Abstract
It is generally believed that variation in sperm phenotype within a single ejaculate has no consequences for offspring performance, because sperm phenotypes are thought not to reflect sperm genotypes. We show that variation in individual sperm function within an ejaculate affects the performance of the resulting offspring in the Atlantic salmon Salmo salar. We experimentally manipulated the time between sperm activation and fertilization in order to select for sperm cohorts differing in longevity within single ejaculates of wild caught male salmon. We found that within-ejaculate variation in sperm longevity significantly affected offspring development and hence time until hatching. Whether these effects have a genetic or epigenetic basis needs to be further evaluated. However, our results provide experimental evidence for transgenerational effects of individual sperm function.
Keywords: gamete selection, epigenetics, haploid selection, sperm competition, fish
1. Introduction
The female reproductive tract and egg coating are often provided with barriers against sperm, and fertilization is often preceded by a demanding ‘obstacle course’ for the sperm cells [1]. Why females have evolved to complicate fertilization is puzzling, especially considering that hostility towards sperm may result in infertility [2]. One possibility is that females benefit from having higher quality males fertilizing their eggs [1,3], a hypothesis, which relies upon polyandry and a positive association between male success in sperm competition and offspring quality across males [4,5]. However, the facts that the association between the competitiveness of a male's ejaculate and the fitness of his offspring can be negative [5] and that fertilization seems to be similarly difficult in many monandrous species suggest that other mechanisms may be at play.
One possibility is that, within a single ejaculate of a given male, sperm phenotype may affect zygote fitness such that sperm screening results in more vigorous offspring and thus benefits females [1,3]. Marked phenotypic variation across sperm cells within a single ejaculate is ubiquitous, both in terms of sperm morphology [6–8] and performance [9]. However, sperm are generally considered products of the diploid paternal genome with no endogenous haploid gene expression, and sperm phenotype is thus thought not to reflect the haploid genome they carry [10]. Nevertheless, several mechanisms, both genetic and epigenetic, can potentially generate a relationship between sperm phenotype and zygote performance (see §4).
Here, we tested the hypothesis that within-ejaculate variation in sperm phenotype affects offspring performance in the Atlantic salmon Salmo salar. We enquired whether those sperm cells within a single ejaculate that survive for a longer time father offspring that differ from those fathered by other sperm cells in the same ejaculate. In order to produce different sperm cohorts within a single ejaculate of a given male, we experimentally varied the time between sperm activation and fertilization. We then assessed the effects of sperm cohort on offspring performance.
2. Material and methods
Sperm and eggs were obtained from wild-caught S. salar. Male and female gametes are activated by contact with water, which allows full experimental control over the selection of parents, the timing of gamete activation, sperm density and egg numbers. We performed in vitro fertilizations for all experiments (electronic supplementary material). In the sperm selection experiment, we selected for cohorts of sperm within an ejaculate based on intra-ejaculate variation in sperm ‘longevity’ (i.e. the time over which a sperm is motile). Under natural spawning conditions, gamete release is synchronous and sperm activity decreases to zero over the course of less than 1 min [11]. Hence, any delay between activation and fertilization selects for longer lived sperm cells. We employed a split design, in which we divided an ejaculate and a clutch of eggs into three equal parts (hereafter referred to as subclutches) and exposed each to one of three treatments (n = 26): sperm were activated and eggs added after 0 s (all sperm active), 20 s (ca 50% sperm active) or 40 s (few sperm active).
Fertilization success in S. salar decreases with reduced concentration of active sperm and fertilization success thus gradually decreased across our treatments (electronic supplementary material). A shortage of active sperm may generate competition between eggs, and eggs better able to attract sperm may also result in better offspring. In a second experiment, we tested for a potential effect of such egg competition. We manipulated the concentration of sperm available in four treatments and diluted 1000, 200, 50 or 5 µl of the same ejaculate in 100 ml of water to fertilize a subclutch of eggs using a split design (n = 11) without delay between activation and fertilization.
In both experiments, we assessed time from fertilization until hatching. At hatching, digital images were taken to measure the standard length at hatching. To assess growth rate in alevins after hatching in the sperm longevity experiment, we measured standard length (tip of head to basis of tail fin) of alevins both at hatching and three weeks after hatching.
3. Results
Sperm fertilizing eggs after 20 s sired offspring that hatched significantly earlier than sperm fertilizing eggs after zero or 40 s (table 1 and figure 1). Moreover, the pattern of the effect of sperm longevity differed across parental pairs, as revealed by a significant interaction between pair and treatment (electronic supplementary material, figure S1), which may be due to genetic and/or environmental differences across pairs. To assess the relative contribution of these two sources of variation, we performed further analyses, which suggest that both genetic and environmental effects are involved (see the electronic supplementary material for details).
Table 1.
Mixed model ANOVAs (REML estimation) comparing time until hatching between treatments of (a) sperm selection and (b) egg competition.
| response variable | fixed effect | F | d.f. | p | variance components ± s.e. |
|---|---|---|---|---|---|
| (a) sperm selection |
treatment |
3.69 |
2,49.2 |
0.032 |
pair ID 11.05 ± 3.88 treatment × pair ID 7.04 ± 1.52 |
| (b) egg competition |
treatment |
3.39 |
2,30.2 |
0.031 |
pair ID 0.66 ± 0.30 treatment × pair ID 0.03 ± 0.01 |
Figure 1.
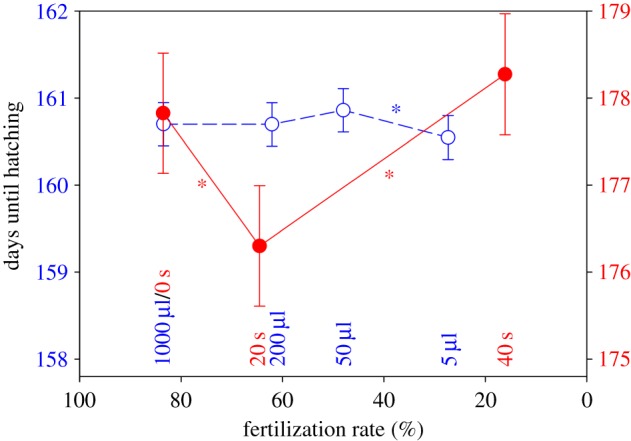
Variation in embryo development time in response to variation in (i) sperm longevity and (ii) sperm dilution. Here, mean (±s.e.) duration of embryo development is plotted against the realized fertilization rate across treatment groups. Embryo development time was significantly affected by variation in sperm longevity (solid symbols, solid line and right ordinate). Sperm dilution had a significant effect on embryo development time (unfilled symbols, broken line and left ordinate). Asterisks indicate significant differences between treatments. (Online version in colour.)
Furthermore, we found a relatively small but significant effect of egg competition on embryo development time (table 1 and figure 1). However, time until hatching decreased with greater sperm dilution, which contrasts with the increase in time until hatching in the 40 s treatment with the lowest fertilization rate.
At hatching, alevins in the 40 s treatment were larger than alevins in the other two treatments but there was no difference in the size of their yolk sack (table 2). The same pattern was found three weeks after hatching (table 2), suggesting no difference in growth rate between the three treatments (table 2). However, effects on alevin size at hatching could in part be caused by sperm dilution effects, because we found larvae to be significantly larger in the 5 μl treatment than in the 1000 μl treatment (treatment effect: F1,7 = 6.19, p = 0.042; pair ID: variance component = 0.52 ± 0.14).
Table 2.
Mixed model ANOVAs (REML estimation) comparing offspring size at hatching and growth rate between sperm selection treatments.
| response variable | fixed effect | F | d.f. | p | variance components ± s.e. |
|---|---|---|---|---|---|
| standard length at hatching | treatment | 5.41 | 2,31 | 0.001 | pair ID 0.69 ± 0.30 |
| age | 3.52 | 1,31 | 0.07 | ||
| yolk sack size at hatching | treatment | 0.12 | 2,31 | 0.89 | pair ID 23.68 ± 0.09 |
| age | 0.14 | 1,31 | 0.71 | ||
| standard length 21 days after hatching | treatment | 7.27 | 2,30 | 0.003 | pair ID 0.51 ± 0.24 |
| age | 2.91 | 1,30 | 0.10 | ||
| temperature | 34.73 | 1,30 | <0.001 | ||
| yolk sack size 21 days after hatching | treatment | 1.12 | 2,16 | 0.35 | pair ID 12.09 ± 4.88 treatment × pair ID 1.32 ± 0.47 |
| age | 1.60 | 1,16 | 0.22 | ||
| temperature | 28.85 | 1,16 | 0.001 | ||
| growth rate | treatment | 1.69 | 2,31 | 0.20 | pair ID 0.0015 ± 0.0002 |
| temperature | 5.44 | 1,31 | 0.03 |
4. Discussion
We found that sperm of intermediate longevity sired offspring with the most rapid development. This could not be attributed to egg competition caused by sperm dilution. Offspring size was also affected by sperm longevity but this effect was at least partly attributable to variation in fertilization success and hence to egg competition. We stress that the date of emergence from the gravel bed is a key life-history trait in salmon, which may be under strong selection: early hatching offspring will emerge early and will hence have an advantage when establishing themselves on the feeding grounds in competition with other juveniles [12].
There are at least four different, not mutually exclusive, mechanisms that could explain the effects of within-ejaculate variation in sperm performance on offspring performance. First, the empirical evidence for haploid gene expression is growing [13,14], suggesting that we may need to re-evaluate the possibility that translation of the haploid set of genes within a sperm affects sperm phenotypes and is correlated with offspring performance [15–17]. However, haploid gene expression in animals is highly debated [17]. Second, pre- and postmeiotic sperm senescence [18,19] in conjunction with the production of ejaculates with mixed-aged sperm could generate a relationship between sperm performance and mutational load in individual sperm. An intriguing facet of our findings is that the relationship between development time and sperm selection treatment was nonlinear (figure 1). This may reflect the balance between within-ejaculate sperm selection for long-lived sperm (whereby average sperm quality would increase with sperm age [1,3]) and post-activation sperm senescence [18–20]. Sperm longevity appears to have a positive effect on offspring in a broadcast spawning ascidian [21] but negative effects on offspring fitness in kittiwakes, Rissa tridactyla [22]. However, the importance of sperm senescence in salmon where sperm are active for just under a minute remains to be tested and further experiments are therefore needed to evaluate this hypothesis. Third, epigenetic effects that affect sperm phenotype may travel from the sperm into the zygote and show transgenerational effects [23,24]. Finally, it has been suggested that ‘sloppy’ spermatogenesis by the diploid male, who effectively fails to produce very large numbers of cells without some error [6], may play a role: apart from morphological and functional defects, ‘abnormal’ sperm may also be more prone to carry defects at the molecular level (e.g. DNA fragmentation [25]), which may affect zygote performance. Our results suggest that we need to improve our understanding of the physiological and molecular mechanisms that generate variation in sperm phenotypes in order to appreciate the direct and evolutionary effects of sperm selection.
The fact that males transfer such an enormous number of sperm in each mating has been attributed to between-ejaculate sperm competition [26]. Our results suggest that within-ejaculate competition between sperm may contribute not only to the evolution of female ‘barriers’ to sperm [1–3] but also to the evolution of sperm numbers [27,28]. If sperm phenotype affects offspring performance, this may result in indirect selection on ejaculate size favouring males that produce large numbers of sperm even in the absence of between-ejaculate sperm competition (i.e. under strict genetic monogamy). Under this scenario, males that produce a larger number of sperm would father offspring with, on average, a higher fitness. This effect will in theory be stronger, the higher the mutational load is among sperm [27,28].
Acknowledgements
We thank the staff at Älvkarleby for technical assistance and M. Gage and C. Cornwallis for comments on an earlier draft.
Data accessibility
Data available from the dryad digital repository: http://doi.org/10.5061/dryad.5f3f0 [29].
Funding statement
This study was supported by the Swiss National Foundation (S.I.), the Wenner-Gren Foundation (S.I.), the Swedish Research Council (S.I., G.A.) and the European Research Council (G.A.; AdG-294333).
References
- 1.Birkhead TR, Møller AP, Sutherland WJ. 1993. Why do females make it so difficult for males to fertilize their eggs? J. Theor. Biol. 161, 51–60 (doi:10.1006/jtbi.1993.1039) [Google Scholar]
- 2.Morrow EH, Arnqvist G, Pitcher TE. 2002. The evolution of infertility: does hatching rate in birds coevolve with female polyandry? J. Evol. Biol. 15, 702–709 (doi:10.1046/j.1420-9101.2002.00445.x) [Google Scholar]
- 3.Keller L, Reeve HK. 1995. Why do females mate with multiple males? The sexually selected sperm hypothesis. Adv. Study Behav. 24, 291–315 (doi:10.1016/S0065-3454(08)60397-6) [Google Scholar]
- 4.Hosken DJ, Garner TWJ, Tregenza T, Wedell N, Ward PI. 2003. Superior sperm competitors sire higher-quality young. Proc. R. Soc. Lond. B 270, 1933–1938 (doi:10.1098/rspb.2003.2443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilde T, Foged A, Schilling N, Arnqvist G. 2009. Postmating sexual selection favors males that sire offspring with low fitness. Science 324, 1705–1706 (doi:10.1126/science.1171675) [DOI] [PubMed] [Google Scholar]
- 6.Holt WV, Van Look KJW. 2004. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction 127, 527–535 (doi:10.1530/rep.1.00134) [DOI] [PubMed] [Google Scholar]
- 7.Immler S, Calhim S, Birkhead TR. 2008. Increased postcopulatory sexual selection reduces the intramale variation in sperm design. Evolution 62, 1538–1543 (doi:10.1111/j.1558-5646.2008.00393.x) [DOI] [PubMed] [Google Scholar]
- 8.Maroto-Morales A, Ramon M, Garcia-Alvarez O, Soler A, Fernandez-Santos M, Roldan E, Gomendio M, Perez-Guzman M, Garde J. 2012. Morphometrically-distinct sperm subpopulations defined by a multistep statistical procedure in ram ejaculates: intra- and interindividual variation. Theriogenology 77, 1529–1539 (doi:10.1016/j.theriogenology.2011.11.020) [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick JL, Garcia-Gonzalez F, Evans JP. 2010. Linking sperm length and velocity: the importance of intramale variation. Biol. Lett. 6, 797–799 (doi:10.1098/rsbl.2010.0231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hecht N. 1998. Molecular mechanisms of male germ cell differentiation. Bioessays 20, 555–561 (doi:10.1002/(SICI)1521-1878(199807)20:7<555::AID-BIES6>3.0.CO;2-J) [DOI] [PubMed] [Google Scholar]
- 11.Dziewulska K, Rzemieniecki A, Domagala J. 2010. Motility and energetic status of Atlantic salmon (Salmo salar L.) sperm after refrigerated storage. J. Appl. Ichthyol. 26, 668–673 (doi:10.1111/j.1439-0426.2010.01538.x) [Google Scholar]
- 12.Chapman D. 1962. Aggressive behavior in juvenile coho salmon as a cause of emigration. J. Fish. Res. Board Can. 19, 1047–1080 (doi:10.1139/f62-069) [Google Scholar]
- 13.Zheng Y, Deng X, Martin-DeLeon P. 2001. Lack of sharing of Spam1 (Ph-20) among mouse spermatids and transmission ratio distortion. Biol. Reprod. 64, 1730–1738 (doi:10.1095/biolreprod64.6.1730) [DOI] [PubMed] [Google Scholar]
- 14.Vibranovski M, Chalopin D, Lopes H, Long M, Karr T. 2010. Direct evidence for postmeiotic transcription during Drosophila melanogaster spermatogenesis. Genetics 186, 431–433 (doi:10.1534/genetics.110.118919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph SB, Kirkpatrick M. 2004. Haploid selection in animals. Trends Ecol. Evol. 19, 592–597 (doi:10.1016/j.tree.2004.08.004) [Google Scholar]
- 16.Immler S. 2008. Sperm competition and sperm cooperation: the potential role of diploid and haploid expression. Reproduction 135, 275–283 (doi:10.1530/REP-07-0482) [DOI] [PubMed] [Google Scholar]
- 17.Higginson D, Pitnick S. 2011. Evolution of intra-ejaculate sperm interactions: do sperm cooperate? Biol. Rev. 86, 249–270 (doi:10.1111/j.1469-185X.2010.00147.x) [DOI] [PubMed] [Google Scholar]
- 18.Pizzari T, Dean R, Pacey A, Moore HD, Bonsall MB. 2008. The evolutionary ecology of pre-and post-meiotic sperm senescence. Trends Ecol. Evol. 23, 131–140 (doi:10.1016/j.tree.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 19.Reinhardt K. 2007. Evolutionary consequences of sperm cell aging. Q. Rev. Biol. 82, 375–393 (doi:10.1086/522811) [DOI] [PubMed] [Google Scholar]
- 20.Siva-Jothy M. 2000. The young sperm gambit. Ecol. Lett. 3, 172–174 (doi:10.1046/j.1461-0248.2000.00146.x) [Google Scholar]
- 21.Crean A, Dwyer J, Marshall D. 2012. Fertilization is not a new beginning: the relationship between sperm longevity and offspring performance. PLoS ONE 7, e49167 (doi:10.1371/journal.pone.0049167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White J, Wagner R, Helfenstein F, Hatch S, Mulard H, Naves L, Danchin E. 2008. Multiple deleterious effects of experimentally aged sperm in a monogamous bird. Proc. Natl Acad. Sci. USA 105, 13 947–13 952 (doi:10.1073/pnas.0803067105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dadoune J-P. 2009. Spermatozoal RNAs: what about their functions? Microsc. Res. Tech. 72, 536–551 (doi:10.1002/jemt.20697) [DOI] [PubMed] [Google Scholar]
- 24.Jenkins T, Carrell D. 2012. The sperm epigenome and potential implications for the developing embryo. Reproduction 143, 727–734 (doi:10.1530/REP-11-0450) [DOI] [PubMed] [Google Scholar]
- 25.Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. 2003. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J. Androl. 24, 59–66 [PubMed] [Google Scholar]
- 26.Parker GA. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP.), pp. 3–54 London, UK: Academic Press [Google Scholar]
- 27.Parker GA, Begon ME. 1993. Sperm competition games: sperm size and number under gametic control. Proc. R. Soc. Lond. B 253, 255–262 (doi:10.1098/rspb.1993.0111) [DOI] [PubMed] [Google Scholar]
- 28.Manning JT, Chamberlain AT. 1994. Sib competition and sperm competitiveness: an answer to ‘why so many sperms?’ and the recombination/sperm number correlation. Proc. R. Soc. Lond. B 256, 177–182 (doi:10.1098/rspb.1994.0067) [DOI] [PubMed] [Google Scholar]
- 29.Immler S, Hotzy C, Alavioon G, Petersson E, Arnqvist G. 2014. Sperm variation within a single ejaculate affects offspring development in Atlantic salmon. Dryad Digital Repository (doi:10.5061/dryad.5f3f0) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the dryad digital repository: http://doi.org/10.5061/dryad.5f3f0 [29].


