (Fig. 7D) and depended on cell preference (F = 5.37, P = 0.001) but not area (F = 0.89, P = 0.3). Preferred, near-flank, and far-flank neurons showed significant repulsive shifts in V1 (mean = 0.11; P < 0.0001, difference from 0) and MT (mean = 0.06; P = 0.03, difference from 0). There was no difference between V1 and MT at any stimulus offset (P > 0.5). Changes in tuning bandwidth changes also depended on the cell's preference (Fig. 7E; F = 4.77, P = 0.003) but not area (F = 0.48, P = 0.5).
In summary, we found that brief adaptation with large gratings had similar effects in V1 and MT. In both areas, adaptation affected primarily the initial response epoch and led to a strong loss of responsivity and repulsive shifts in preference. This is in contrast to the effects of prolonged adaptation with large gratings, which led to a weaker loss of responsivity and attractive shifts in both areas (Fig. 2). This is because prolonged, but not brief, adaptation weakens surround suppression. Thus effects of adaptation are similar in V1 and MT for responses measured with small and large gratings and after brief or prolonged adaptation.
DISCUSSION
We found that the effects of adaptation depended strongly on stimulus size and adaptation duration but were similar in V1 and MT. In both V1 and MT, prolonged adaptation with small and large stimuli generated opposite shifts in preference and different degrees of response reduction. In both areas, brief adaptation with large stimuli resulted in strong response reduction and repulsive shifts in preference. Our findings strongly indicate that previously reported disparities between adaptation effects in V1 (Dragoi et al. 2000, 2002; Muller et al. 1999) and MT (Kohn and Movshon 2004) are the result of differences in stimulus size (Patterson et al. 2013; Wissig and Kohn 2012) and do not reflect a fundamental difference in how these networks adjust to recent stimulus history. Previous V1 and MT studies used stimuli tailored to the receptive field properties of neurons in each area. Because of the larger spatial receptive fields of MT neurons, larger stimuli were used there, giving rise to the difference with effects previously reported in V1.
Although adaptation effects were not noticeably different between V1 and MT, we cannot exclude the possibility that there is some quantitative difference that would only be apparent with a larger sample of neurons. However, our sample size and statistical power was sufficient to reveal a strong influence of adaptation duration and stimulus size. Thus, if there are differences in adaptation effects between V1 and MT, they are substantially weaker than the influence of these other factors. We note also that our finding of similar effects in V1 and MT apply to our stimulus ensemble but do not imply that effects in these two areas need always be similar.
Several comparisons did reveal effects that were slightly different in the two areas. For instance, following prolonged adaptation with small stimuli, off-flank adapted cells shifted more strongly toward the adapter in MT. This difference might be explained by motion opponency in MT, an inhibitory input provided by cells with the opposite direction preference (Snowden et al. 1991). If off-flank adapted MT neurons received weaker inhibition from cells with the near-opposite preference (since these would be preferred adapted and thus their responsivity strongly reduced), this could cause disinhibition leading to attractive shifts. One might expect this effect to be particularly strong for small stimuli for which adaptation causes a stronger response reduction. This could also explain why we observed no shift on average, rather than repulsive shifts, in the awake MT data when small stimuli were used: for a substantial proportion of these neurons, the adapter was far from the preferred direction.
In addition, we found that prolonged adaptation effects in MT of awake monkeys also depend on stimulus size. This is consistent with the size dependence of perceptual aftereffects measured in human studies. For instance, the motion aftereffect induced by small adapters is stronger than that induced by large ones (Murakami and Shimojo 1995; Sachtler and Zaidi 1993; Tadin et al. 2003). Similarly, the perceptual effects of an adapter of a fixed size increase with eccentricity, perhaps because of weaker surround effects in larger, peripheral receptive fields (Johnston and Wright 1983; Murakami and Shimojo 1995; Tadin et al. 2003; see also Wissig and Kohn 2012). Although our awake data revealed a robust size modulation of adaptation effects, they did not allow us to establish that effects were the same in the two experimental preparations. Our sample size was insufficient for this purpose, and the adaptation duration, which can strongly influence effects (Fig. 7; Patterson et al. 2013), also differed between the anesthetized and awake experiments. However, previous work that affords a more straightforward comparison of adaptation effects in awake and anesthetized animals failed to find notable differences either in V1 (Dragoi et al. 2000; Muller et al. 1999; compared with Dragoi et al. 2002) or MT (Priebe et al. 2002).
To our knowledge, our study is the first to use the same stimulus ensemble to compare how adaptation alters tuning at successive stages of visual processing. Several previous studies, however, have compared adaptation effects on responsivity and contrast or coherence sensitivity across stages of the visual system. For instance, studies of V1 contrast adaptation failed to find changes in the LGN under similar stimulus conditions (Movshon and Lennie 1979; Ohzawa et al. 1985). Nelson (1991a) studied the time course of suppression induced in V1 by the brief presentation of a bar stimulus; a similar approach revealed some suppressive effects in the LGN, but these could not fully explain those in V1 (Nelson 1991b). McLelland and colleagues studied responses to afterimages induced by prolonged presentation of static images and found these decayed much more quickly in cortex (McLelland et al. 2010) than in the LGN (McLelland et al. 2009). Crowder et al. (2006) report similar effects of contrast adaptation in V1 and V2 of cat visual cortex. Finally, Priebe et al. (2002) showed that the spatial specificity of adaptation-induced changes MT responsivity could not be explained by effects in V1. However, several of these studies tailored stimuli to each neuron. This complicates the interpretation, because any similarity or difference between areas can reflect the preferences of neurons there (leading to systematic differences in the stimuli used), rather than how the areas adjust to a particular input. Perhaps more importantly, these previous studies did not explore how adaptation altered tuning, our present focus.
Previous work suggests that the effects of prolonged adaptation with drifting gratings in MT are inherited from early visual areas (Kohn and Movshon 2003): adapting a portion of an MT neuron's spatial receptive field with a small stimulus results in a strong change in contrast sensitivity at that location but not at other locations within the receptive field. Such spatial specificity suggests that effects are induced where receptive fields are smaller than in MT, such as V1, and these are then inherited by MT. Our finding that adaptation with small gratings caused a greater loss of MT responsivity than adaptation with large gratings is consistent with MT effects being inherited from early visual cortex. V1 responses were substantially stronger to small gratings than to large ones, and adaptation with small gratings resulted in a stronger loss of V1 responsivity. This is because large gratings recruit surround suppression; adaptation weakens the influence of the surround, leading to a disinhibition that partially offsets the adaptation-induced loss of responsivity within the receptive field. In MT, small gratings provided substantially weaker drive than large gratings, because the larger stimulus was not sufficiently big to recruit strong surround suppression in MT. Despite evoking weaker responses in MT, small gratings caused a stronger loss of responsivity, as they did in V1. This can be attributed to effects originating in V1 or elsewhere in the early visual system.
It is important to note that the similar way in which V1 and MT tuning preferences are altered for stimuli of different sizes and adapters of different durations is not by itself strong evidence that MT effects are inherited from V1. First, the similarity we observed was evident when effects were measured relative to preadaptation tuning bandwidth; in absolute terms, the shifts in MT preference were thus roughly 50% larger than in V1. Second, previous work has shown that altering feedforward input to a recurrent network can give rise to distinct tuning effects there (Compte and Wang 2006; Kohn and Movshon 2004). In a network with strong recurrent connections, the tuning of an individual neuron can reflect dynamic interactions among neurons with different tuning properties. These interactions can manifest themselves in many different ways, for instance, as motion opponency or spatial inhomogeneities within the receptive field (Richert et al. 2013). If one subpopulation of neurons within such a recurrent network receives weakened feedforward input, these interactions are changed. This can result, for instance, in reduced lateral inhibition to neighboring neurons, causing their tuning to shift toward the adapter. Thus the similar effects of our adapting stimulus ensemble on the tuning of V1 and MT neurons do not follow directly from the suggestion that MT passively inherits effects from V1. Rather, it suggests a common active strategy for adjusting to recent sensory drive in these two networks, perhaps implemented through distinct circuit mechanisms.
Our explanation for the size dependence of adaptation effects is that the surround is weakened after prolonged stimulation (Wissig and Kohn 2012; Patterson et al. 2013). Previous modeling work by Teich and Qian (2003) showed that changes in the relative strength of recurrent excitatory and inhibitory connections can generate opposite shifts in neuronal preferences and a range of effects on responsivity. Our explanation is in no way inconsistent with this model. Our explanation provides insight into the plasticity triggered by stimuli of different size and duration, but does specify how this occurs; the Teich and Qian model is specific about the mechanisms underlying changes in tuning but does not explain under what stimulus conditions these are likely to be recruited. It is tempting to relate these, for instance, by equating a weakened surround with weaker inhibition, but this is simplistic and likely incorrect. The mechanisms of surround suppression remain poorly understood (Angelucci and Bressloff 2006), and recent work has provided inconsistent answers as to its relationship to inhibitory input (Haider et al. 2010; Ozeki et al. 2009).
Although our primary focus is to understand how the visual system adapts to its environment, our findings have important implications for functional MRI (fMRI) studies that use adaptation as a tool to infer selectivity. In such studies, feature selectivity is inferred from the reduced blood oxygen level-dependent response that follows the repeated presentation of two identical stimuli compared with the response that follows the presentation of two stimuli that differ along some feature dimension (Krekelberg et al. 2006a). Our finding that large stimuli cause less response reduction than small stimuli (Fig. 2, A and B) shows that this inference is only valid if one takes into account the size of the stimulus compared with the typical receptive field size in the area of interest. With this knowledge, the finding that fMRI adaptation increases along the visual hierarchy is easily understood as the result of an increase in receptive field size, rather than a counterintuitive increase in orientation selectivity or a true change in adaptability (see Wissig and Kohn 2012 for further discussion). Our findings may also explain why some high-level feature changes (e.g., faces vs. houses) result in selective fMRI adaptation in high-level areas such as the fusiform face area but not in V1, even though pictures of faces likely differ from pictures of houses in local orientation content (Krekelberg et al. 2006a). Future fMRI adaptation studies should therefore take average receptive field size into account or vary image size, especially when comparing selectivity across areas.
GRANTS
This work was supported by National Eye Institute Grants EY016774 and EY017605 and Research to Prevent Blindness. C. A. Patterson and S. C. Wissig were supported in part by National Institutes of Health Medical Scientist Training Program Grant T32 GM007288.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.P. and A.K. conception and design of research; C.A.P., J.D., S.C.W., and B.K. performed experiments; C.A.P. analyzed data; C.A.P. and A.K. interpreted results of experiments; C.A.P. and A.K. prepared figures; C.A.P. and A.K. drafted manuscript; C.A.P., J.D., S.C.W., B.K., and A.K. edited and revised manuscript; C.A.P., J.D., S.C.W., B.K., and A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Amin Zandvakili, Xiaoxuan Jia, and Seiji Tanabe for assistance with data collection.
REFERENCES
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol 52: 1106–1130, 1984 [DOI] [PubMed] [Google Scholar]
- Angelucci A, Bressloff PC. Contribution of feedforward, lateral, and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res 154: 93–120, 2006 [DOI] [PubMed] [Google Scholar]
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron 36: 909–919, 2002 [DOI] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci 28: 157–189, 2005 [DOI] [PubMed] [Google Scholar]
- Brown SG, Masland RH. Spatial scale and cellular substrate of contrast adaptation by retinal ganglion cells. Nat Neurosci 4: 44–51, 2001 [DOI] [PubMed] [Google Scholar]
- Camp AJ, Tailby C, Solomon SG. Adaptable mechanisms that regulate the contrast response of neurons in the primate lateral geniculate nucleus. J Neurosci 29: 5009–5021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Barlow HB, O'Keefe LP, Poirson AB, Movshon JA. Adaptation to contingencies in macaque primary visual cortex. Philos Trans R Soc Lond B Biol Sci 352: 1149–1154, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol 88: 2530–2546, 2002 [DOI] [PubMed] [Google Scholar]
- Chander D, Chichilnisky EJ. Adaptation to temporal contrast in primate and salamander retina. J Neurosci 21: 9904–9916, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Dan Y, Li CY. Stimulation of non-classical receptive field enhances orientation selectivity in the cat. J Physiol 564: 233–243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Wang XJ. Tuning curve shift by attention modulation in cortical neurons: a computational study of its mechanisms. Cereb Cortex 16: 761–778, 2006 [DOI] [PubMed] [Google Scholar]
- Crowder NA, Price NS, Hietanen MA, Dreher B, Clifford CW, Ibbotson MR. Relationship between contrast adaptation, and orientation tuning in V1 and V2 of cat visual cortex. J Neurophysiol 95: 271–283, 2006 [DOI] [PubMed] [Google Scholar]
- Dragoi V, Sharma J, Miller EK, Sur M. Dynamics of neuronal sensitivity in visual cortex and local feature discrimination. Nat Neurosci 5: 883–891, 2002 [DOI] [PubMed] [Google Scholar]
- Dragoi V, Sharma J, Sur M. Adaptation-induced plasticity of orientation tuning in adult visual cortex. Neuron 28: 287–298, 2000 [DOI] [PubMed] [Google Scholar]
- El-Shamayleh Y, Movshon JA. Neuronal responses to texture-defined form in macaque visual area V2. J Neurosci 31: 8543–8555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsen G, Shen YS, Yao H, Spor G, Li C, Dan Y. Dynamic modification of cortical orientation tuning mediated by recurrent connections. Neuron 36: 945–954, 2002 [DOI] [PubMed] [Google Scholar]
- Ghisovan N, Nemri A, Shumikhina S, Molotchnikoff S. Long adaptation reveals mostly attractive shifts of orientation tuning in cat primary visual cortex. Neuroscience 164: 1274–1283, 2009 [DOI] [PubMed] [Google Scholar]
- Haider B, Krause MR, Duque A, Yu Y, Touryan J, Mazer JA, McCormick DA. Synaptic and network mechanisms of sparse and reliable visual cortical activity during nonclassical receptive field stimulation. Neuron 65: 107–121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann TS, Bremmer F, Albright TD, Krekelberg B. Receptive field positions in area MT during slow eye movements. J Neurosci 31: 10437–10444, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Wright MJ. Visual motion and cortical velocity. Nature 304: 436–438, 1983 [DOI] [PubMed] [Google Scholar]
- Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol 97: 3155–3164, 2007 [DOI] [PubMed] [Google Scholar]
- Kohn A, Movshon JA. Neuronal adaptation to visual motion in area MT of the macaque. Neuron 39: 681–691, 2003 [DOI] [PubMed] [Google Scholar]
- Kohn A, Movshon JA. Adaptation changes the direction tuning of macaque MT neurons. Nat Neurosci 7: 764–772, 2004 [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Albright TD. Motion mechanisms in macaque MT. J Neurophysiol 93: 2908–2921, 2005 [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Boynton GM, van Wezel RJ. Adaptation: from single cells to BOLD signals. Trends Neurosci 29: 250–256, 2006a [DOI] [PubMed] [Google Scholar]
- Krekelberg B, van Wezel RJ, Albright TD. Adaptation in macaque MT reduces perceived speed, and improves speed discrimination. J Neurophysiol 95: 255–270, 2006b [DOI] [PubMed] [Google Scholar]
- Liu Y, Murray SO, Jagadeesh B. Time course and stimulus dependence of repetition-induced response suppression in inferotemporal cortex. J Neurophysiol 101: 418–436, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland D, Ahmed B, Bair W. Responses to static visual images in macaque lateral geniculate nucleus: implications for adaptation, negative afterimages, and visual fading. J Neurosci 29: 8996–9001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland D, Baker PM, Ahmed B, Bair W. Neuronal responses during and after the presentation of static visual stimuli in macaque primary visual cortex. J Neurosci 30: 12619–12631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Lennie P. Pattern-selective adaptation in visual cortical neurones. Nature 278: 850–852, 1979 [DOI] [PubMed] [Google Scholar]
- Movshon JA, Newsome WT. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J Neurosci 16: 7733–7741, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science 285: 1405–1408, 1999 [DOI] [PubMed] [Google Scholar]
- Murakami I, Shimojo S. Modulation of motion aftereffect by surround motion, and its dependence on stimulus size and eccentricity. Vision Res 35: 1835–1844, 1995 [DOI] [PubMed] [Google Scholar]
- Nelson SB. Temporal interactions in the cat visual system. I. Orientation-selective suppression in the visual cortex. J Neurosci 11: 344–356, 1991a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB. Temporal interactions in the cat visual system. II. Suppressive and facilitatory effects in the lateral geniculate nucleus. J Neurosci 11: 357–368, 1991b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat's visual system. J Neurophysiol 54: 651–667, 1985 [DOI] [PubMed] [Google Scholar]
- Ozeki H, Finn IM, Schaffer ES, Miller KD, Ferster D. Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron 62: 578–592, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CA, Wissig SC, Kohn A. Distinct effects of brief and prolonged adaptation in primary visual cortex of macaque monkeys. J Neurosci 33: 532–543, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Churchland MM, Lisberger SG. Constraints on the source of short-term motion adaptation in macaque area MT. I. the role of input and intrinsic mechanisms. J Neurophysiol 88: 354–369, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert M, Albright TD, Krekelberg B. The complex structure of receptive fields in the middle temporal area. Front Syst Neurosci 7: 2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F. Temporal contrast adaptation in salamander bipolar cells. J Neurosci 21: 9445–9454, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach DL, Hawken MJ, Shapley R. Dynamics of orientation tuning in macaque primary visual cortex. Nature 387: 281–284, 1997 [DOI] [PubMed] [Google Scholar]
- Sachtler WL, Zaidi Q. Effect of spatial configuration on motion aftereffects. J Opt Soc Am A 10: 1433–1449, 1993 [DOI] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron 49: 307–318, 2006 [DOI] [PubMed] [Google Scholar]
- Schlack A, Krekelberg B, Albright TD. Recent history of stimulus speeds affects the speed tuning of neurons in area MT. J Neurosci 27: 11009–11018, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol 79: 3272–3278, 1998 [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Treue S, Erickson RG, Andersen RA. The response of area MT and V1 neurons to transparent motion. J Neurosci 11: 2768–2785, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SG, Peirce JW, Dhruv NT, Lennie P. Profound contrast adaptation early in the visual pathway. Neuron 42: 155–162, 2004 [DOI] [PubMed] [Google Scholar]
- Stocker AA, Simoncelli EP. Noise characteristics and prior expectations in human visual speed perception. Nat Neurosci 9: 578–585, 2006 [DOI] [PubMed] [Google Scholar]
- Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature 424: 312–315, 2003 [DOI] [PubMed] [Google Scholar]
- Teich AF, Qian N. Learning and adaptation in a recurrent model of V1 orientation selectivity. J Neurophysiol 89: 2086–2100, 2003 [DOI] [PubMed] [Google Scholar]
- Van Wezel RJ, Britten KH. Motion adaptation in area MT. J Neurophysiol 88: 3469–3476, 2002 [DOI] [PubMed] [Google Scholar]
- Webster MA. Adaptation and visual coding. J Vis 11: 3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissig SC, Kohn A. The influence of surround suppression on adaptation effects in primary visual cortex. J Neurophysiol 107: 3370–3384, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lisberger SG. Relationship between adapted neural population responses in MT and motion adaptation in speed and direction of smooth-pursuit eye movements. J Neurophysiol 101: 2693–2707, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Contrast adaptation in subthreshold and spiking responses of mammalian Y-type retinal ganglion cells. J Neurosci 25: 860–868, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]