Abstract
Predicting ecological impacts of invasive species and identifying potentially damaging future invaders are research priorities. Since damage by invaders is characterized by their depletion of resources, comparisons of the ‘functional response’ (FR; resource uptake rate as a function of resource density) of invaders and natives might predict invader impact. We tested this by comparing FRs of the ecologically damaging ‘world's worst’ invasive fish, the largemouth bass (Micropterus salmoides), with a native equivalent, the Cape kurper (Sandelia capensis), and an emerging invader, the sharptooth catfish (Clarias gariepinus), with the native river goby (Glossogobius callidus), in South Africa, a global invasion hotspot. Using tadpoles (Hyperolius marmoratus) as prey, we found that the invaders consumed significantly more than natives. Attack rates at low prey densities within invader/native comparisons reflected similarities in predatory strategies; however, both invasive species displayed significantly higher Type II FRs than the native comparators. This was driven by significantly lower prey handling times by invaders, resulting in significantly higher maximum feeding rates. The higher FRs of these invaders are thus congruent with, and can predict, their impacts on native communities. Comparative FRs may be a rapid and reliable method for predicting ecological impacts of emerging and future invasive species.
Keywords: biological invasions, impact prediction, invasive species, predator–prey, resource use, Type II functional responses
1. Introduction
Biological invasions are occurring globally at an increasing rate [1,2] and are recognized as major causes of biodiversity loss [3]. Invasions also pose significant economic threats [4], and there is growing pressure to prioritize their prevention and mitigation [2]. However, impacts of invasive species are often speculative [2,5] and consequently it is a major challenge to make objective and robust predictions of the impacts of existing, emerging and potentially invasive species [6–9].
A promising method in predicting invasive species impacts draws on the realization that invaders are commonly associated with rapid and efficient resource exploitation [8,10,11]. Comparisons of the functional response (FR; resource uptake rate as a function of resource density) of invasive and native consumers may thus reveal invader impacts on native prey species. The relative impacts of multiple invaders could also be assessed this way, a particularly useful exercise where invaders are newly emerging and hence have little or no impact history [7]. Here, we test these ideas with assessment of the FRs of an invasive fish species whose impacts have been well documented, plus an emerging fish invader, when compared with trophically analogous native fish in South Africa.
Largemouth bass (Micropterus salmoides) and sharptooth catfish (Clarias gariepinus) are known invaders in southern Africa. Whereas the ecological impacts of largemouth bass are well established, with negative effects on native invertebrate and fish communities [12,13], impacts of the sharptooth catfish, although evident, are not yet well quantified [14,15]. Since trophically analogous native fish species exist in South Africa, the Cape kurper (Sandelia capensis) and the river goby (Glossogobius callidus), we experimentally derived the predatory FRs of the two invaders and the two natives. We tested the hypothesis that high-impact invaders are characterized by significantly higher FRs than co-evolved natives and this corroborates field impacts, and propose such comparisons can facilitate predictions of the likely impact of emerging invaders by relating findings to those with known impacts.
2. Material and methods
Fish were sourced in the Eastern Cape, South Africa, in April/May 2013: bass were collected from Jameson (33°43′04″ S; 26°26′23″ E) and Douglas Dams (33°19′16″ S; 26°31′15″ E); catfish were supplied by the Camdeboo Satellite Aquaculture Project, Graaff-Reinet; native kurper and goby were collected from the Blindekloof stream (33°43′1.42″ S; 25°17′27.41″ E) and Ndlambe Dam (33°10′17″ S; 26°55′00″ E). Each species was housed separately in 600 l holding tanks in a closed re-circulating system. Fish were acclimated for 1 week prior to experiments and maintained on a diet of earthworms to standardize prior experience.
FR experiments were conducted in 15 square 300 l fibreglass tanks, part of the same flow-through system as the holding tanks (water flow, 1 l min−1; mean temperature±s.e., 18.05 ± 0.08°C; one reading per hour). Fish (n = 18 per species) were size-matched with respect to total length (TL) and gape height (GH) (TL cm ± s.e., bass 8.39 ± 0.15, catfish 7.86 ± 0.07, kurper 7.27 ± 0.20, goby 7.68 ± 0.09; GH mm ± s.e., bass 12.67 ± 0.38, catfish 10.05 ± 0.12, kurper 11.09 ± 0.31, goby 11.68 ± 0.39). Prey were tadpoles of the painted reed frog (Hyperolius marmoratus) (26.2 ± 0.75 mm mean body length ± s.e.), which all fish species readily consumed. Individual fish were randomly selected 48 h prior to use, allocated to experimental tanks and held without food to allow for standardization of hunger levels. Individual fish were then presented with tadpoles at six prey densities (2, 4, 8, 16, 32 and 64), with three replicates per density. Replicates were initiated at 16h00 and prey consumption was examined after 16 h. Controls were three replicates of each prey density in the absence of predators.
Differences in overall prey consumption between invasive and native comparator fish species were assessed using generalized linear models (GLMs) with quasi-Poisson error distribution in ‘R’ that were simplified via a step-deletion process. We determined FR types using logistic regression of the proportion of prey consumed against initial prey density [16] and modelled FRs using the Rogers random predator equation for a Type II response, which accounts for non-replacement of prey as they are consumed [17]. FR data were bootstrapped (n = 30) and the parameters attack rate a, handling time h and maximum feeding rate 1/hT (T = experimental time) compared using GLMs.
3. Results
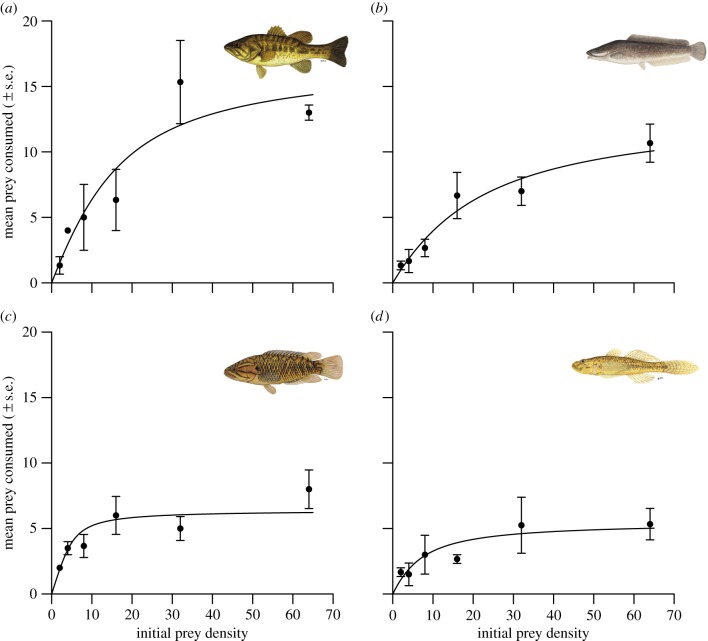
Control tadpole survival was greater than 98% after 16 h, thus experimental deaths were attributed to fish predation. Each invasive species consumed significantly more prey than the comparator native species (bass versus kurper, z = 3.32, p < 0.001; catfish versus goby, z = 2.94, p < 0.001; figure 1a–d). There was a significant effect of predator species on attack rate a (F3,116 = 128.57, p < 0.001; figure 2a); although attack rate differed between bass and kurper (z = 10.44, p < 0.001; figure 2a), this was similar for the catfish and goby (z = 0.21, p = 0.97; figure 2a), and attack rate was clearly more similar within invader/native comparisons than between them (figure 2a). Fish species had a significant effect on handling time h (F3,116 = 314.21, p < 0.001; figure 2b), which was driven by invaders having lower handling times than the comparator native species (bass versus kurper, z = 24.53, p < 0.001; catfish versus goby, z = 18.46, p < 0.001; figure 2b). This consequently translated into significant differences in maximum feeding rates (1/hT) (F3,116 = 291.24, p < 0.001; figure 2c), which were significantly higher in invaders than for native comparators (bass versus kurper, z = 24.49, p < 0.001; catfish versus goby, z = 16.23, p < 0.001; figure 2c). This is further evidenced by the higher FR asymptotes of the invasive fish (figure 1).
Figure 1.
FRs of invasive (a) largemouth bass and (b) sharptooth catfish; and native (c) Cape kurper and (d) river goby towards tadpole prey (modelled by the Rogers random predator equation for a Type II response). Data are mean numbers of prey consumed at each density ± s.e.; n = 3 per density. (Online version in colour.)
Figure 2.
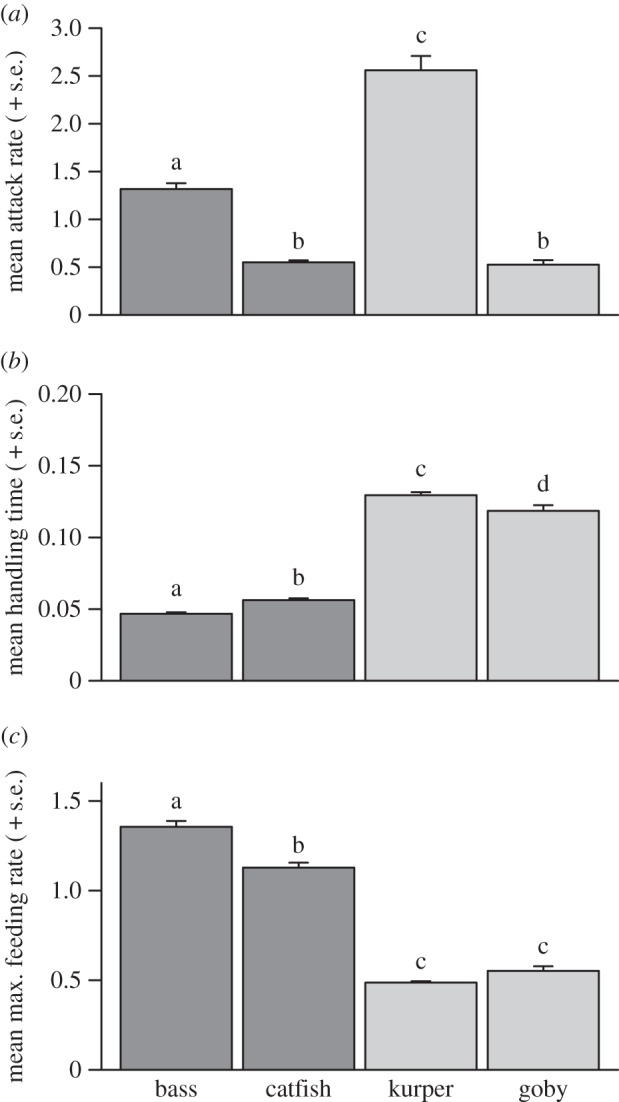
Mean (+s.e.) (a) attack rate a, (b) handling time h and (c) maximum feeding rate 1/hT derived from bootstrapping (n = 30). Different letters indicate significant differences (Tukeys contrasts, p < 0.01). Dark grey bars denote invader, whereas light grey bars denote native.
4. Discussion
A major challenge in invasion biology is the development of predictive methodologies that can forecast the ecological impacts of existing, emerging and potential invasive species. The FR is useful in this regard, as it may be an inherent feature of a species that enables assessment of impact relative to natives and/or other invaders [7]. Higher attack rates and/or lower handling times (and thus higher maximum feeding rates) of invaders may be due to characteristic differences in resource acquisition ability and/or to naivety of, for example, native prey with respect to a novel invasive predator. Indeed, the lack of coevolutionary history between invader and native resource is likely to lead to differences in their interaction strength when compared with native–native consumer–resource pairings [7,9].
Here, the FR of the largemouth bass, an invasive fish with well-established field impacts [12], was significantly higher in comparison with the native equivalent, the Cape kurper. This finding corroborates reported field impacts, whereby in areas where bass are present, native prey species populations are often decimated and other native fish species are absent [13,18]. Similarly, a heightened FR was observed in the emergent invader, the sharptooth catfish, in relation to the comparator native species, the river goby. This higher FR of the invader, a species where impact has not been quantified [14], predicts potentially similar negative field impacts by this invasive species as inferred by recent research [15]. Our results also support the inclusion of the largemouth bass in the ‘100 of the World's Worst Invasive Alien Species’ list.
Within the invader/native comparisons there were, however, differences with respect to the FR parameters. Classically defined as the attack rate, a serves as a measure of relative predatory efficiency at low prey densities. Whereas similar values of a were found for the dorsoventrally flattened, benthic fish (catfish/goby), thus validating the comparison between two similar predatory strategies, there was a significant divergence in the bass/kurper comparison. This observation of a greater predatory efficiency of the kurper at low prey densities does not, however, translate to the higher prey densities where their feeding rate is much lower in comparison with the invasive bass. Such efficiency may reflect a necessity for the native species to be an effective consumer at low prey densities, as opposed to the bass which are able to forage on greater numbers of prey at higher densities. This is supported further by a greater consumption of tadpoles overall by bass in comparison with the kurper. Indeed, at higher prey densities, a divergence in the species feeding rates occurred and, indeed, the rate of consumption by invaders was up to three times that of the native species.
Higher FRs may be a reliable measure and predictor of ecological impact as the FR describes the per capita effect of a consumer on a resource, a major determinant of the population dynamics of resources, such as prey [19]. Additionally, the FR-based prediction is beneficial owing to its relative ease of derivation [7]. Furthermore, the classic formula for the derivation of the impact of invaders, by Parker et al. [6], contains terms for the per capita effect (potentially the FR), the abundance (potentially a proxy for the numerical response), as well as the actual or predicted geographical range of the invader. Thus, the FR could, by itself or in any combination with other parameters in the Parker equation, be valuable for assessing invader impact, currently and in the future. In the present example of invasive fish in South Africa, FRs clearly corroborate and could have predicted their field impacts and can thus provide an objective tool for assigning risk to particular invaders.
Data accessibility
Raw data can be found in the electronic supplementary material.
Funding statement
This research was funded by the DST-NRF Centre of Excellence for Invasion Biology. D.M.R. received support from the National Research Foundation (grant no. 85417). The Department of Economic Development, Environmental Affairs and Tourism (Cacadu Region) is thanked for issuing research permits. J.D. acknowledges support from the Natural Environment Research Council (NERC) and the Leverhulme Trust. H. Kaiser is thanked for the use of the experimental facility at the Department of Ichthyology and Fisheries Science, Rhodes University.
References
- 1.Wonham MJ, Carlton JT. 2005. Trends in marine biological invasions at local and regional scales: the Northeast Pacific Ocean as a model system. Biol. Invasions 7, 369–392 (doi:10.1007/s10530-004-2581-7) [Google Scholar]
- 2.Simberloff D, et al. 2013. Impacts of biological invasions: what's what and the way forward. Trends Ecol. Evol. 28, 58–66 (doi:10.1016/j.tree.2012.07.013) [DOI] [PubMed] [Google Scholar]
- 3.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 4.Born W, Rauschmayer F, Bräuer I. 2005. Economic evaluation of biological invasions—a survey. Ecol. Econ. 55, 321–336 (doi:10.1016/j.ecolecon.2005.08.014) [Google Scholar]
- 5.Ricciardi A. 2003. Predicting the impacts of an introduced species from its invasion history: an empirical approach applied to zebra mussel invasions. Freshwater Biol. 48, 972–981 (doi:10.1046/j.1365-2427.2003.01071.x) [Google Scholar]
- 6.Parker IM, et al. 1999. Impact : toward a framework for understanding the ecological effects of invaders. Biol. Invasions 1, 3–19 (doi:10.1023/A:1010034312781) [Google Scholar]
- 7.Dick JTA, et al. In press Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol. Invasions. (doi:10.1007/s10530-013-0550-8) [Google Scholar]
- 8.Dick JTA, et al. 2013. Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol. Invasions 15, 837–845 (doi:10.1007/s10530-012-0332-8) [Google Scholar]
- 9.Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL. 2013. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 83, 263–282 (doi:10.1890/13-0183.1) [Google Scholar]
- 10.Funk JL, Vitousek PM. 2007. Resource-use efficiency and plant invasion in low-resource systems. Nature 446, 1079–1081 (doi:10.1038/nature05719) [DOI] [PubMed] [Google Scholar]
- 11.Chapple DG, Simmonds SM, Wong BBM. 2012. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 27, 57–64 (doi:10.1016/j.tree.2011.09.010) [DOI] [PubMed] [Google Scholar]
- 12.Maezono Y, Kobayashi R, Kusahara M, Miyashita T. 2005. Direct and indirect effects of exotic bass and bluegill on exotic and native organisms in farm ponds. Ecol. Appl. 15, 638–650 (doi:10.1890/02-5386) [Google Scholar]
- 13.Weyl PSR, de Moor FC, Hill MP, Weyl OLF. 2010. The effect of largemouth bass Micropterus salmoides on aquatic macro-invertebrate communities in the Wit River, Eastern Cape, South Africa. Afr. J. Aquat. Sci. 35, 273–281 (doi:10.2989/16085914.2010.540776) [Google Scholar]
- 14.Cambray JA. 2003. The need for research and monitoring on the impacts of translocated sharptooth catfish, Clarias gariepinus, in South Africa. Afr. J. Aquat. Sci. 28, 191–195 (doi:10.2989/16085910309503786) [Google Scholar]
- 15.Kadye WT, Booth AJ. 2012. An invader within an altered landscape: one catfish, two rivers and an inter-basin water transfer scheme. River Res. Appl. 29, 1131–1146 (doi:10.1002/rra.2599) [Google Scholar]
- 16.Juliano SA. 2001. Nonlinear curve fitting: predation and functional response curves. In Design and analysis of ecological experiments (eds Scheiner SM, Gurevitch J.), pp. 178–196 Oxford, UK: Oxford University Press [Google Scholar]
- 17.Rogers D. 1972. Random search and insect population models. J. Anim. Ecol. 41, 369–383 (doi:10.2307/3474) [Google Scholar]
- 18.Ellender BR, Weyl OLF, Swartz ER. 2011. Invasion of a headwater stream by non-native fishes in the Swartkops River system, South Africa. Afr. Zool. 46, 39–46 (doi:10.3377/004.046.0116) [Google Scholar]
- 19.Wootton JT. 1997. Estimates and tests of per capita interaction strength: diet, abundance, and impact of intertidally foraging birds. Ecol. Monogr. 67, 45–64 (doi:10.1890/0012-9615(1997)067[0045:EATOPC]2.0.CO;2) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data can be found in the electronic supplementary material.