Abstract
Light is an essential environmental factor required for photosynthesis, but it also mediates signals to control plant development and growth and induces stress tolerance. The photosynthetic organelle (chloroplast) is a key component in the signalling and response network in plants. This theme issue of Philosophical Transactions of the Royal Society of London B: Biology provides updates, highlights and summaries of the most recent findings on chloroplast-initiated signalling cascades and responses to environmental changes, including light and biotic stress. Besides plant molecular cell biology and physiology, the theme issue includes aspects from the cross-disciplinary fields of environmental adaptation, ecology and agronomy.
Oxygenic photosynthetic organisms carry out the most intriguing reaction on Earth, namely the conversion of light energy from the sun into chemical energy, which also results in oxygen as a by-product. The photosynthetic end products (sugars) drive most processes in living cells on Earth. As photosynthetic organisms represent the basis of our daily life (food, energy, materials), effects on their primary productivity have an impact on the society in various aspects, for instance economy, ecological sustainability and even our lifestyle. Photosynthetic organisms, particularly plants which are essentially sessile, have to constantly deal with changes in a wide range of abiotic and biotic factors in their immediate environment on a seasonal as well as daily basis. The chloroplast is a light-driven energy factory, but besides this primary mission it perceives signals from surroundings to adjust plant development and induce adaptation to ever-changing environmental cues.
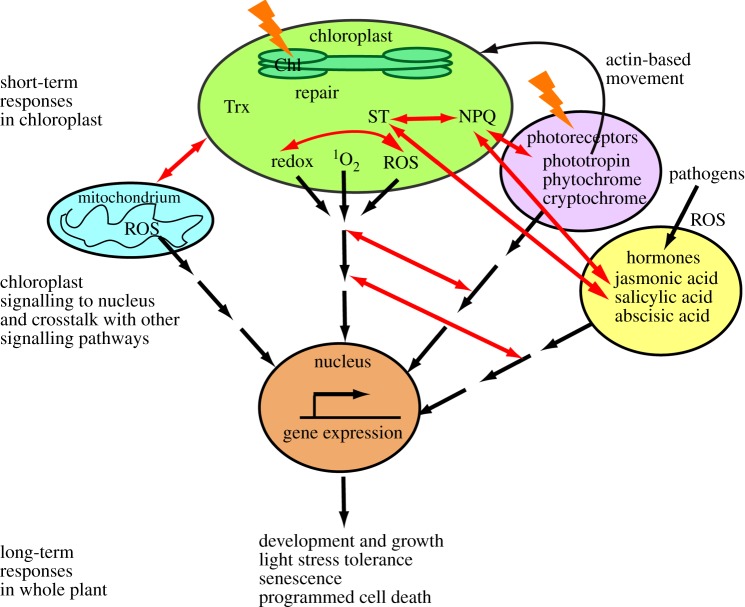
The signalling cascades start from various chloroplast processes but merge later or crosstalk with each other and with other signalling cascades (figure 1). For example, acclimation of plants to excess light conditions may also simultaneously increase the tolerance to other abiotic stress factors [1]. Recently, chloroplasts were also recognized to perceive and mediate signals that promote tolerance against plant pathogens (immune defence) or that are involved in hormone perception [2]. Resolving the crosstalk between the cascades is most important for understanding physiological responses in plants under ever-changing environments, and for predicting how plants survive under natural growth conditions.
Figure 1.
Overview of light-induced chloroplast signalling and response mechanisms, covered by papers in this theme issue. Chl, chlorophyll; NPQ, non-photochemical quenching; ROS, reactive oxygen species; ST, state transition; Trx, thioredoxin. (Online version in colour.)
This theme issue of Philosophical Transactions of the Royal Society of London B: Biology covers the most recent findings and updates on the molecular short-term mechanisms used by the chloroplast to adjust its function to changes in light conditions, and on the signalling pathways that induce long-term adaptive responses, such as stress tolerance and immune defence in plants (figure 1). It focuses on the current understanding of the crosstalk between signalling networks activated by chloroplasts and mitochondria, light receptors and those induced by biotic stress. It also focuses on the variation of the adaptive mechanisms in natural population and on their agricultural and ecological impacts. Thus, besides plant molecular cell biology and physiology, the theme issue includes aspects from the cross-disciplinary fields of environmental adaptation, ecology and agronomy. It consists of 10 research articles and nine reviews covering the following four topics: (i) short-term adaptive responses in chloroplasts, (ii) chloroplast-to-nucleus signalling and crosstalk with other signalling pathways, (iii) natural variation of regulatory mechanisms to allow for adaptation and (iv) agricultural and ecological perspective of light responses in chloroplasts.
Light signals perceived by chlorophyll (Chl) in the thylakoid membrane and by photoreceptors in the cytosol activate various short-term adaptive responses including enzyme regulation, photoprotection and repair (figure 1). Ebenhöh et al. [3] propose a mathematical model for relative contributions of non-photochemical quenching (NPQ) and state transition (ST) in light acclimation. The paper by Cazzaniga et al. [4] identifies photoreceptor-dependent chloroplast movement as an additional pathway used to dissipate the excess absorbed energy, whereas Ruban & Belgio [5] investigate NPQ in relation to maximum light intensity tolerated by plants. Bertrand et al. [6] investigate the different mechanisms involved in NPQ relaxation in diatoms. Nikkanen & Rintamäki [7] and Kirchhoff [8] review the current knowledge on chloroplast processes regulated by thioredoxins under changing light environment and processes in the thylakoid membrane associated with the photosystem II repair cycle in high light stress, respectively.
Together with adjustments of metabolic processes and induction of photoprotective mechanisms, light initiates signalling to the nucleus for gene expression, resulting in various long-term adaptive responses, including development and growth, stress and programmed cell death (figure 1). Larkin [9] provides an updated insight into the impact of the GENOMES UNCOUPLED genes on plastid-to-nucleus signalling and reviews the influence of plastids on light receptor signalling and development, whereas the contribution by Blanco et al. [10] searches for new components integrating mitochondrial and plastid retrograde signals that regulate plant energy metabolism. Alsharafa et al. [11] investigate the kinetics of events involved in initiation of high light acclimation, and Tikkanen et al. [12] show that chloroplast signalling interacts with both reactive oxygen species (ROS) and hormonal signalling. ROS signalling is also highlighted in the papers by Heyno et al. (hydrogen peroxide) [13] and by Zhang et al. (singlet oxygen) [14]. Foyer et al. [15] introduce a chloroplast protein belonging to the WHIRLY family and propose that the redox state of the photosynthetic electron transport chain triggers the movement of this protein from the chloroplast to the nucleus where it regulates the gene expression leading to cross tolerance, including light acclimation and immune defence. Gorecka et al. [16] identify novel components for crosstalk of immune reaction-induced signalling networks with two short-term photoprotective mechanisms, ST and NPQ. Trotta et al. [17] further review the increasing evidence for crosstalk between light-induced chloroplast signalling and immune reactions in plants.
To allow for adaptation to a changing environment, natural selection of existing genetic variation takes place. Flood, Yin et al. [18] report natural variation in photosystem II protein phosphorylation in the model plant Arabidopsis thaliana and propose a possible role in the adaptation to diverse environments. In addition, Serõdio et al. [19] review the current knowledge of adaptation of macroalgal chloroplasts to life in sea slug following ingestion. Finally, the review by Darko et al. [20] uses selected examples to show how artificial lighting can be used to improve plant growth in agriculture and for production of functional food and materials, whereas Demmig-Adams et al. [21] provide an ecophysiological perspective of light responses in the chloroplast to optimize its function and of the whole plant in a changing environment.
This research on light-induced signalling and response is developing in many directions, as reflected by the broad field coverage of the papers of this theme issue. It highlights and summarizes the present knowledge from the individual chloroplast reactions to the variation of the adaptive mechanisms in natural populations and on their agricultural and ecological impacts.
Acknowledgements
We thank all the authors who have contributed to this theme issue and the reviewers for their valuable comments and suggestions. We hope that the readers enjoy the high-quality papers of this theme issue and that these publications inspire the scientists in the field to make new discoveries in future.
Funding statement
We thank for support the Swedish Research Council (to C.S.), Academy of Finland (to E.R.), the Carl and Thecla Lamberg Foundation (to C.S., E.R.) and the University of Le Mans (to B.S.).
References
- 1.Barajas-Lopez de D, Blanco NE, Strand A. 2013. Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim. Biophys. Acta 1833, 425–437. ( 10.1016/j.bbamcr.2012.06.020) [DOI] [PubMed] [Google Scholar]
- 2.Bartoli CG, Casalongue CA, Simontacchi M, Marquez-Garcia B, Foyer CH. 2013. Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ. Exp. Bot. 94, 73–88. ( 10.1016/J.Envexpbot.2012.05.003) [DOI] [Google Scholar]
- 3.Ebenhöh O, Fucile G, Finazzi G, Rochaix J-D, Goldschmidt-Clermont M. 2014. Short-term acclimation of the photosynthetic electron transfer chain to changing light: a mathematical model. Phil. Trans. R. Soc. B 369, 20130223 ( 10.1098/rstb.2013.0223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dall'Osto L, Cazzaniga S, Wada M, Bassi R. 2014. On the origin of a slowly reversible fluorescence decay component in the Arabidopsis npq4 mutant. Phil. Trans. R. Soc. B 369, 20130221 ( 10.1098/rstb.2013.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruban AV, Belgio E. 2014. The relationship between maximum tolerated light intensity and photoprotective energy dissipation in the photosynthetic antenna: chloroplast gains and losses. Phil. Trans. R. Soc. B 369, 20130222 ( 10.1098/rstb.2013.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roháček K, Bertrand M, Moreau B, Jacquette B, Caplat C, Morant-Manceau A, Schoefs B. 2014. Relaxation of the non-photochemical chlorophyll fluorescence quenching in diatoms: kinetics, components and mechanisms. Phil. Trans. R. Soc. B 369, 20130241 ( 10.1098/rstb.2013.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikkanen L, Rintamäki E. 2014. Thioredoxin-dependent regulatory networks in chloroplasts under fluctuating light conditions. Phil. Trans. R. Soc. B 369, 20130224 ( 10.1098/rstb.2013.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchhoff H. 2014. Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Phil. Trans. R. Soc. B 369, 20130225 ( 10.1098/rstb.2013.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin RM. 2014. Influence of plastids on light signalling and development. Phil. Trans. R. Soc. B 369, 20130232 ( 10.1098/rstb.2013.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco NE, Guinea-Díaz M, Whelan J, Strand Å. 2014. Interaction between plastid and mitochondrial retrograde signalling pathways during changes to plastid redox status. Phil. Trans. R. Soc. B 369, 20130231 ( 10.1098/rstb.2013.0231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsharafa K, Vogel MO, Oelze M-L, Moore M, Stingl N, König K, Friedman H, Mueller MJ, Dietz K-J. 2014. Kinetics of retrograde signalling initiation in the high light response of Arabidopsis thaliana. Phil. Trans. R. Soc. B 369, 20130424 ( 10.1098/rstb.2013.0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tikkanen M, Gollan PJ, Mekala N, Isojärvi J, Aro E-M. 2014. Light-harvesting mutants show differential gene expression upon shift to high light as a consequence of photosynthetic redox and reactive oxygen species metabolism. Phil. Trans. R. Soc. B 369, 20130229 ( 10.1098/rstb.2013.0229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyno E, Innocenti G, Lemaire SD, Issakidis-Bourguet E, Krieger-Liszkay A. 2014. Putative role of the malate valve enzyme NADP–malate dehydrogenase in H2O2 signalling in Arabidopsis. Phil. Trans. R. Soc. B 369, 20130228 ( 10.1098/rstb.2013.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Apel K, Kim C. 2014. Singlet oxygen-mediated and EXECUTER-dependent signalling and acclimation of Arabidopsis thaliana exposed to light stress. Phil. Trans. R. Soc. B 369, 20130227 ( 10.1098/rstb.2013.0227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foyer CH, Karpinska B, Krupinska K. 2014. The functions of WHIRLY1 and REDOX-RESPONSIVE TRANSCRIPTION FACTOR 1 in cross tolerance responses in plants: a hypothesis. Phil. Trans. R. Soc. B 369, 20130226 ( 10.1098/rstb.2013.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorecka M, Alvarez-Fernandez R, Slattery K, McAusland L, Davey PA, Karpinski S, Lawson T, Mullineaux PM. 2014. Abscisic acid signalling determines susceptibility of bundle sheath cells to photoinhibition in high light-exposed Arabidopsis leaves. Phil. Trans. R. Soc. B 369, 20130234 ( 10.1098/rstb.2013.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trotta A, Rahikainen M, Konert G, Finazzi G, Kangasjärvi S. 2014. Signalling crosstalk in light stress and immune reactions in plants. Phil. Trans. R. Soc. B 369, 20130235 ( 10.1098/rstb.2013.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flood PJ, Yin L, Herdean A, Harbinson J, Aarts MGM, Spetea C. 2014. Natural variation in phosphorylation of photosystem II proteins in Arabidopsis thaliana: is it caused by genetic variation in the STN kinases? Phil. Trans. R. Soc. B 369, 20130499 ( 10.1098/rstb.2013.0499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seroôdio J, Cruz S, Cartaxana P, Calado R. 2014. Photophysiology of kleptoplasts: photosynthetic use of light by chloroplasts living in animal cells. Phil. Trans. R. Soc. B 369, 20130242 ( 10.1098/rstb.2013.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darko E, Heydarizadeh P, Schoefs B, Sabzalian MR. 2014. Photosynthesis under artificial light: the shift in primary and secondary metabolism. Phil. Trans. R. Soc. B 369, 20130243 ( 10.1098/rstb.2013.0243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demmig-Adams B, Stewart JJ, Adams WW., III 2014. Multiple feedbacks between chloroplast and whole plant in the context of plant adaptation and acclimation to the environment. Phil. Trans. R. Soc. B 369, 20130244 ( 10.1098/rstb.2013.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]