Abstract
Background:
Carbon nanotubes (CNTs) have a large variety of applications in tissue engineering and biomedical devices. The biocompatibility and cytotoxicity of CNTs have been studied widely, however, up until now; there was uncertainty on how nanosized materials behave in the human body and stem cells. The current study describes the functionalized carbon nanotubes on adipose-derived stem cells (ADSCs) for viability and proliferation purposes in vitro.
Materials and Methods:
After chemical modification of the CNTs, the ADSCs were cultured in Dulbecco's Modified Eagle's. Medium (DMEM) having doses of 0.1, 1, 10, 20, 50, and 100 μg/ml of CNTs. On the third and seventh days of the experiment, the cellular viability, proliferation, and stemness were determined, using the MTT, trypan Blue, and flow cytometry assays in variable CNTs dosage.
Results:
In doses of 0.1 and 1 μg/ml, the expression of the surface markers were similar to the control groups on day three, but decreased in higher dosages on day seven. The viability of both groups was the same on day three, but in comparison to the control groups, was found to decrease in the higher dosages on day seven.
Conclusion:
The effect of CNTs on the viability and proliferation of ADSCs is a function of time and the doses used. Through further investigation by using these particles, we expect that we should be able to increase the viability and proliferation of ADSCs.
Keywords: Adipose-derived stem cells, carbon nanotubes, cell viability, proliferation
INTRODUCTION
In the twenty-first century, nanotechnology is one of the key technologies promoting health and improving quality of life, by enabling new health care technologies,[1,2] and accordingly, there have been huge advances and increased funding for technological research on nanomaterials.[3] In particular, carbon nanotubes (CNTs), one of the most representative nanomaterials were first reported by Somia Iijima, in 1991.[4] CNTs consist of carbon atoms with a cylindrical hollow nanostructure that look like wire meshwork. On account of their unique properties such as, mechanical, physical, electronic, and chemical, CNT applications have been studied in biomedical engineering and medical chemistry.[5,6,7] CNTs can be divided into two main types; single-wall nanotubes (SWNTs) and multiwall nanotubes (MWNTs); these also have a high surface area, high mechanical strength, ultra-light weight, rich electronic properties, and excellent chemical and thermal stability.[8] The insolubility of carbon nanotubes in aqueous media has been a major problem for biological and biomedical applications.[9] The recent development in methods to chemically modify and functionalize carbon nanotubes has made it possible to solubilize and disperse carbon nanotubes in a medium,[10,11] thus, carbon nanotubes can now be functionalized to complete improved properties and functions such as biocompatibility and biomolecular recognition capabilities.[12,13] Although CNTs have been widely studied in the physical, electronic, and chemical areas, a few studies concerning biomedical applications have been reported on human cells, which may be probably due to the increased risk of cytotoxicity.[14,15,16] Therefore, investigating the effect of carbon nanotubes on stem cells and their interaction mechanism in-vitro and in-vivo is very important, and it is necessary to research it. Recent studies have also demonstrated that culturing stem cells on CNT scaffolds appears to be a viable strategy to modulate stem cell lineage commitment toward other cells[17,18] and CNT patterns have enormous potential as a new platform for basic research and applications, using stem cells for regenerative medicine and tissue engineering.[19] However, some controversy exists with regard to the biocompatible character of SWNTs and MWNTs, with some in vitro studies reporting that CNTs are cytotoxic.[16,20] Additionally, some studies suggested CNTs to be excellent substrates for cellular growth.[7,21] Mooney et al., reported that CNT suspension has good biocompatibility with mesenchymal stem cells (MSCs) and supports proliferation as well as differentiation of MSCs in the presence of an induction medium.[22] Webster et al., reported that carbon nanofibers increased osteoblast functions.[23] Moreover, Aoki et al. reported cell proliferation on multiwall CNTs.[24,25] Daxiang Cui et al., showed the effects of single-walled carbon nanotubes (SWCNTs) on HEK293 cells, which inhibit proliferation and decrease cell adhesive ability in a dose- and time-dependent manner.[26] In another study, Lam et al., investigated the potential pulmonary toxicity of SWNTs in mice and considered that chronic inhalation and/or exposure to SWNTs could be a serious occupational health hazard.[27] Therefore, extensive research is necessary to study the effect of carbon nanotubes on cells, organs, or whole organisms in culture media. The main purpose of this study is to determine the effects of functionalized CNTs on adipose derived-stem cells (ADSCs) in vitro for viability and proliferation, and therefore, laying down a basis for further related investigation.
MATERIALS AND METHODS
Functionalization of carbon nanotubes
Carbon nanotubes (CNTs) were obtained from the Nanostructure and Amorphous Material Co. (Product No. 1281 YJS). The CNTs were not soluble (or dispersible) in deionized (DI) water or alcohol even after prolonged sonication. Suspensions were formed where the CNTs quickly agglomerated and settled down to the bottom of the bottle. In a typical example, 30 mg of as-received pure CNTs was suspended in 60 ml of a 1:1 mixture of concentrated sulfuric acid 98% and nitric acid 70%, (Merck), in a 100 ml round bottom flask, equipped with a condenser, and refluxed for one hour. Following that, the resulting dispersion was diluted in water and centrifuged for 10 minutes and the supernatant was removed. Then the resulting solid was diluted with 1200 ml of deionized water and collected on a membrane filter. Finally, the sample was dried in a vacuum at 80°C overnight.[28,29] Functionalization of the CNTs was determined by fourier transform infrared (FTIR) spectroscopy.
Dispersions of carbon nanotubes
The preparation of CNTs dispersed in DMEM supplemented with 5% FBS (DMEM/FBS) used a sonication/centrifugation protocol described by Chin et al.,[30] Briefly, 1 mg of the as-received CNTs-containing powder was dispensed into an Eppendorf tube containing 1 ml of DMEM/FBS, vortexed for ~ one minute, and the probe sonicated for 10 minutes at 0°C (The tip of the probe sonicator was placed one-third of the distance below the surface of the 1 ml suspension).
The resulting black suspension was centrifuged in an Eppendorf tube for 2 minutes at 16,000 g (14,000 rpm). The upper 75% of the supernatant was recovered without disturbing the sediment and placed in a clean tube for a second centrifugation for 2 min at 16,000 g (14,000 rpm). The upper 75% of the second supernatant was carefully recovered to afford DMEM–CNT dispersion.
Isolation and culture of adipose-derived stem cells
The adipose-derived stem cells (ADSCs) were isolated from the subcutaneous abdominal adipose tissue harvested from five patients (25-45 years). Approval was obtained from the patients previously. The adipose tissue was then enzymatically dissociated for 30 minutes at 37°C, using 0.075% collagenase type I (Sigma) and washed with PBS (Sigma). After neutralization of the collagenase with DMEM-LG (Sigma) and 10% fetal bovine serum (FBS) (Invitrogen) the cell solution was centrifuged at 1500 rpm for 10 minutes. The stromal cell pellet was resuspended in the culture medium containing DMEM-LG supplemented with 10% FBS, 1% penicillin/streptomycin (Gibco) cultures were maintained at sub-confluent levels in a 37°C incubator with 5% CO2, and the medium was replaced every three days. When the cells reached 80% confluence, they were passaged with trypsin/EDTA (0.05% trypsin/0.53 mM EDTA) (sigma) solution.
Cell viability and proliferation assay
The ADSCs were cultured in medium DMEM-LG with 10% FBS and 1% penicillin/streptomycin at 37°C in 5% CO2 incubator for three and seven days. CNTs with different concentrations of 0.1, 1, 10, 20, 50, and100 μg/ml were added into the culture medium. ADSCs without CNTs were used as the control. The medium was exchanged once per three days and the assay was carried out in triplicate.
Trypan blue assay
Trypan blue (TB) is a vital dye. The reactivity of TB is based on the fact that the chromophore is negatively charged and does not interact with the cell unless the membrane is damaged. Therefore, all the cells, which exclude the dye, are viable. Cells were released with trypsin-EDTA, rinsed, and resuspended (dilute cells in complete medium without serum to an approximate concentration of 1 × 105 to 2 × 105 cells per ml) in a screw cap test tube. Ten microliters of the cell were taken out into a new Eppendorf tube, and 10 μl trypan blue was added (0.4% TB stain (fresh and filtered) in phosphate buffered saline) and mixed gently again. Cell suspension of about 10 μl containing TB was drawn up and filled in the hemocytometer. Dead cells stained blue with TB could be counted separately for a viability count. The cell count was done with the following formula: Cell viability (%) = Live cell count - Blank/Total cell count - Blank × 100.
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide assay
The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide (MTT)(Sigma) survival assay was used to evaluate the ADSCs viability. They were treated with increasing concentrations in each nanotube, prepared in the medium, on days three and seven. Briefly, the medium of each well plate was removed, rinsed with PBS, and replaced with 800 μl of serum-free medium and 80 μl MTT solution (5 mg/ml in PBS). Then it was incubated for four hours at 37°C in a 5% CO2 humidified incubator, so that, purple formazan crystals formed in the plate. The medium was removed and 800 μl of dimethylsulfoxide (DMSO) (Sigma) was added to each well and incubated in a dark place for two hours. The DMSO dissolved the formazan crystals and created a purple color. Then 100 μl of the solution was transferred to a 96-well plate and absorbance of each well was read at 570 nm with the ELISA reader (Hiperion MPR4). These experiments at least were carried out in triplicate. Three different experiments were independently repeated, thrice, for each experiment[3]
Flow cytometry
The cell surface markers of ADSCs that were treated with CNTs for three and seven days, determined by flow cytometry. After treating with trypsin-EDTA, the obtained cells were centrifuged. The cell pellets were washed with PBS and incubated with antibody against the CD56 (IQ Product), CD 44 (DAKO Cytomation) was conjugated with phycoerythrin (PE), CD16 and CD 90(IQ Product) were conjugated with fluoroisothiocyanate (FITC) for 30 minutes in the dark at 4°C. For isotype control, nonspecific FITC conjugated IgG was substituted for the primary antibodies. Flow cytometry was performed with an FAC scan flow cytometer (Becton Dickinson, San Jose, CA).
Electron microscopy
Transmission electron microscopy (TEM) was used to detect the possible morphological changes on the CNT specimens depending on the oxidative treated samples. TEM was performed on a LEO-906E at 40-120 KV; sample preparation involved sonicating materials in DI water and methanol for at least half an hour and then putting a drop of the resulting suspension onto a carbon film supported by copper grids.[29,30,31]
Statistics
The Kolmogrov-Smirnov test was used for assessing the normal distribution of variables and ANOVA (one-way-analysis of variance) with the LSD post hoc test was used for comparison of the MTT and Trypan Blue results in the different groups.
RESULTS
Characterizations of carbon nanotubes
Microscopic analyses
The TEM images of the reflux functionalized CNT bundles are shown in Figure 1. On the basis of the TEM results obtained from more dilute dispersions in methanol, it is evident that the bundles are weakly held together, as the images show large numbers of individual tubes and thin bundles.
Figure 1.
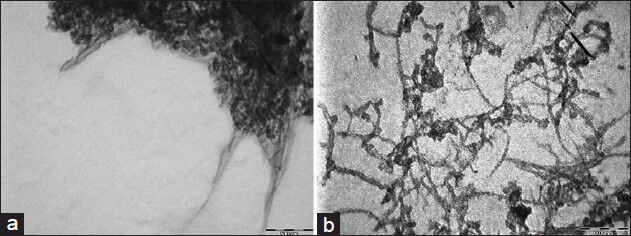
TEM images of CNTs, (a) pristine, (b) after mixed acid reflux
Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) spectra of pristine and reflux functionalized CNTs were obtained to determine the structure of the chemical groups form on the nanotube sidewalls and tube ends. The FTIR spectrum [Figure 2] showed a number of infrared peaks, which were assigned as follows: The peak at 3600-3200 cm−1 was due to carboxylate O–H stretching and 2960-2930 cm−1 was assigned to aliphatic C–H stretching. Also the peak at 1620-1450 cm−1 was due to carboxylate O–H bending. The peaks at 1162 and 1114 cm−1 were due to C–O stretches and C–H bending. The peaks at 1629 and 1717 cm−1 may also be linked to carboxylate C = O or to aromatic C = C stretches. The FTIR was performed on a FT/IR-6300 (400-4000 cm−1), JASCO, Japan.
Figure 2.
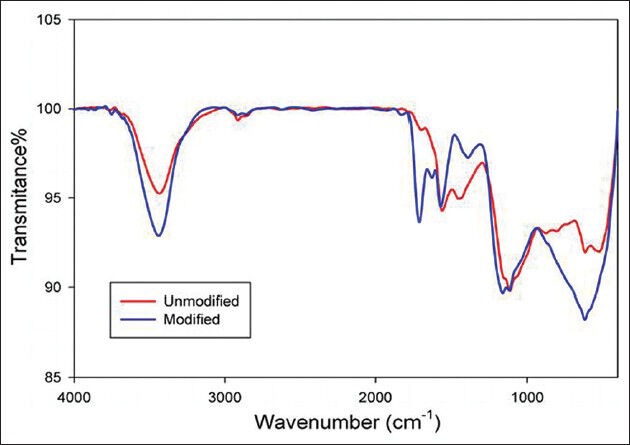
Comparison of the FT-IR spectra for pristine (red) CNTs and after mixed acid reflux (blue)
Raman spectroscopy
Raman spectroscopy is a valuable technique for detection of the molecular structure. Figure 3 shows the Raman spectroscopy of raw and pure CNTs. This technique is a good method to recognize the carbonic components. Raman spectra at a high frequency range: Two peaks can be seen, which are the characteristics of CNTs. One of the peaks is related to the graphite band (G band) and the other is related to the irregular and disorder band (D band). It is shown that the D band and G band peaks are at 1331 cm−1 and 1574 cm−1, respectively. These peaks show that the nanotube structure may not be damaged by acidic treatment. The difference between the Raman spectrum of acidic CNTs and initial CNTs shows that when acidic treatment is performed on CNTs, the G band peak increases. This event shows that some of carbon bonds are broken by acidic treatment and the desired carboxylic (−COOH) and hydroxyl (−OH) functional groups have been created. The Raman spectra has been performed on a Nicolet Almega XR Dispersive Raman Spectrometer.
Figure 3.
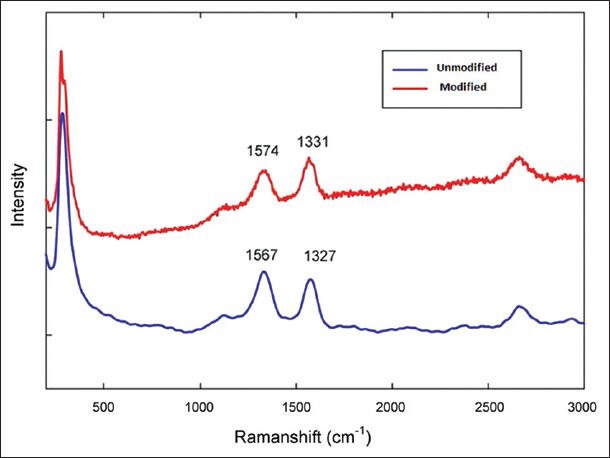
Comparison of the Raman spectra for pristine (blue) CNTs and after mixed acid reflux (red)
Effect of carbon nanotubes on the viability and proliferation of ADSCs
Trypan blue assay
Carbon nanotube
The medium (DMEM) produced significant cytotoxicity at concentrations of 50 (43.2% inhibition) and 100 (56.3% inhibition) μg/ml, as explained using the trypan blue assay, on day three. Also on day seven, the concentrations of 50 (64% inhibition) and 100 (75.7% inhibition) μg/ml were significant in comparison to the control [Figure 4].
Figure 4.
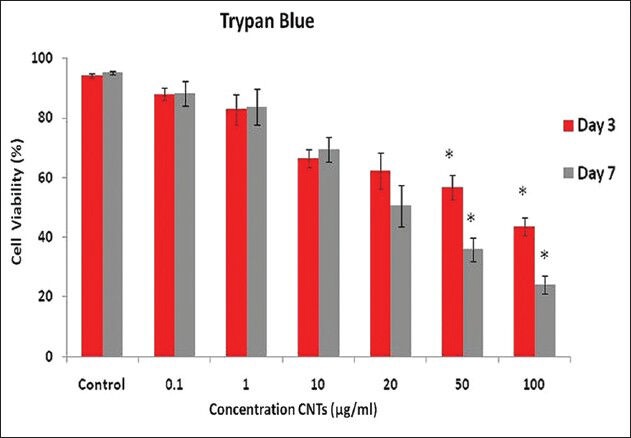
Cell number was measured by the Trypan blue dye exclusion method, and the percentage of cell growth was calculated as a ratio of the number of CNT-treated cells and control cells. Results were mean ± SD of the triplicate experiments. Denoted a significant difference from the control (P ≤ 0.05)
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide assay
The cell viability was positively correlated with the degree of MTT reduction; the cell viability of CNT-treated ADSCs was evaluated by using the MTT reduction assays. As indicated in Figure 5, treatment of ADSCs with various concentrations of CNTs (0.1, 1, 10, 20, 50, and 100 μg/ml) caused a time- and dose-dependent decrease in cell numbers, relative to the control cultures. The cell viability from 20 μg/ml CNTs downward was significant, and a maximum inhibition effect was recorded on the third and seventh days, at 100 μg/ml CNTs, in comparison to the control.
Figure 5.
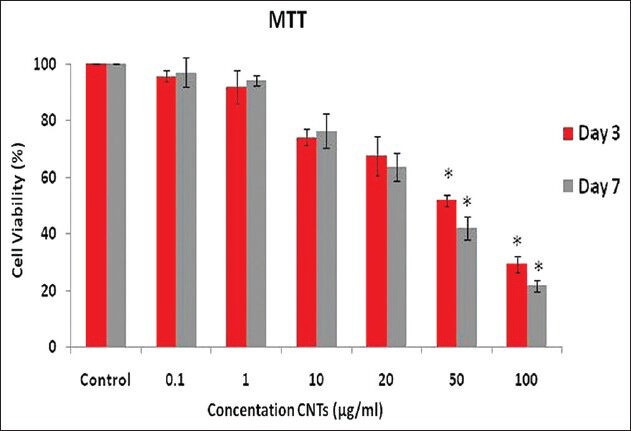
ADSC viability measured by MTT assay, and the percentage of cell viability was calculated as a ratio of the OD of CNT-treated cells and control cells (P ≤ 0.001). Results were mean ± SD of the triplicate experiments, denoted a significant difference from the control (P ≤ 0.05)
Flow cytometry
Flowcytometric analysis of the undifferentiated human adipose derived stem cells (ADSCs) was performed after treatment by the CNTs. The results showed that the cells expressed hematopoietic phenotypic markers (as a negative control) for CD16, for CD 56 weakly (1%), while CD44 and CD90 were expressed at a high level (94%) [Figure 6].
Figure 6.
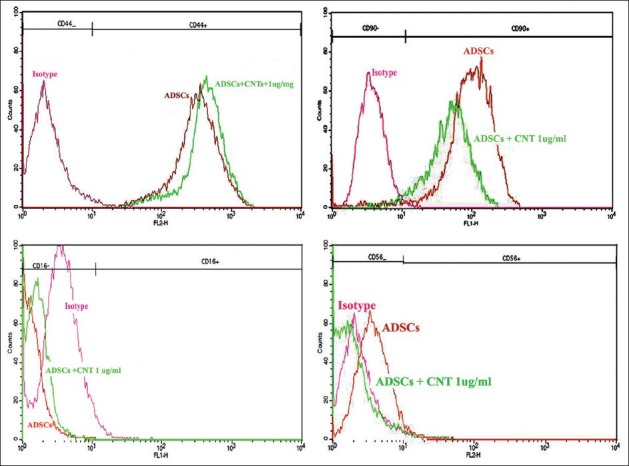
Flow cytometric analysis of human ADSCs using specific FITC and PE coupled antibodies against surface markers. The histograms corresponding to the isotype control, ADSCs, and ADSCs + CNTs are demonstrated by red, orange, and green lines
DISCUSSION
Carbon nanotubes are certainly known as modern tools in nanobiotechnology, and because of their unique properties, have great potential applications in suitable substrates, for cell growth in tissue engineering, biomedical devices, and as vectors for gene transfection.[32,33] Our present study mainly looks at the effect of CNTs on viability and proliferation of ADSCs in vitro. The evaluated parameters in this study include the mitochondrial activities, total cell content, and surface CD marker response after three and seven days of exposure. The present study also employs FTIR, Raman spectroscopy, and TEM, to describe the raw and functionalized CNT samples. One of the major problems in CNT evaluation is the nanotubes tendency to agglomerate owing to the substantial van der Waals attraction, so they exhibit low dispersibility (hydrophobic) in water and other solvents.[3,34] However, chemical-treated CNTs show hydrophilic behaviors. Acid-washed CNTs show better dispersibility in comparison to sonication alone. This may be due to the OH groups formed in the acid-washed case that makes hydrogen bind with water molecules.[35]
A typical FTIR spectrum of CNTs treated with the acid mixture (H2SO4/HNO3) for one hour shows that a new peak appears around 1717 cm−1. It is normally assigned to the C = O strength vibration in the COOH group, which means that the acid-mixture treatment will introduce some C = O groups at the end or side of the CNTs. Meanwhile, the hydrogen binding formation between the −COOH groups became more effective. We also found that initially, in the treated CNTs, the peak is around 1564 cm−1, which is assigned to the C = C groups; its shift to a higher frequency around 1574 cm−1, may suggest a change (oxidation) in the structure of the CNTs. Jin Zhang, et al.,[36] indicates the effect of chemical oxidation on the structure of SWNTs by using different oxidants. The oxidation procedure is characterized by using FTIR spectroscopy, which is consistent in our findings. In the Raman spectroscopy both samples of CNTs possess the characteristic Raman peaks around 1325 and 1570 cm1, corresponding to the D and G bands. The D band represents disordered or amorphous carbons, while the G band indicates graphite or ordered carbons in the CNTs. Therefore, the D/G intensity ratio can be taken as a crude measurement for the extent of functionalization. It has been observed that the peak intensity of the D band, for modified CNTs, increases compared to the unmodified nanotubes, which is an indication of functionalization, during which the ordered SP2 hybridized carbons are converted to SP3 carbon. However, no significant alternation is observed for the G band. Looking at the D/G ratios of unmodified and modified CNTs, it is observed that the value increases from 0.92 (pristine CNTs) to 0.99 (acid modified CNTs), which is a clear indication of the covalent modification of CNTs.
According to our finding, as significant cytotoxicity was demonstrated at the dose of 50 and 100 μg/ml of CNTs by TB and MTT assays, the cells were subsequently exposed to these concentrations for three and seven days, and light microscopy was employed to assess the nanomaterial interaction in the ADSCs and dose-dependent morphological alteration. Our observation showed that the CNTs could inhibit the proliferation of ADSCs, induce cell apoptosis, and decrease the cellular adhesive ability in a time- and dose-dependent manner. Even as a CNT concentration of 100 μg/ml induced death of ADSCs in three and seven days, 1 μg/ml of CNTs and less than that in the medium appeared to be safe for cells. This finding was consistent with that of Daxiang Cui et al.,[16] who reported that in the SWCNTs effect on HEK293 cells, cell G1 arrest occurred after 25 μg/ml exposure of SWCNTs in the medium, and this arrest was accompanied by a dramatic decrease in the number of cells in the S phase. Also Bottini et al.,[37] showed the cytotoxicity effect of MWCNT carboxylic on T lymphocyte and T leukemia cells, which led to 50% cell death after 24 hours, with the use of TB. This study is consistent with our result on day three at the concentration of 50 μg/ml. As mentioned, ADSCs were evaluated by the MTT assay and results are shown in our study. Cell viability, with the maximum inhibition effect on day seven, was at a concentration of 100 μg/ml. In this regard, Tian et al.,[38] showed that the effect of SWCNTs on cell viability of human keratinocyte cells at the concentration of 100 μg/ml gave 79, 50, and 31% viability after days one, three, and five, respectively, which conforms to our results. Another study has also reported functionalized CNT (hydroxylic (−OH) and carboxcylic (−COOH)) reduced cell viabilitssssy to 33% with use of the MTT assay.[39,40]
CONCLUSION
In conclusion, exposure of ADSCs to a wide dose range of CNTs (0.1, 1, 10, 20, 50, 100 μg/ml) for days three and seven, after dispersibility, revealed that low-dose CNTs (0.1 and 1μg/ml) increased viability and proliferation in mild ranges, but in a high dose they had the toxicity and could inhibit proliferation and reduce the viability of ADSCs by inducing cell apoptosis and decreasing the cellular adhesive ability. This study has demonstrated the impact of CNTs on the biological systems, important information for future safe applications of CNTs, and also the design of biocompatible nanomaterials in tissue engineering.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Feynman RP. There's plenty of room at the bottom. Science. 1991;254:1300–1. [Google Scholar]
- 2.Siegel RW. Creating nanophase materials. Sci Am. 1996;275:74–9. [Google Scholar]
- 3.Davoren M, Herzog E, Casey A, Cottineau B, Chambers G, Byrne HJ, et al. In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol In vitro. 2007;21:438–48. doi: 10.1016/j.tiv.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–8. [Google Scholar]
- 5.Uo M, Tamura K, Sato Y, Yokoyama A, Watari F, Totsuka Y, et al. The cytotoxicity of metal encapsulating carbon nanocapsules. Small. 2005;1:816–9. doi: 10.1002/smll.200400143. [DOI] [PubMed] [Google Scholar]
- 6.Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes: The route toward applications. Science. 2002;297:787–92. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 7.Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun (Camb) 2004;7:16–7. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Thordarson P, Gooding JJ, Ringer SP, Braet F. Carbon nanotubes for biological and biomedical applications. Nanotechnology. 2007;18:412001. [Google Scholar]
- 9.Teker K, Sirdeshmukh R, Sivakumar K, Lu S, Wickstrom E, Wang HN, et al. Applications of carbon nanotubes for cancer research. Nano Biotechnology. 2005;1:171–82. [Google Scholar]
- 10.Thordarson P, Le Droumaguet B, Velonia K. Well-defined protein – polymer conjugates-synthesis and potential applications. Appl Microbiol Biotechnol. 2006;73:243–54. doi: 10.1007/s00253-006-0574-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Lee GS, Zettl A, Bertozzi CR. Biomimetic engineering of carbon nanotubes by using cell surface mucin mimics. Angew Chem Int Ed Engl. 2004;43:6111–6. doi: 10.1002/anie.200460620. [DOI] [PubMed] [Google Scholar]
- 12.Shim M, Kam NW, Chen RJ, Li Y, Dai H. Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Lett. 2002;2:285–8. [Google Scholar]
- 13.Bahr JL, Tour JM. Covalent chemistry of single-wall carbon nanotubes. J Mater Chem. 2002;12:1952–8. [Google Scholar]
- 14.Masciangioli T, Zhang WX. Peer Reviewed: Environmental technologies at the nanoscale. Environ Sci Technol. 2003;37:102–8. doi: 10.1021/es0323998. [DOI] [PubMed] [Google Scholar]
- 15.Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- 16.Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol lett. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Chao TI, Xiang S, Chen CS, Chin WC, Nelson A, Wang C, et al. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2009;384:426–30. doi: 10.1016/j.bbrc.2009.04.157. [DOI] [PubMed] [Google Scholar]
- 18.Park SY, Namgung S, Kim B, Im J, Kim J, Sun K, et al. Carbon nanotube monolayer patterns for directed growth of mesenchymal stem cells. Adv Mater. 2007;19:2530–4. [Google Scholar]
- 19.Lu Y, Chen S. Micro and nano-fabrication of biodegradable polymers for drug delivery. Adv Drug Deliv Rev. 2004;56:1621–33. doi: 10.1016/j.addr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, et al. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39:1378–83. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 21.Bianco A, Hoebeke J, Godefroy S, Chaloin O, Pantarotto D, Briand JP, et al. Cationic carbon nanotubes bind to CpG oligodeoxynucleotides and enhance their immunostimulatory properties. J Am Chem Soc. 2005;127:58–9. doi: 10.1021/ja044293y. [DOI] [PubMed] [Google Scholar]
- 22.Mooney E, Dockery P, Greiser U, Murphy M, Barron V. Carbon nanotubes and mesenchymal stem cells: Biocompatibility, proliferation and differentiation. Nano Lett. 2008;8:2137–43. doi: 10.1021/nl073300o. [DOI] [PubMed] [Google Scholar]
- 23.Webster TJ, Waid MC, McKenzie JL, Price RL, Ejiofor JU. Nano-biotechnology: Carbon nanofibres as improved neural and orthopaedic implants. Nanotechnology. 2003;15:48. doi: 10.1088/0957-4484/15/1/009. [DOI] [PubMed] [Google Scholar]
- 24.Aoki N, Yokoyama A, Nodasaka Y, Akasaka T, Uo M, Sato Y, et al. Cell culture on a carbon nanotube scaffold. J Biomed Nanotechnol. 2005;1:402–5. [Google Scholar]
- 25.Aoki N, Yokoyama A, Nodasaka Y, Akasaka T, Uo M, Sato Y, et al. Strikingly extended morphology of cells grown on carbon nanotubes. Chem Lett. 2006;35:508–9. [Google Scholar]
- 26.Cui D, Tian F, Coyer SR, Wang J, Pan B, Gao F, et al. Effects of Antisense-Myc-Conjugated Single-Walled Carbon Nanotubes on HL-60Cells. J Nanosci Nanotechnol. 2007;7:4–5. doi: 10.1166/jnn.2007.348. [DOI] [PubMed] [Google Scholar]
- 27.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–34. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Iqbal Z, Mitra S. Rapidly functionalized, water-dispersed carbon nanotubes at high concentration. J Am Chem Soc. 2006;128:95–9. doi: 10.1021/ja053003q. [DOI] [PubMed] [Google Scholar]
- 29.Datsyuk V, Kalyva M, Papagelis K, Parthenios J, Tasis D, Siokou A, et al. Chemical oxidation of multiwalled carbon nanotubes. Carbon. 2008;46:833–40. [Google Scholar]
- 30.Chin SF, Baughman RH, Dalton AB, Dieckmann GR, Draper RK, Mikoryak C, et al. Amphiphilic helical peptide enhances the uptake of single-walled carbon nanotubes by living cells. Exp Biol Med (Maywood) 2007;232:1236–44. doi: 10.3181/0612-RM-284. [DOI] [PubMed] [Google Scholar]
- 31.Zheng B, Li Y, Liu J. CVD synthesis and purification of single-walled carbon nanotubes on aerogel-supported catalyst. Applied Physics A. Mater Sci Processi. 2002;74:345–8. [Google Scholar]
- 32.Zhang D, Yi C, Zhang J, Chen Y, Yao X, Yang M. The effects of carbon nanotubes on the proliferation and differentiation of primary osteoblasts. Nanotechnology. 2007;18:475102. doi: 10.1007/978-1-60761-579-8_5. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Tam UC, Czlapinski JL, Lee GS, Rabuka D, Zettl A, et al. Interfacing carbon nanotubes with living cells. J Am Chem Soc. 2006;128:6292–3. doi: 10.1021/ja060276s. [DOI] [PubMed] [Google Scholar]
- 34.Ruelle B, Peeterbroeck S, Gouttebaron R, Godfroid T, Monteverde F, Dauchot JP, et al. Functionalization of carbon nanotubes by atomic nitrogen formed in a microwave plasma Ar+N2 and subsequent poly (ε-caprolactone) grafting. J Mater Chem. 2007;17:157–9. [Google Scholar]
- 35.Zhao C, Ji L, Liu H, Hu G, Zhang S, Yang M, et al. Functionalized carbon nanotubes containing isocyanate groups. J Solid State Chem. 2004;177:4394–8. [Google Scholar]
- 36.Zhang J, Zou H, Qing Q, Yang Y, Li Q, Liu Z, et al. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J Phy Chem B. 2003;107:3712–8. [Google Scholar]
- 37.Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–6. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Tian F, Cui D, Schwarz H, Estrada GG, Kobayashi H. Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol in vitro. 2006;20:1202–12. doi: 10.1016/j.tiv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–5. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 40.Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168:121–31. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]


