Abstract
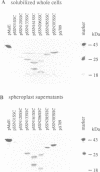
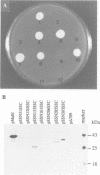
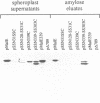
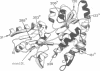
The maltose binding protein (MBP or MalE) of Escherichia coli is the periplasmic component of the transport system for malto-oligosaccharides. It is synthesized in the cytoplasm with an N-terminal signal peptide that is cleaved upon export. We examined whether active MBP could assemble into an active protein in bacteria, from N- and COOH-terminal complementary protein fragments encoded by distinct, engineered segments of its structural gene. We found export and functional periplasmic assembly of MBP fragments, despite the complex polypeptide chain topology of this protein, if two conditions were satisfied. First, each of the two fragments must carry a signal peptide. Second, the boundaries between the two fragments must correspond to a permissive site within the protein. Functional assembly of active MBP occurred in five cases where these conditions were met: sites after residues 133, 161, 206, 285 and 303; but not in three other cases where the break junction corresponded to a non-permissive site: after residues 31, 120 and 339. Thus, permissive sites which were initially characterized because they could accept extensive genetic insertion/deletion modifications without loss of most biological properties provide a means of defining complementing protein fragments. This observation opens a way to study genetically the relationships between protein export and folding into the periplasm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Andersson H., von Heijne G. A 30-residue-long "export initiation domain" adjacent to the signal sequence is critical for protein translocation across the inner membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9751–9754. doi: 10.1073/pnas.88.21.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkowitz R. A., Joly J. C., Wickner W. Translocation can drive the unfolding of a preprotein domain. EMBO J. 1993 Jan;12(1):243–253. doi: 10.1002/j.1460-2075.1993.tb05650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr Export of the periplasmic maltose-binding protein of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):401–439. doi: 10.1007/BF00763175. [DOI] [PubMed] [Google Scholar]
- Bassford P., Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. doi: 10.1038/277538a0. [DOI] [PubMed] [Google Scholar]
- Betton J. M., Desmadril M., Yon J. M. Detection of intermediates in the unfolding transition of phosphoglycerate kinase using limited proteolysis. Biochemistry. 1989 Jun 27;28(13):5421–5428. doi: 10.1021/bi00439a016. [DOI] [PubMed] [Google Scholar]
- Betton J. M., Martineau P., Saurin W., Hofnung M. Location of tolerated insertions/deletions in the structure of the maltose binding protein. FEBS Lett. 1993 Jun 28;325(1-2):34–38. doi: 10.1016/0014-5793(93)81409-s. [DOI] [PubMed] [Google Scholar]
- Bibi E., Kaback H. R. In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4325–4329. doi: 10.1073/pnas.87.11.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman A. I., Puziss J. W., Bassford P. J., Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993 Mar;12(3):879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev I. K., Ray P. H., Novak P. Minimum substrate sequence for signal peptidase I of Escherichia coli. J Biol Chem. 1990 Nov 25;265(33):20069–20072. [PubMed] [Google Scholar]
- Duplay P., Bedouelle H., Fowler A., Zabin I., Saurin W., Hofnung M. Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10606–10613. [PubMed] [Google Scholar]
- Duplay P., Hofnung M. Two regions of mature periplasmic maltose-binding protein of Escherichia coli involved in secretion. J Bacteriol. 1988 Oct;170(10):4445–4450. doi: 10.1128/jb.170.10.4445-4450.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplay P., Szmelcman S., Bedouelle H., Hofnung M. Silent and functional changes in the periplasmic maltose-binding protein of Escherichia coli K12. I. Transport of maltose. J Mol Biol. 1987 Apr 20;194(4):663–673. doi: 10.1016/0022-2836(87)90243-9. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Klotz U. Affinity chromatographic isolation of the periplasmic maltose binding protein of Escherichia coli. FEBS Lett. 1978 Oct 15;94(2):213–217. doi: 10.1016/0014-5793(78)80940-5. [DOI] [PubMed] [Google Scholar]
- Herold M., Leistler B., Hage A., Luger K., Kirschner K. Autonomous folding and coenzyme binding of the excised pyridoxal 5'-phosphate binding domain of aspartate aminotransferase from Escherichia coli. Biochemistry. 1991 Apr 16;30(15):3612–3620. doi: 10.1021/bi00229a004. [DOI] [PubMed] [Google Scholar]
- Ito K., Beckwith J. R. Role of the mature protein sequence of maltose-binding protein in its secretion across the E. coli cytoplasmic membrane. Cell. 1981 Jul;25(1):143–150. doi: 10.1016/0092-8674(81)90238-5. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A. Molecular chaperones and protein translocation across the Escherichia coli inner membrane. Mol Microbiol. 1991 Jan;5(1):19–22. doi: 10.1111/j.1365-2958.1991.tb01821.x. [DOI] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron(III) hydroxamate transport of Escherichia coli: restoration of iron supply by coexpression of the N- and C-terminal halves of the cytoplasmic membrane protein FhuB cloned on separate plasmids. Mol Gen Genet. 1990 Sep;223(3):379–384. doi: 10.1007/BF00264443. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laminet A. A., Plückthun A. The precursor of beta-lactamase: purification, properties and folding kinetics. EMBO J. 1989 May;8(5):1469–1477. doi: 10.1002/j.1460-2075.1989.tb03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio R., Vogel Z., Wess J. Reconstitution of functional muscarinic receptors by co-expression of amino- and carboxyl-terminal receptor fragments. FEBS Lett. 1993 Mar 15;319(1-2):195–200. doi: 10.1016/0014-5793(93)80066-4. [DOI] [PubMed] [Google Scholar]
- Martineau P., Guillet J. G., Leclerc C., Hofnung M. Expression of heterologous peptides at two permissive sites of the MalE protein: antigenicity and immunogenicity of foreign B-cell and T-cell epitopes. Gene. 1992 Apr 1;113(1):35–46. doi: 10.1016/0378-1119(92)90667-e. [DOI] [PubMed] [Google Scholar]
- Martineau P., Szmelcman S., Spurlino J. C., Quiocho F. A., Hofnung M. Genetic approach to the role of tryptophan residues in the activities and fluorescence of a bacterial periplasmic maltose-binding protein. J Mol Biol. 1990 Jul 5;214(1):337–352. doi: 10.1016/0022-2836(90)90165-I. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Kimura E., Mizushima S. Complementation of two overlapping fragments of SecA, a protein translocation ATPase of Escherichia coli, allows ATP binding to its amino-terminal region. J Biol Chem. 1990 May 25;265(15):8760–8765. [PubMed] [Google Scholar]
- Minard P., Hall L., Betton J. M., Missiakas D., Yon J. M. Efficient expression and characterization of isolated structural domains of yeast phosphoglycerate kinase generated by site-directed mutagenesis. Protein Eng. 1989 Oct;3(1):55–60. doi: 10.1093/protein/3.1.55. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Park S., Liu G., Topping T. B., Cover W. H., Randall L. L. Modulation of folding pathways of exported proteins by the leader sequence. Science. 1988 Feb 26;239(4843):1033–1035. doi: 10.1126/science.3278378. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986 Sep 12;46(6):921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Randall L. L. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell. 1983 May;33(1):231–240. doi: 10.1016/0092-8674(83)90352-5. [DOI] [PubMed] [Google Scholar]
- Reed K. E., Cronan J. E., Jr Escherichia coli exports previously folded and biotinated protein domains. J Biol Chem. 1991 Jun 25;266(18):11425–11428. [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Shiba K., Schimmel P. Functional assembly of a randomly cleaved protein. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1880–1884. doi: 10.1073/pnas.89.5.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H. A. Active transport of maltose in Escherichia coli K12. Role of the periplasmic maltose-binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J Biol Chem. 1982 May 25;257(10):5455–5461. [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Spurlino J. C., Lu G. Y., Quiocho F. A. The 2.3-A resolution structure of the maltose- or maltodextrin-binding protein, a primary receptor of bacterial active transport and chemotaxis. J Biol Chem. 1991 Mar 15;266(8):5202–5219. doi: 10.2210/pdb1mbp/pdb. [DOI] [PubMed] [Google Scholar]
- Tani K., Tokuda H., Mizushima S. Translocation of ProOmpA possessing an intramolecular disulfide bridge into membrane vesicles of Escherichia coli. Effect of membrane energization. J Biol Chem. 1990 Oct 5;265(28):17341–17347. [PubMed] [Google Scholar]
- Taniuchi H., Parr G. R., Juillerat M. A. Complementation in folding and fragment exchange. Methods Enzymol. 1986;131:185–217. doi: 10.1016/0076-6879(86)31042-5. [DOI] [PubMed] [Google Scholar]
- Toyama H., Tanizawa K., Yoshimura T., Asano S., Lim Y. H., Esaki N., Soda K. Thermostable alanine racemase of Bacillus stearothermophilus. Construction and expression of active fragmentary enzyme. J Biol Chem. 1991 Jul 25;266(21):13634–13639. [PubMed] [Google Scholar]
- Wetlaufer D. B. Folding of protein fragments. Adv Protein Chem. 1981;34:61–92. doi: 10.1016/s0065-3233(08)60518-5. [DOI] [PubMed] [Google Scholar]
- Wetlaufer D. B. Nucleation, rapid folding, and globular intrachain regions in proteins. Proc Natl Acad Sci U S A. 1973 Mar;70(3):697–701. doi: 10.1073/pnas.70.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- Wrubel W., Stochaj U., Sonnewald U., Theres C., Ehring R. Reconstitution of an active lactose carrier in vivo by simultaneous synthesis of two complementary protein fragments. J Bacteriol. 1990 Sep;172(9):5374–5381. doi: 10.1128/jb.172.9.5374-5381.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. R., Schachman H. K. In vivo formation of active aspartate transcarbamoylase from complementing fragments of the catalytic polypeptide chains. Protein Sci. 1993 Jun;2(6):1013–1023. doi: 10.1002/pro.5560020614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon J. M., Betton J. M. Protein folding in vitro and in the cellular environment. Biol Cell. 1991;71(1-2):17–23. doi: 10.1016/0248-4900(91)90047-q. [DOI] [PubMed] [Google Scholar]