Abstract
Land plants live in a challenging environment dominated by unpredictable changes. A particular problem is fluctuation in sunlight intensity that can cause irreversible damage of components of the photosynthetic apparatus in thylakoid membranes under high light conditions. Although a battery of photoprotective mechanisms minimize damage, photoinhibition of the photosystem II (PSII) complex occurs. Plants have evolved a multi-step PSII repair cycle that allows efficient recovery from photooxidative PSII damage. An important feature of the repair cycle is its subcompartmentalization to stacked grana thylakoids and unstacked thylakoid regions. Thus, understanding the crosstalk between stacked and unstacked thylakoid membranes is essential to understand the PSII repair cycle. This review summarizes recent progress in our understanding of high-light-induced structural changes of the thylakoid membrane system and correlates these changes to the efficiency of the PSII repair cycle. The role of reversible protein phosphorylation for structural alterations is discussed. It turns out that dynamic changes in thylakoid membrane architecture triggered by high light exposure are central for efficient repair of PSII.
Keywords: PSII core phosphatase, photoinhibition, photosynthesis, photosystem II repair, STN8, thylakoid membrane
1. Introduction
Plants are integrated in a complex and highly dynamic network of environmental factors that determines their fitness and survival. A particular challenge for plants is that some of these factors vary unpredictably. A prime example is sunlight intensity that randomly changes by several orders of magnitude during the day [1]. This fluctuation in solar radiation has a significant impact on photosynthetic light reactions harboured in the thylakoid membrane system inside chloroplasts. In particular, high light intensities can induce severe damage by formation of toxic side products (reactive oxygen species, ROS) in the photosynthetic apparatus. Therefore, in anticipation of unforeseen alterations in sunlight intensity, plants have evolved a battery of constitutive or inducible photoprotective mechanisms [2–4]. These mechanisms include, for example, paraheliotropism that orients the leaf parallel to the sunlight direction [5], chloroplast avoidance movement that relocates chloroplasts within the plant cell to minimize light exposure [6], ROS scavenging systems [7–9] and high energy quenching that converts harvested light energy safely into heat [3,10,11].
Although these multi-level photoprotective mechanisms help to minimize the excitation pressure on the photosynthetic machinery, damage is unavoidable. The main target of photoxidative damage is the photosystem II (PSII) supercomplex [12]. PSII photodamage occurs at all light intensities [13] but leads to net inhibition of photosynthesis only if the rate of damage exceeds the rate of repair, i.e. under high light stress. In plants, the PSII supercomplex is organized as a dimer in which each monomer binds three so called minor light-harvesting complexes II (LHCII), CP24, CP26 and CP29, and one or two trimeric major LHCII complexes [14,15]. This massive approximately 1.4 MDa-sized holocomplex [15] is mainly localized in strictly stacked grana thylakoid areas. Grana are a characteristic feature of higher plants and some green algae thylakoid membranes [16]. Tight grana stacking is visualized by the small distance (approx. 3.5 nm) by which two grana membrane pairs are separated on the stromal side (stromal gap) and by the luminal width of only 4.5 nm ([17,18], see also figure 2). A consequence of grana formation is structural subcompartmentalization of the thylakoid membrane system into tightly stacked and exposed unstacked regions. This has a profound impact for the lateral distribution of protein complexes between these subcompartments because some of them have bulky protrusions that prevent them from entering stacked grana regions by steric hindrance. This is realized for PSI [19] and the ATPase [20] that have bulky stromal protrusions. Consequently, these protein complexes are confined to unstacked stroma lamellae and to unstacked grana margins and end membranes, whereas LHCII and PSII with flat stromal surfaces are concentrated in stacked grana regions [21,22]. Grana formation is mainly driven by interactions between stromal protein moieties of LHCIIs localized in adjacent stacked membranes [23]. It was suggested that the formation of semi-crystalline protein arrays in grana thylakoids that consist of PSII–LHCII supercomplexes can promote grana stacking [17]. A puzzling problem of grana formation was how membranes can bend sharply at the periphery of the grana cylinder (grana margins). Very recently, a small protein family (CURT) was identified that can facilitate membrane bending and plays a central role in grana formation [24]. Beyond the lateral asymmetric distribution of protein complexes involved in energy transformation, grana stacking also has important consequences for the sublocalization of low abundance protein complexes that are involved in PSII repair. This aspect, together with high-light-triggered architectural alterations of thylakoid membranes on the micrometre and supramolecular level, will be addressed in this review. It will be demonstrated that this unique membrane system responds dynamically to light stress and that these structural alterations are required for the repair of PSII.
Figure 2.
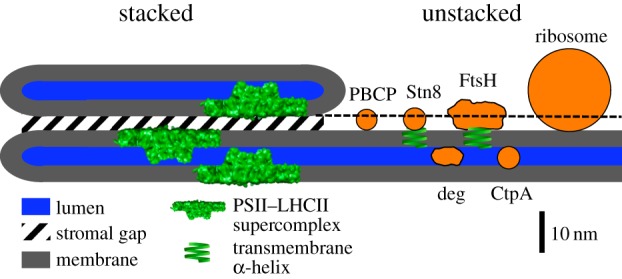
Structural model of the thylakoid membrane and components involved in PSII repair. The model represents the structural relationship between thylakoid membrane features and the sizes of proteins. The sizes and contours of the Deg and FtsH proteases were adapted from [50,51]. For PBCP, STN8 and CtpA, the sizes were calculated from their relative molecular mass (RMM). The RMM of a protein is proportional to its volume. Assuming a spherical protein contour, the relative change in diameter for protein 1 (dprotein1) to protein 2 (dprotein2) can be derived from RMM by solving the following equation for dprotein1: dprotein1/dprotein2 = (RMMprotein1)1/3/(RMMprotein2)1/3. The absolute diameter for protein 1 can be calculated if the relationship between d and RMM is known for a reference protein (protein 2). PC was selected as a reference protein. Its RMM is 10.5 kDa and its dimensions are 3 × 3 × 4 nm [52]. For the calculations, a mean diameter of 3.5 nm was used. Based on this number, the diameters of PBCP, STN8 and CtpA were calculated: PBCP: 32 kDa [40] ≥ approximately 5.1 nm; STN8 kinase; 56 kDa, extrinsic part 40.5 kDa ≥ approximately 5.5 nm; CtpA, 43 kDa [44] ≥ approximately 5.6 nm. (Online version in colour.)
2. PSII repair cycle
The critical role of efficient PSII repair is exemplified by the estimate that without repair, the yield of photosynthesis would drop below 5% [25]. In light of this number, it is not surprising that one of the most efficient repair mechanisms, the PSII repair cycle, evolved to maintain photosynthetic performance [26–28]. The mechanism of how PSII is damaged by light is under debate and beyond the scope of this review; the reader is referred to recent review articles [28–30]. Whatever the exact mechanism is, it finally leads to an impaired D1 subunit that is the main target of photooxidative damage [31]. Together with the D2 subunit, D1 forms the heterodimeric reaction centre of PSII that binds all cofactors involved in electron transport [32]. The bottom line is that the PSII repair cycles degrade the damaged D1 and replace it by a de novo synthesized subunit leading to a fully functional recovered PSII holocomplex.
One challenge for D1 replacement is that this subunit is buried in the middle of the LHCII–PSII holocomplex [15]. Therefore, disassembly of the holocomplex is required to make the damaged D1 subunit accessible to proteases and to allow insertion of the newly synthesized copy [27,33]. Consequently, an important step in the PSII repair cycle is the disassembly of the PSII holocomplex. The multi-step PSII repair cycle is outlined in figure 1. The mainstream model is that after high-light-induced damage, the PSII subunits D1, D2, CP43 and psbH are phosphorylated (figure 1(2)), catalysed mainly by the STN8 kinase [34,35] and possibly by the STN7 kinase [36–38]. As evidence exists that in STN7/STN8 double mutants, the disassembly of the PSII holocomplex is inhibited [33,39–41], it was hypothesized that phosphorylation of core subunits triggers disassembly (figure 1(3)). It is further assumed that the stripped down and phosphorylated PSII complex is the form that diffuses from stacked to unstacked thylakoid regions (figure 1(4)) where the repair machinery is localized. It was proposed that before degradation of the damaged D1 subunit can proceed, dephosphorylation is required (figure 1(5); [42]). Recently, a PSII core phosphatase (PBCP) was identified that catalyses PSII dephosphorylation [43]. Proteolytic D1 degradation is catalysed by two types of proteases (figure 1(6)). The FtsH proteases operate from the stromal side, whereas Deg proteases work mainly from the luminal side [44–46]. A new copy of the plastom-encoded D1 subunit is translated at chloroplast 70S ribosomes. The nascent precursor D1 protein is processed by a C-terminal processing enzyme (CtpA) localized in the thylakoid lumen [47] and the mature D1 subunit is inserted into the PSII core complex (figure 1(7)). The cycle is closed by reassembly and functionalization of the PSII holocomplex and back-migration to stacked grana (figure 1(8)).
Figure 1.
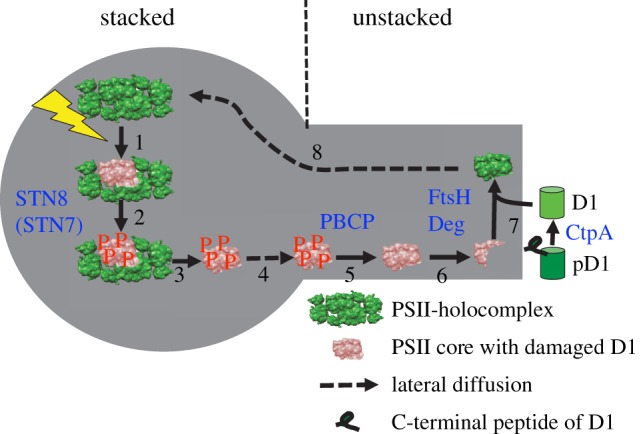
PSII repair cycle. The repair cycle is subcompartmentalized to stacked and unstacked thylakoid regions. After high light induces photodamage mainly to the D1 subunit of PSII (1), the holocomplex is phosphorylated by STN8 kinase (2). The phosphorylation triggers monomerization and detachment of LHCII from the PSII core (3) followed by lateral migration from stacked thylakoids to unstacked regions (4). Before the damaged D1 subunit is degraded by FtsH and Deg proteases (6), the PSII core is dephosphorylated by PBCP phosphatase (5). However, it is under debate whether dephosphorylation is required for D1 degradation. A new synthesized D1 copy is inserted in the PSII core (7). Before D1 is inserted, the precursor D1 protein (pD1) is processed by CtpA (7). Finally, the PSII holocomplex reassembles and migrates back to stacked grana (8). (Online version in colour.)
3. High-light-induced changes in overall membrane architecture
As illustrated in figure 1, the repair of photodamaged PSII requires coordination of partial reactions of the repair cycle localized in stacked and unstacked membrane regions. Therefore, the structural relationship of grana core, grana margins and stroma lamellae is relevant for efficient PSII repair. In recent years, it has come into focus that high light stress is accompanied by changes in macroscopic thylakoid folding [39,48,49]. From these studies, a partial destacking of grana was concluded, i.e. a vertical separation of grana membrane pairs at the stromal side and lateral reduction of the grana diameter were identified under high light conditions. Furthermore, the width of the thylakoid lumen also changes dynamically. A swelling in light-adapted plants was identified [18]. As detailed below, these changes make sense in view of the structural constraints given by the tight stacking of grana membranes. Figure 2 compares the structural relationship between key proteins involved in PSII repair (see also figure 1) and ultrastructural data of stacked grana membranes derived from electron microscopy. The molecular contours of FtsH and Deg proteases were derived from high-resolution structures. For PBCP, STN8 kinase and CtpA, no such structural data are available. Therefore, their sizes were calculated from their relative molecular masses as explained in figure 2 legend. These sizes should be taken as an estimate, in particular, because a spherical contour of these proteins is assumed, but this may not be realized. However, figure 2 allows discussion of structural constraints realized in stacked grana for accessibility of proteins involved in PSII repair.
Figure 2 suggests that all repair proteins could be excluded from stacked grana regions because either the stromal gap of approximately 3.5 nm is too small to allow access of ribosomes, FtsH, STN8 and PBCP (dashed line in figure 2) or the width of the lumen (of approx. 4.5 nm) combined by macromolecular crowding by extrinsic parts of PSII (water-splitting system) restricts inflow of Deg proteases and CtpA. For the lumen-hosted small protein plastocyanin (PC, 10.5 kDa, 3 × 3 × 4 nm), we identified severe restriction of its mobility in stacked grana [18]. As Deg proteases (diameter of hexamer is approx. 9 nm) and CtpA (43 kDa) are significantly larger than PC, it is likely that they are sterically excluded from the densely packed grana lumen. For the stroma side, it is safe to conclude that the accessibility of the FtsH protease and ribosomes to grana core regions is highly restricted. The situation for PBCP and STN8 is less clear. Their sizes (assuming spherical shape) are only slightly larger than the stromal gap (figure 2). However, it has to be taken into account that PSII has approximately 1 nm protrusion on the stromal side [32] that would further reduce the stromal gap. The fact that PBCP and STN8 are of similar size as the stromal gap in grana opens possibilities that small changes in the gap width can have significant impact on the accessibility of the phosphatase and kinase to stacked grana. This has to be explored in future studies. Overall, steric restrictions as realized in grana core thylakoids reduce the access of proteins involved in the PSII repair cycle. It follows that two domains are established, one that contains active PSII (stacked) and one responsible for repair processes (unstacked). This kind of structural differentiation could be advantageous under conditions where PSII repair is of minor significance (i.e. under low light), because it can avoid unwanted turnover of PSII. However, under high light stress, the system must activate its performance for efficient repair that calls for structural rearrangements. So far, these considerations mainly addressed the relationship between grana core and stroma lamellae. The role of grana margins is unclear but as they are at the interface between the two other thylakoid regions, it is likely that they play a pivotal role for the PSII repair cycle [53]. More studies are required to unravel the function of grana margins for protein repair in thylakoid membranes.
As mentioned above, high light intensities induce architectural alterations of the thylakoid folding. These changes can work synergistically to facilitate repair of damaged PSII. The swelling of the lumen could allow Deg proteases to get in contact with PSII. Similarly, vertical unstacking of grana thylakoids by an increase of the stromal gap enables better accessibility for FtsH, STN8 and PBCP to grana stacks. Furthermore, a lateral partial destacking of grana allows direct contact of PSII localized in former stacked regions to STN8, PBCP and FtsH. Thus, the flexible nature of the overall thylakoid membrane seems to be key for initiation of PSII repair processes. The capability of the thylakoid membrane system for massive structural reorganizations was also shown by atomic force microscopy and scanning electron microscopy studies [54]. It is noteworthy that the architectural changes mentioned above are not the only control point for the activation of the PSII repair cycle. In particular, evidence exists that the activity of the STN8 kinase is dependent on the redox state of the plastoquinone pool [39,55]. Furthermore, the active forms of the FtsH and Deg proteases are hexamers [45,46,50]. For the Deg protease, it was found that oligomerization is triggered by acidic pH values in the thylakoid lumen [50]. As such, the structural rearrangements of thylakoid membranes should be seen as part of a complex regulatory network that controls the repair processes in thylakoid membranes.
4. High-light-induced changes in supramolecular protein organization
Another structural level that has a strong dependency on light intensity is the protein arrangement in thylakoid membranes, i.e. the supramolecular level. A well known phenomenon is that increasing the light intensity leads to alterations of the LHC/electron transport complexes ratio [56,57]. Under high light intensity, the abundance of LHCII decreases, whereas the amount of PSII and cytochrome b6f complexes increases. In consequence, this acclimation to elevated sunlight intensities leads to lower light-harvesting capacities and increased electron transport capacities.
In addition to the dynamic control of the protein composition in thylakoid membranes, the degree of supramolecular ordering is also dependent on the light intensity. In stacked grana, proteins can be organized in a disordered way or in highly ordered two-dimensional semi-crystalline arrays [58]. These arrays are constituted by the LHCII–PSII holocomplex. Electron microscopic analysis revealed that the proportion of protein arrays decreases under high light [59]. This is in accordance with previous studies showing that PSII arrays are formed under low light [57]. It seems likely that highly ordered semi-crystalline protein arrays are disadvantageous for PSII repair under high light. This might be explained by the fact that the mobility of PSII complexes localized in semi-crystalline arrays is highly restricted and therefore the required mobilization to enter the repair processes is hindered. But this hypothesis awaits experimental verification.
Concerning the PSII repair cycle, an important observation is that the mobility of grana-hosted pigment protein complexes (PSII and LHCII) increases under high light stress. By using fluorescence recovery after photobleaching (FRAP), it was measured that high light treatment increases the rate of protein exchange between different grana discs [60] and the mobility within isolated grana membranes [49]. The significance for the PSII repair cycle is given by the fact that under non-stressed conditions, protein (PSII) mobility in stacked grana is very low [60–62]. Based on this low PSII mobility, it is conceptually hard to understand how damaged PSII in core grana can escape to reach the repair machinery in distant stroma lamellae. The FRAP data indicate that a high-light-induced switch in protein mobility ensures efficient diffusion of damaged PSII out of stacked thylakoid regions. The higher protein mobility could be caused by PSII disassembly and/or by electrostatic effects that are triggered by protein phosphorylation. This is addressed in §5.
5. Role of protein phosphorylation
As summarized in §2, it is accepted that photoinhibition leads to phosphorylation of PSII core subunits. Although the significance of core phosphorylation for the PSII repair cycle was challenged [34], it is now in agreement that PSII phosphorylation increases its mobility as required for swift repair, i.e. that it facilitates PSII repair [33,37–40]. What is unknown is the molecular mechanism(s) of PSII mobilization by protein phosphorylation. Based on recent data, two levels can be envisioned how core phosphorylation induces mobilization. The first level is that phosphorylation leads to partial destacking of grana. As discussed in §3, this allows better contact between STN8, PBCP and FtsH to damaged PSII. Whether a link exists between protein phosphorylation and the swelling of the lumen is unknown. If a link exists, then it must be an indirect one because PSII core phosphorylation by STN8 takes places exclusively on the stromal side [37]. However, concerning partial destacking, two models were postulated that explain how protein phosphorylation triggers unstacking. The ‘surface charge model’ [63] hypothesizes that addition of negative charges by protein phosphorylation alters the balance between electrostatic repulsion and van der Waals attraction of paired thylakoid membrane surfaces. By contrast, the ‘molecular recognition model’ [64] predicts a local impact of phosphorylation on the protein conformation. The altered conformation of phosphorylated proteins leads to changed recognition motifs between proteins, inducing their separation. In accordance with both models is that partial destacking by PSII core phosphorylation is mechanistically very similar to partial destacking triggered by LHCII phosphorylation under state transition that is catalysed mainly by the STN7 kinase [37,38,65]. Interestingly, the degree of partial destacking under state transition in low light [66] is similar to the degree of destacking under high light stress [49] indicating a similar mechanism. This points to the possibility that the overall phosphorylation level of grana-hosted proteins determines the degree of stacking [67]. The second level of an interrelationship between protein phosphorylation and PSII mobilization is that core phosphorylation could be involved in the disassembly of the holocomplex (§2). The same mechanistic models as discussed for grana destacking can be applied to explain how phosphorylation leads to monomerization and unbinding of LHCII from PSII. It is attractive to assume that smaller disassembled PSII monomer allows faster diffusion through crowded grana membranes to reach the repair machinery in unstacked thylakoid regions. However, this has to be considered carefully because computer simulations predict that in membranes with smaller protein complexes, lateral diffusion is more impaired than in membranes with larger holocomplexes [68]. In conclusion, protein phosphorylation can work on several structural levels, but so far it is unknown what mechanisms or what combinations are responsible for allowing efficient contact between damaged PSII in grana and its repair machinery.
Acknowledgements
Robert Yarbrough is acknowledged for proofreading the manuscript.
Funding statement
H.K. receives support from the National Science Foundation (NSF-MCB115871), the United States-Israel Binational Agricultural Research and Development Fund (BARD US-4334-10), the US National Institute of Food and Agriculture (NIFA, grant no. 2011-68005-30416), the US Department of Agriculture (ARC grant no. WNP00775) and Washington State University.
References
- 1.Külheim C, Ågren J, Jansson S. 2002. Rapid regulation of light harvesting and plant fitness in the field. Science 297, 91–93. ( 10.1126/science.1072359) [DOI] [PubMed] [Google Scholar]
- 2.Niyogi KK. 1999. Photoprotection revisited: genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333–359. ( 10.1146/annurev.arplant.50.1.333) [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Wakao S, Fischer BB, Niyogi KK. 2009. Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260. ( 10.1146/annurev.arplant.58.032806.103844) [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Badger MR. 2011. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 16, 53–60. ( 10.1016/j.tplants.2010.10.001) [DOI] [PubMed] [Google Scholar]
- 5.Ehleringer J, Forseth I. 1980. Solar tracking by plants. Science 210, 1094–1098. ( 10.1126/science.210.4474.1094) [DOI] [PubMed] [Google Scholar]
- 6.Wada M, Kagawa T, Sato Y. 2003. Chloroplast movement. Annu. Rev. Plant Biol. 54, 455–468. ( 10.1146/annurev.arplant.54.031902.135023) [DOI] [PubMed] [Google Scholar]
- 7.Asada K. 1999. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639. ( 10.1146/annurev.arplant.50.1.601) [DOI] [PubMed] [Google Scholar]
- 8.Neely WC, Martin JM, Barker SA. 1988. Products and relative reaction rates of the oxidation of tocopherols with singlet molecular oxygen. Photochem. Photobiol. 48, 423–428. ( 10.1111/j.1751-1097.1988.tb02840.x) [DOI] [PubMed] [Google Scholar]
- 9.Di Mascio P, Devasagayam TP, Kaiser S, Sies H. 1990. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem. Soc. Trans. 18 1054–1056. [DOI] [PubMed] [Google Scholar]
- 10.Ruban AV, Johnson MP, Duffy CD. 2012. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta 1817, 167–181. ( 10.1016/j.bbabio.2011.04.007) [DOI] [PubMed] [Google Scholar]
- 11.Jahns P, Holzwarth AR. 2012. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 1817, 182–193. ( 10.1016/j.bbabio.2011.04.012) [DOI] [PubMed] [Google Scholar]
- 12.Kyle DJ, Ohad I, Arntzen CJ. 1984. Membrane protein damage and repair: selective loss of quinone-protein function in chloroplast membranes. Proc. Natl Acad. Sci. USA 181, 4070–4074. ( 10.1073/pnas.81.13.4070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasil O, Adir N, Ohad I. 1992. Dynamics of photosystem II: mechanism of photoinhibition and recovery processes. In The photosystems: structure, function and molecular biology (ed. Barber J.), pp. 295 Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 14.Jansson S. 1999. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4, 1360–1385. ( 10.1016/S1360-1385(99)01419-3) [DOI] [PubMed] [Google Scholar]
- 15.Caffarri S, Kouril R, Kereiche S, Boekema EJ, Croce R. 2009. Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 28, 3052–3063. ( 10.1038/emboj.2009.232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nevo R, Chuartzman SG, Tsabari O, Reich Z. 2009. Architecture and plasticity of thylakoid membrane networks. In Lipids in photosynthesis (eds Wada H, Murata N.), pp. 295–328. Berlin, Germany: Springer. [Google Scholar]
- 17.Daum B, Nicastro D, Austin J, II, McIntosh R, Kühlbrandt W. 2010. Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea. Plant Cell 22, 1299–1312. ( 10.1105/tpc.109.071431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhoff H, Hall C, Wood M, Herbstová M, Tsabari O, Nevo R, Charuvi D, Shimoni E, Reich Z. 2011. Dynamic control of protein diffusion within the granal thylakoid lumen. Proc. Natl Acad. Sci. USA 108, 20 248–20 253. ( 10.1073/pnas.1104141109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amunts A, Nelson N. 2009. Plant photosystem I design in the light of evolution. Structure 17, 637–650. ( 10.1016/j.str.2009.03.006) [DOI] [PubMed] [Google Scholar]
- 20.Abrahams J, Leslie AGW, Lutter R, Walker JE. 1994. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628. ( 10.1038/370621a0) [DOI] [PubMed] [Google Scholar]
- 21.Andersson B, Anderson JM. 1980. Lateral heterogeneity in the distribuition of chlorophyll-protein complexes of the thylakoid membranes of spinach. Biochim. Biophys. Acta 593, 427–440. ( 10.1016/0005-2728(80)90078-X) [DOI] [PubMed] [Google Scholar]
- 22.Albertsson P-A. 2001. A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 6, 349–354. ( 10.1016/S1360-1385(01)02021-0) [DOI] [PubMed] [Google Scholar]
- 23.Mullet JE. 1983. The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J. Biol. Chem. 258, 9941–9948. [PubMed] [Google Scholar]
- 24.Armbruster U, et al. 2013. Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature. Plant Cell 25, 2661–2678. ( 10.1105/tpc.113.113118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melis A. 1999. Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci. 4, 130–135. ( 10.1016/S1360-1385(99)01387-4) [DOI] [PubMed] [Google Scholar]
- 26.Mulo P, Sirpio S, Suorsa M, Aro EM. 2008. Auxiliary proteins involved in the assembly and sustenance of photosystem II. Photosynth. Res. 98, 489–501. ( 10.1007/s11120-008-9320-3) [DOI] [PubMed] [Google Scholar]
- 27.Nixon PJ, Michoux F, Yu J, Boehm M, Komenda J. 2010. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 106, 1–16. ( 10.1093/aob/mcq059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nath K, Jajoo A, Poudyal RS, Timilsina R, Park YS, Aro EM, Nam HG, Lee CH. 2013. Towards a critical understanding of the photosystem II repair mechanism and its regulation during stress conditions. FEBS Lett. 587, 3372–3381. ( 10.1016/j.febslet.2013.09.015) [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S, Murata N. 2008. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13, 178–182. ( 10.1016/j.tplants.2008.01.005) [DOI] [PubMed] [Google Scholar]
- 30.Vass I, Cser K. 2009. Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci. 14, 200–205. ( 10.1016/j.tplants.2009.01.009) [DOI] [PubMed] [Google Scholar]
- 31.Ohad I, Kyle DJ, Arntzen CJ. 1984. Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptide in chloroplast membranes. J. Cell Biol. 99, 481–485. ( 10.1083/jcb.99.2.481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umena Y, Kawakami K, Shen J-R, Kamiya N. 2011. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–61. ( 10.1038/nature09913) [DOI] [PubMed] [Google Scholar]
- 33.Tikkanen M, Nurmi M, Kangasjärvi S, Aro EM. 2008. Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim. Biophys. Acta 1777, 1432–1437. ( 10.1016/j.bbabio.2008.08.004) [DOI] [PubMed] [Google Scholar]
- 34.Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D. 2005. Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437, 1179–1182. ( 10.1038/nature04016) [DOI] [PubMed] [Google Scholar]
- 35.Vainonen JP, Hansson M, Vener AV. 2005. STN8 protein kinase in Arabidopsis thaliana is specific in phosphorylation of photosystem II core proteins. J. Biol. Chem. 280, 33 679–33 686. ( 10.1074/jbc.M505729200) [DOI] [PubMed] [Google Scholar]
- 36.Fristedt R, Vener AV. 2011. High light induced disassembly of photosystem II supercomplexes in Arabidopsis requires STN7-dependent phosphorylation of CP29. PLoS ONE 6, e24565 ( 10.1371/journal.pone.0024565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pesaresi P, Pribil M, Wunder T, Leister D. 2011. Dynamics of reversible protein phosphorylation in thylakoids of flowering plants: the roles of STN7, STN8 and TAP38. Biochim. Biophys. Acta 1807, 887–896. ( 10.1016/j.bbabio.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 38.Rochaix JD, Lemeille S, Shapiguzov A, Samol I, Fucile G, Willig A, Goldschmidt-Clermont M. 2012. Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment. Phil. Trans. R. Soc. B 367, 3466–3474. ( 10.1098/rstb.2012.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fristedt R, Willig A, Granath P, Crèvecoeur M, Rochaix J-D, Vener AV. 2009. Phosphorylation of photosystem II controls functional macroscopic folding of photosynthetic membranes in Arabidopsis. Plant Cell 21, 3950–3964. ( 10.1105/tpc.109.069435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nath K, et al. 2013. Loss-of-function of OsSTN8 suppresses the photosystem (PS) II core protein phosphorylation and interferes with PSII repair mechanism in rice (Oryza sativa). Plant J. 76, 675–686. ( 10.1111/tpj.12331) [DOI] [PubMed] [Google Scholar]
- 41.Dietzel L, Bräutigam K, Steiner S, Schüffler K, Lepetit B, Grimm B, Schöttler MA, Pfannschmidt T. 2011. Photosystem II supercomplex remodeling serves as an entry mechanism for state transitions in Arabidopsis. Plant Cell 23, 2964–2977. ( 10.1105/tpc.111.087049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koivuniemi A, Aro EM, Andersson B. 1995. Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry 34, 16 022–16 029. ( 10.1021/bi00049a016) [DOI] [PubMed] [Google Scholar]
- 43.Samol I, Shapiguzov A, Ingelsson B, Fucile G, Crèvecoeur M, Vener AV, Rochaix JD, Goldschmidt-Clermont M. 2012. Identification of a photosystem II phosphatase involved in light acclimation in Arabidopsis. Plant Cell 24, 2596–2609. ( 10.1105/tpc.112.095703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato Y, Sakamoto W. 2009. Protein quality control in chloroplasts: a current model of D1 protein degradation in the photosystem II repair cycle. J. Biochem. 146, 463–469. ( 10.1093/jb/mvp073) [DOI] [PubMed] [Google Scholar]
- 45.Schuhmann H, Adamska I. 2012. Deg proteases and their role in protein quality control and processing in different subcellular compartments of the plant cell. Physiol. Plant. 145, 224–234. ( 10.1111/j.1399-3054.2011.01533.x) [DOI] [PubMed] [Google Scholar]
- 46.Wagner R, Aigner H, Funk C. 2012. FtsH proteases located in the plant chloroplast. Physiol. Plant. 145, 203–214. ( 10.1111/j.1399-3054.2011.01548.x) [DOI] [PubMed] [Google Scholar]
- 47.Che Y, Fu A, Hou X, McDonald K, Buchanan BB, Huang W, Luan S. 2013. C-terminal processing of reaction center protein D1 is essential for the function and assembly of photosystem II in Arabidopsis. Proc. Natl Acad. Sci. USA. 110, 16 247–16 252. ( 10.1073/pnas.1313894110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khatoon M, et al. 2009. Quality control of photosystem II. J. Biol. Chem. 284, 2543–2552. ( 10.1074/jbc.M109.007740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbstova M, Tietz S, Kinzel C, Turkina MV, Kirchhoff H. 2012. Architectural switch in plant photosynthetic membranes induced by light stress. Proc. Natl Acad. Sci. USA 109, 20 130–20 135. ( 10.1073/pnas.1214265109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kley J, et al. 2011. Structural adaptation of the plant protease Deg1 to repair photosystem II during light exposure. Nat. Struct. Mol. Biol. 18, 728–731. ( 10.1038/nsmb.2055) [DOI] [PubMed] [Google Scholar]
- 51.Suno R, Niwa H, Tsuchiya D, Zhang X, Yoshida M, Morikawa K. 2006. Structure of the whole cytosolic region of ATP-dependent protease FtsH. Mol. Cell 22, 575–585. ( 10.1016/j.molcel.2006.04.020) [DOI] [PubMed] [Google Scholar]
- 52.Guss JM, Harrowell PR, Murata M, Norris VA, Freeman HC. 1986. Crystal structure analysis of reduced (CuI) poplar plastocyanin at six pH values. J. Mol. Biol. 192, 361–387. ( 10.1016/0022-2836(86)90371-2) [DOI] [PubMed] [Google Scholar]
- 53.Kettunen R, Tyystjärvi E, Aro EM. 1995. Do grana margins of thylakoid membranes form a functional domain during repair cycle of photosystem II? In Photosynthesis: from light to biosphere, vol. 4 (ed. Mathis P.), pp. 331–334. Dordrecht, The Netherlands: Kluwer. [Google Scholar]
- 54.Chuartzman SG, Nevo R, Shimoni E, Charuvi D, Kiss V, Ohad I, Brumfeld V, Reich Z. 2008. Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 20, 1029–1039. ( 10.1105/tpc.107.055830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bennett J. 1991. Protein phosphorylation in green plant chloroplasts. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 281–311. ( 10.1146/annurev.pp.42.060191.001433) [DOI] [Google Scholar]
- 56.Anderson JM. 1988. Thylakoid membrane organisation in sun/shade acclimation, Aust. J. Plant Physiol. 15, 11–26. ( 10.1071/PP9880011) [DOI] [Google Scholar]
- 57.Kirchhoff H, Haase W, Wegner S, Danielsson R, Ackermann R, Albertsson P-A. 2007. Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplasts. Biochemistry 46, 11 169–11 176. ( 10.1021/bi700748y) [DOI] [PubMed] [Google Scholar]
- 58.Dekker JP, Boekema EJ. 2005. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 1706, 12–39. ( 10.1016/j.bbabio.2004.09.009) [DOI] [PubMed] [Google Scholar]
- 59.Kouřil R, Wientjes E, Bultema JB, Croce R, Boekema EJ. 2013. High-light versus low-light; effect of light acclimation on photosystem composition and organization in Arabidopsis thaliana. Biochim. Biophys. Acta 1827, 411–419. ( 10.1016/j.bbabio.2012.12.003) [DOI] [PubMed] [Google Scholar]
- 60.Goral TK, Johnson MP, Kirchhoff H, Ruban AV, Mullineaux CW. 2010. Visualizing the diffusion of chlorophyll-proteins in higher plant thylakoid membranes: effects of photoinhibition and protein phosphorylation. Plant J. 62, 948–959. [DOI] [PubMed] [Google Scholar]
- 61.Kirchhoff H, Tremmel I, Haase W, Kubitscheck U. 2004. Supramolecular photosystem II organization in grana thylakoid membranes: evidence for a structured arrangement. Biochemistry 43, 9204–9213. ( 10.1021/bi0494626) [DOI] [PubMed] [Google Scholar]
- 62.Kirchhoff H, Haferkamp S, Allen JF, Epstein D, Mullineaux CW. 2008. Significance of macromolecular crowding for protein diffusion in thylakoid membranes of chloroplasts. Plant Physiol. 146, 1571–1578. ( 10.1104/pp.107.115170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barber J. 1982. Influence of surface charges on thylakoid structure and function. Annu. Rev. Plant Physiol. 33, 261–295. ( 10.1146/annurev.pp.33.060182.001401) [DOI] [Google Scholar]
- 64.Allen JF, Forsberg J. 2001. Molecular recognition in thylakoid structure and function. Trend Plant Sci. 6, 317–326. ( 10.1016/S1360-1385(01)02010-6) [DOI] [PubMed] [Google Scholar]
- 65.Allen JF. 1992. Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098, 275–335. ( 10.1016/S0005-2728(09)91014-3) [DOI] [PubMed] [Google Scholar]
- 66.Kyle DJ, Staehelin LA, Arntzen CJ. 1983. Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch. Biochem. Biophys. 222, 527–541. ( 10.1016/0003-9861(83)90551-9) [DOI] [PubMed] [Google Scholar]
- 67.Fristed R, Granath P, Vener AV. 2010. A protein phosphorylation threshold for functional stacking of plant photosynthetic membranes. PLoS ONE 5, e10963 ( 10.1371/journal.pone.0010963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tremmel I, Kirchhoff H, Weis E, Farquhar GD. 2003. Dependence of the plastoquinone diffusion coefficient on the shape, size, density of integral thylakoid proteins. Biochim. Biophys. Acta 1607, 97–109. ( 10.1016/j.bbabio.2003.09.004) [DOI] [PubMed] [Google Scholar]


