Abstract
Background:
It has been shown that plants from the family Rhamnaceae possess anticancer activity. In this study, we sought to determine if Ziziphus spina-christi, a species from this family, has cytotoxic effect on cancer cell lines.
Materials and Methods:
Using maceration method, different extracts of leaves of Z. spina-christi were prepared. Hexane, chloroform, chloroform-methanol (9:1), methanol-water (7:1) methanol, butanol and water were used for extraction, after preliminary phytochemical analyses were done. The cytotoxic activity of the extracts against Hela and MDA-MB-468 tumor cells was evaluated by MTT assay. Briefly, cells were seeded in microplates and different concentrations of extracts were added. After incubation of cells for 72 h, their viability was evaluated by addition of tetrazolium salt solution. After 3 h medium was aspirated, dimethyl sulfoxide was added and absorbance was determined at 540 nm with an ELISA plate reader. Extracts were considered cytotoxic when more than 50% reduction on cell survival was observed.
Results:
Hexane, chloroform, chloroform-methanol, butanol, methanol-water and aqueous extracts of Z. spina-christi significantly and concentration-dependently reduced viability of Hela and MAD-MB-468 cells. In the both cell lines, chloroform-methanol extract of Z. spina-christi was more potent than the other extracts.
Results:
From the finding of this study it can be concluded that Z. spina-christi is a good candidate for further study for new cytotoxic agents.
Keywords: Cytotoxicity; Hela; MDA-MB-468; 3-(4,5-dimethylthiazol-2-yl)-2; 5-diphenyl tetrazolium bromide assay; Ziziphus spina-christi
INTRODUCTION
Cancer, one of the leading causes of death worldwide, accounts for about 13% of all deaths in 2008.[1] During the last decades, we are encountering with increase in the incidence of cancer.[2] Despite the progress in our knowledge about cancer, recent strategies for cancer treatment were not very effective. Therefore, scientists are still trying to find new compounds for the treatment of this disease. It has been shown that plants provide important resources for a large number of compounds with anti-tumor activity.[3,4,5] In recent years, a significant percentage of anti-cancer drugs are in clinical trials that are dependent on natural resources.[6]
During the last few decades, chemo preventive and chemotherapeutic compounds including colchicine, vincristine, vinblastine, podophyllotoxin and taxol were isolated from various plants and used against various types of cancers.[7,8,9,10]
Cytotoxic activity of plants from family Rhamnaceae has been shown in several studies.[5,11] The family of Rhamnaceae consists of about 50-60 genera and approximately 870-900 species. They are flowering plants, mostly trees, shrubs and some vines with worldwide distribution, but are more common in the subtropical and tropical regions. Ziziphus, one of the genus of the family of Rhamnaceae, consist of more than 40 species of spiny shrubs and small trees.
It has been shown that Ziziphus jujube mill, a member of the genus Ziziphus, possess different effects including antimicrobial, antioxidant, immunostimulant, antidiabetic, antihyperglycemic and anticancer effects.[12,13]
One of the active component of Ziziphus species e.g., Z. mauritiana, Z. rugosa and Z. oenoplia is betulinic acid. Studies have shown that betulinic acid selectively inhibited the growth of human melanoma cell line in vitro and it is in clinical or preclinical development for cancer therapy.[5]
Z. spina–christi L., commonly known by the Persian name, “Konar” or “Sedr”, is an armed shrub or tree, which widely growth in the southern of Iran. The plant fruit is edible sweet drupe and its leaves have been used as stomachic, emollient, antiulcer, disinfectant and antifungal in the Iranian traditional medicine. Z. spina–christi is well known among people as a good hair and body detergent.[14,15,16,17] The phytochemical and pharmacological studies of the Z. spina–christi have been the subject of some research in the recent years. These studies confirmed that essential oil such as geranyl acetate, methyl hexadecanoate and methyl octadecanoate, peptide and cyclopeptide alkaloids like spinanine-A, tanines, sterols like β-sitosterol, flavonoids such as rutin and quercetin derivatives, triterpenoid sapogenins and saponins such as betulinic acid are the main constituents of the leaves of Z. spina–christi.[15,18,19] Biological and pharmacological tests have shown antibacterial, antiviral and antidiabetic effects of the extracts or fractions of the leaves of this plant.[14,16,17]
Limited studies have shown that crude extract of Z. spina-christi had cytotoxic effect on tumor cell lines.[11] In this study, we used solvents with different polarity for extraction of ingredients of Z. spina-christi and evaluated their cytotoxic effects on Hela and MDA-MB-468 cells.
MATERIALS AND METHODS
Materials
Compounds used were: Methanol, butanol, chloroform, hexane, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), NaCl, KCl, NaOH, HCl, H2SO4, NaHCO3, Na2HPO4 (Merck, Germany), penicillin/streptomycin (Sigma, USA), RPMI1640, fetal calf serum (FCS), sodium pyruvate, trypsin, L-glutamine (Gibco, Scotland), dimethyl sulfoxide (DMSO) (Fluka, Italy), doxorubicin (Farmitalia, Italy).
Plant material
The leaves of Z. spina-christi were bought from local market of Isfahan, Iran and identified in the School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
Extraction and isolation
One hundred grams of dried powder was extracted by 300 ml of hexane using maceration method.[4] The residue was subjected to more extraction using 300 ml of chloroform, chloroform-methanol (9:1), methanol-water (7-1) and methanol, respectively. Butanolic and aqueous extracts were then obtained from the methanolic extract. The extracts were concentrated by a rotary evaporator and freeze dryer. The extracts were dried in vacuum oven at 40°C for 5 days. Then they were stored at 4°C until used. Ten mg of the solid residues were dissolved in 100 μl of DMSO and diluted to 2 ml with phosphate buffer saline (PBS) and filtered through 0.22 μ microbiological filters. Dilution was continued so that the final concentrations of extracts were 10, 100, 200, 500, 1000 and 5000 μg/ml.
Preliminary phytochemical analyses
The presence of alkaloids, glycosides, flavonoids, tannins, saponins and anthraquinones in the leaves of Z. spina-christi was tested using standard qualitative methods described earlier.[20,21]
Cell lines
Hela (human cervix carcinoma) and MDA-MB-468 (Human breast carcinoma), cell lines were purchased from the cell bank of Pasture Institute, Tehran, Iran.
Maintenance of human cell lines
Cells were grown in RPMI-1640 [supplemented with 10% of FCS, penicillin/streptomycin (50 IU ml−1 and 500 μg ml−1, respectively), sodium pyruvate (1 mM), NaHCO3 and L-glutamine (2 mM)]. Using 0.22 μ microbiological filters, completed media was steril ized and kept at 4°C prior to use. Cells were maintained and grown in RPMI 1640 up to 15 subcultures. A sample of each cell lines was frozen and kept under liquid nitrogen for future studies.
Cytotoxicity evaluation of extracts
Cytotoxic effect of the extracts with different polarity against Hela and MDA-MB-468 human tumor cell lines was determined by a rapid colorimetric assay, using MTT.[22] In this assay soluble MTT is metabolized by mitochondrial enzyme activity of viable tumor cells, into an insoluble colored formazan product. Subsequently formazan were dissolved in DMSO and measured spectrophotometrically at 540 nm. Briefly, 200 μl of cell suspension (5* 104 cells per ml of media) was seeded in 96-well microplates and incubated for 24 h (37°C, 5% CO2 air humidified). Then 20 μl of each concentration of different extracts was added and microplates containing cells and extracts were incubated for another 48 h in the same condition. Doxorubicin was used as a positive control. For the negative control wells contained only the cells in the media with no extracts or doxorubicin. To evaluate cell survival, 20 μl of MTT solution (5 mg/ml in PBS) was added to each well and incubated for 3 h. Then gently 150 μl of old medium containing MTT was replaced by DMSO and pipetted to dissolve any formed formazan crystals. Absorbance was then determined at 540 nm by enzyme-linked immunosorbent assay (ELISA) plate reader. Each extract concentration was assayed in 4 wells and repeated 3-times. Standard curves (absorbance against number of cells) for each cell line were plotted. Percent of cell survival in DMSO (0.5% as negative control) was assumed 100.
Statistical analysis
To perform statistical tests, SIGMASTAT™ (Jandel Software, San Raphael, CA) was used. Analyze-of-variance followed by Student-Newman-Keuls test was used to see the differences among groups. Significance was assumed at the 5% level.
RESULTS
Preliminary phytochemical studies
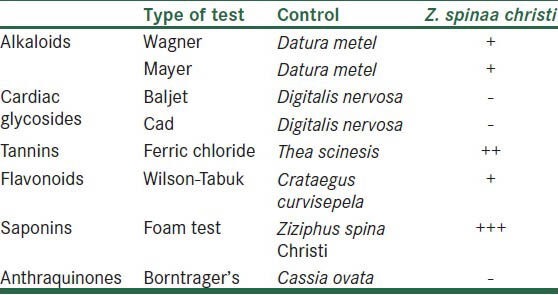
Phytochemical analysis of the extracts of leaves of Z. spina-christi is shown in the Table 1. The results show the presence of alkaloid, tannin, flavonoid and saponin. However, cardiac glycosides and anthraquinone were not detected.
Table 1.
Phytochemical analysis of the leaves of Z.spina christi
Cytotoxic effect of the extracts of Z. spina-christi
The standard curves for Hela and MDA-MB-468 cell lines showed a good linear relationship between absorbance and the number of cells (r2= 0.979 and 0.980 respectively) [Figures 1 and 2]. Doxorubicin (20 μg/ml), a known cytotoxic antibiotic, as a positive control significantly inhibited the growth of Hela and MDA-MB-468 cell lines to less than 25%. Extracts were considered cytotoxic when cell viability decreased to less than 50%.
Figure 1.
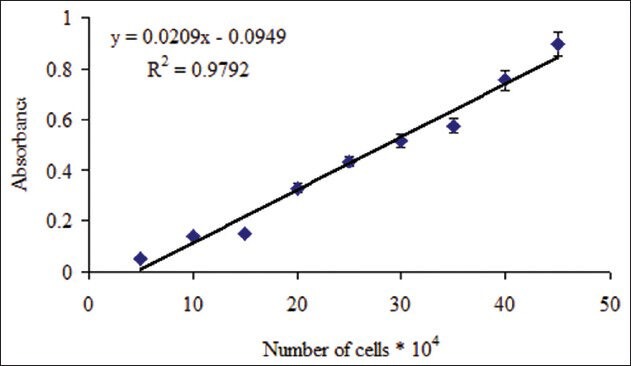
Relationship between absorbance and number of Hela cells Number of viable cells was assessed using MTT assay. Absorbance was determined at 540 nm. Data are presented as mean ± SD, n = 5
Figure 2.
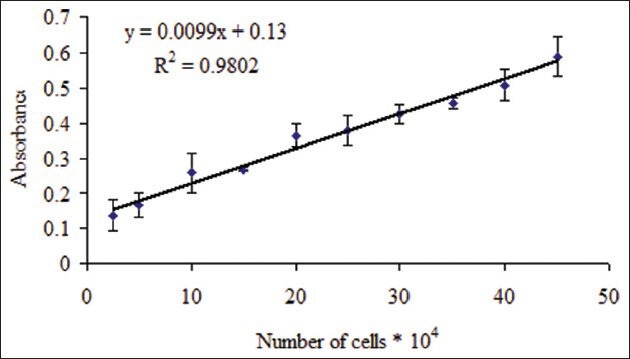
Relationship between absorbance and number of MDA-MB-468 cells Number of viable cells was assessed using MTT assay. Absorbance was determined at 540 nm. Data are presented as mean ± SD, n = 5
The cytotoxic effect of different extracts of Z. spina-christi on Hela and MDA-MB-468 cells were evaluated using MTT-based cytotoxicity method. Hexane, chloroform, chloroform-methanol, butanol, methanol-water and aqueous extracts of Z. spina-christi significantly and concentration-dependently reduced viability of Hela and MAD-MB-468 cells (P < 0.05, Figures 3 and 4). In the both cell lines, chloroform-methanol extracts of Z. spina-christi were more potent than the other extracts.
Figure 3.
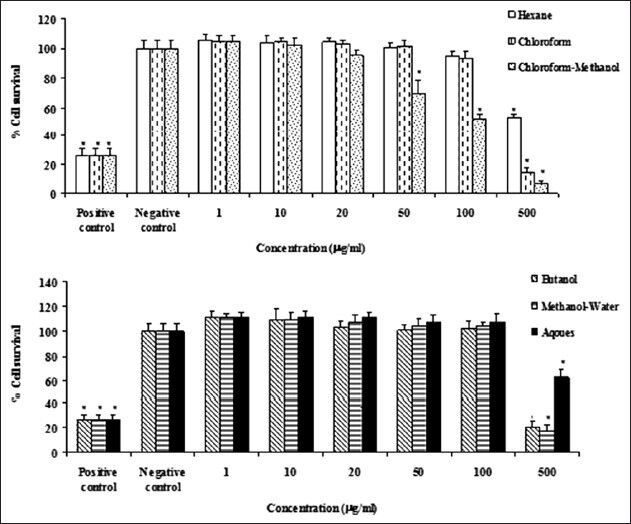
The effects of different extracts of Ziziphus spina-christi on Hela cells viability of the cells was determined by MTT assay. Percent cell survival in the control group was assumed 100. (a) hexane, chloroform and chloroform-methanol extracts. (b) Butanol, methanol-water and aqueous extracts. * = P < 0.05, n = 3
Figure 4.
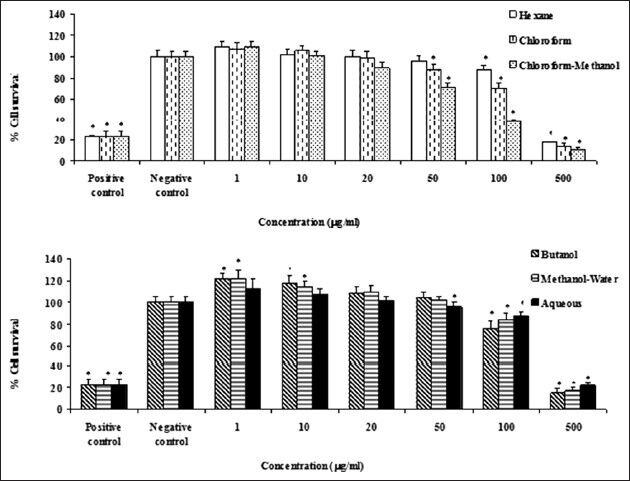
The effects of different extracts of Ziziphus spina-christi on MDA-MB-468 cells viability of the cells was determined by MTT assay. Percent cell survival in the control group was assumed 100. (a) hexane, chloroform and chloroform-methanol extracts. (b) Butanol, methanol-water and aqueous extracts. * = P < 0.05, n = 3
DISCUSSION
Cancer is one of the most important causes of death in many countries. During the last decades, scientists have paid more attention in discovery of new anticancer drugs. Different classes of anticancer drugs are used for cancer treatment among which cytotoxic drugs are very important. Due to the presence of various complex chemical substances such as alkaloids, flavonoids, terpenoids, saponin and phenolic compounds in plants, they are important sources for cytotoxic agents. It has been shown that more than 3,000 species of plants contain anticancer compounds.[7] It has been shown that some species of Ziziphus possess anticancer properties.[2]
In this study, we studied the cytotoxic effects of different extracts of Z. spina-christi on Hela and MDA-MB-468 tumor cell lines, using MTT assay. Hela and MDA-MB-468 are two well known tumor cell lines, which have been widely used for evaluation of cytotoxicity.[22,23,24,25] Standard curves for Hela and MDA-MB-468 cells were plotted that showed good relationship between absorbance and number of cell. Doxorubicin, a known cytotoxic drug, significantly reduced the viability of the both cell lines indicating the validity of the method employed. In order to fractionize compounds with different polarities in this plant, chloroform, chloroform-methanol (9:1), butanol, methanol, methanol-water (7-1) and water were used for extraction. All tested extracts showed significant cytotoxicity against Hela and MDA-MB-468 cell.
However, they were more effective against MDA-MB-468 cells. Several studies have shown that susceptibility of different tumor cell lines to cytotoxic agents is different.[22,26] The order of potency of the extracts against Hela cells was as follow: Chloroform-methanol > chloroform > methanol-water = butanol > hexane >> aqueous. For MDA-MB-468 cells the pattern was: Chloroform-methanol > chloroform = butanol > hexane > aqueous > methanol-water. The cytotoxic effects of chloroform-methanol extract of Z. spina-christi may be associated to the presence of triterpenoid saponin glycoside, cyclopeptide alkaloids such as spinanine A, flavonoides such as rutnine and quercetin.[18,27] Also, the better extraction of alkaloids such as jubanin A, amphibian H and spinanine A may explain the better effect of chloroform and chloroform-methanol extracts of Z. spina-christi on both tested cell lines.[28] It hase been shown that some alkaloids possess good cytotoxic activity against different cell lines[29,30] The cytotoxic effect of butanolic extract could be explained by the presence of saponin such as betulic acid in this extract.[31]
From the findings of this study it can be concluded that Z. spina-christi contain compounds with cytotoxic activity which makes it a promissing candidate for further study to get new cytotoxic agents.
ACKNOWLEDGMENT
This study was supported by a grant from the Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1. [Last accessed on 2013]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en .
- 2.Meghna RA, Narshimha R, Minoo HP. Anti-tumor activity of four ayurvedic herbs in Dalton lymphoma ascites bearing mice and their short-term in vitro cytotoxicity on DLA-cell-line. Afr J Tradit Complement Altern Med. 2008;5:409–18. doi: 10.4314/ajtcam.v5i4.31297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman EJ. Washington DC: CRC Press; 1999. Cancer and the search for selective biochemical inhibitors; pp. 2–18. (57-96). [Google Scholar]
- 4.Evans WC. 15th ed. London: Sounders; 2002. Trease and Evans Pharmacognosy; pp. 394–406. [Google Scholar]
- 5.Kaur R, Kapoor K, Kaur H. Plants as a source of anticancer agents. J Nat Prod Plant Resour. 2011;1:119–24. [Google Scholar]
- 6.Cragg GM, Newman DJ. Antineoplastic agents from natural sources: Achievements and future directions. Expert Opin Investig Drugs. 2000;9:2783–97. doi: 10.1517/13543784.9.12.2783. [DOI] [PubMed] [Google Scholar]
- 7.Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer-an extension of the work of Jonathan Hartwell. J Ethnopharmacol. 2000;73:347–77. doi: 10.1016/s0378-8741(00)00341-x. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava V, Negi AS, Kumar JK, Gupta MM, Khanuja PS. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg Med Chem. 2005;13:5892–908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 9.Mahboobi S, Sellmer A, Beckers T. Development of tubulin inhibitors as antimitotic agents for cancer therapy. Studies Nat Prod Chem. 2006;33:719–50. [Google Scholar]
- 10.Höfle G, Bedorf N, Steinmetz H, Schomburg D, Gerth K, Reichenbach H. Epothilone A and B – novel 16-membered macrolides with cytotoxic activity: Isolation, crystal structure and conformation in solution. Angew Chem Int Ed Engl. 1996;35:1567–9. [Google Scholar]
- 11.Mervat MA, Gendy EL. In vitro, evaluation of medicinal activity of egyptian honey from different floral sources as anticancer and antimycotic infective agents. J Microbial Biochem Technol. 2010;2:118–23. [Google Scholar]
- 12.Fernanda B, Daniela J. Homoharringtonine and related compounds. Toxicon. 2005;45:459–66. [Google Scholar]
- 13.Cragg GM, Kingston DG, Newman D. Boca Raton, Florida: Brunner-Routledge Psychology Press, Taylor and Francis Group; 2005. Anticancer agents from natural products; pp. 47–70. [Google Scholar]
- 14.Abdel-Wahhab MA, Omara EA, Abdel-Gali MM, Hassan NS, Nada SA, Saeed A, et al. A Zizyphus Spina-Christi extract protects against aflatoxin B1-Intitiated hepatic carcinogenicity. Afr J Tradit Complement Altern Med. 2007;4:248–56. [PMC free article] [PubMed] [Google Scholar]
- 15.Shahat AA, Pieters L, Apers S, Nazeif NM, Abdel-Azim NS, Berghe DV, et al. Chemical and biological investigations on Zizyphus spina-christi L. Phytother Res. 2001;9:593–7. doi: 10.1002/ptr.883. [DOI] [PubMed] [Google Scholar]
- 16.Amal H, Eman IA. Effect of Zizyphus leaves extract on mice suffering from ehrlich ascites carcinoma. Nature Science. 2010;8:234–44. [Google Scholar]
- 17.Saied SA, Gebaur J, Hammer K, Buerkert A. Ziziphus spina-christi (L.) Willd: A multipurpose fruit tree. Genet Rosour Crop Evol. 2008;55:929–37. [Google Scholar]
- 18.Anthony C. Dweck FLS FRSC FRSH – Technical Editor. A review of Zizyphus spina-christi. Personal Care. 2005 [Google Scholar]
- 19.Sudhersan C, Hussain J. In vitro clonal propagation of a multipurpose tree, Ziziphus spina-christi (L.) Desf. Turk J Bot. 2003;27:167–71. [Google Scholar]
- 20.Trease GE, Evans WC. London: Bailliere Tindall and Company Publishers; 1983. A textbook of pharmacognosy; pp. 343–83. [Google Scholar]
- 21.Sofowora A. Ibadan: Spectrum Books Ltd; 1993. Medicinal plants and traditional medicine in Africa; p. 150. [Google Scholar]
- 22.Jafarian A, Emami SA, Saeidi M, Sadeghi H. Cytotoxicologic studies of the extract of iranian juniperus sabina and platycladus orientalis on cance cells. J Res Med Sci. 2004;9:7–11. [Google Scholar]
- 23.Sadeghi-Aliabadi H, Emami A, Saidi M, Sadeghi B, Jafarian A. Evaluation of in vitro cytotoxic effects of Juniperus foetidissima and Juniperus sabina extracts against a panel of cancer cells. Iran J Pharm Res. 2009;8:281–6. [Google Scholar]
- 24.Wang X, Yu B, Wu Y, Lee RJ, Lee LJ. Efficient down-regulation of CDK4 by novel lipid nanoparticle-mediated siRNA delivery. Anticancer Res. 2011;31:1619–26. [PubMed] [Google Scholar]
- 25.Papanastasiou AD, Sirinian C, Kalofonos HP. Identification of novel human receptor activator of nuclear factor-kB isoforms generated through alternative splicing: Implications in breast cancer cell survival and migration. Breast Cancer Res. 2012;14:R112. doi: 10.1186/bcr3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monajemi R, Oryan S, Haeri-Roohani A, Ghannadi A, Jafarian A. Cytotoxic effects of essential oils of some Iranian Citrus peels. Iran J Pharm Res. 2005;3:183–7. [Google Scholar]
- 27.Pawlowska AM, Camangi F, Bader A, Braca A. Flovonides of Zizyphus jujuba L. and Zizyphus spina-christi (L.) Willd (Rhamnaceae) fruits. Food Chem. 2009;2:585–862. [Google Scholar]
- 28.AbDEl-Galil FM, Jissry MA. Cyclopeptidealkaloids from Z. spina-christi. Phytochemistry. 2001;3:1348–9. [Google Scholar]
- 29.Guo LL, He HP, Di YT, Li SF, Cheng YY, Yang W, et al. Indole alkaloids from ervatamia chinensis. Phytochemistry. 2012;74:140–5. doi: 10.1016/j.phytochem.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Chanakul W, Tuchinda P, Anantachoke N, Pohmakotr M, Piyachaturawat P, Jariyawat S, et al. Cytotoxic alkaloids from stems, leaves and twigs of Dasymaschalon blumei. Fitoterapia. 2011;82:964–8. doi: 10.1016/j.fitote.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Benammar C, Hichami A, Yessoufou A, Simonin A, Belarbi M, Allali H, et al. Zizyphus lotus L. (Desf.) modulates antioxidant activity and human T-cell proliferation. BMC Complement Altern Med. 2010;10:54. doi: 10.1186/1472-6882-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]


