Abstract
Plants respond to environmental changes by acclimation that activates defence mechanisms and enhances the plant's resistance against a subsequent more severe stress. Chloroplasts play an important role as a sensor of environmental stress factors that interfere with the photosynthetic electron transport and enhance the production of reactive oxygen species (ROS). One of these ROS, singlet oxygen (1O2), activates a signalling pathway within chloroplasts that depends on the two plastid-localized proteins EXECUTER 1 and 2. Moderate light stress induces acclimation protecting photosynthetic membranes against a subsequent more severe high light stress and at the same time activates 1O2-mediated and EXECUTER-dependent signalling. Pre-treatment of Arabidopsis seedlings with moderate light stress confers cross-protection against a virulent Pseudomonas syringae strain. While non-pre-acclimated seedlings are highly susceptible to the pathogen regardless of whether 1O2- and EXECUTER-dependent signalling is active or not, pre-stressed acclimated seedlings without this signalling pathway lose part of their pathogen resistance. These results implicate 1O2- and EXECUTER-dependent signalling in inducing acclimation but suggest also a contribution by other yet unknown signalling pathways during this response of plants to light stress.
Keywords: cross-protection, acclimation, light stress, singlet oxygen signalling, Arabidopsis, Pseudomonas syringae
1. Introduction
Plants are sessile organisms exposed to a highly variable environment that may adversely affect their growth and development [1]. One strategy of plants to cope with environmental stress and mitigate its negative impact is acclimation. Stress acclimation is often triggered by minor environmental changes and results in an enhanced protection against a subsequent more severe stress [2–4]. Such an enhanced stress resistance may be confined to the stress that triggers acclimation or it may provide cross-protection also against other forms of stress [5–7]. In order to acclimate, plants need to monitor and sense continuously their environment. Environmental cues are transformed into signals that are relayed to the nucleus and modulate the plant's capacity to withstand future stress. As plants are continuously challenged not only by one but several environmental factors whose relative impact may change, acclimation is difficult to analyse. Signals activated by environmental changes seem to operate via a signalling network that integrates various cues at the same time. The stability and robustness of such a network has been ascribed to the integration of numerous and redundant pathways with partially overlapping specificities [8]. How these signalling pathways interact and determine stress acclimation in plants is largely unknown.
Chloroplasts act as an important sensor of environmental factors such as high light, low or high temperature and drought that interfere with the photosynthetic electron transport [9,10]. Under these environmental conditions, light absorbed by photosynthetic membranes exceeds the plant's capacity to assimilate CO2 and leads to the over-reduction of the electron transport chain [11,12]. In order to maintain the photosynthetic electron transport chain in an at least partially oxidized state, photosynthetic organisms have evolved several ways to dissipate excess light energy that may result in increased production of reactive oxygen species (ROS) [13–15]. The term ‘ROS’ is used to describe the effects of several chemically distinct ROS. While oxygen in its ground state is chemically inert, it may be converted to ROS by either electron or energy transfer reactions. The former leads by stepwise reduction to the formation of superoxide, hydrogen peroxide (H2O2) and hydroxyl radical [16], whereas the latter results in the formation of singlet oxygen (1O2) [17]. Depending on their concentration, each of these ROS may cause oxidative damage or is believed to initiate signalling. In plants under light stress, concentrations of these ROS increase almost simultaneously, thus making it difficult to link a given ROS to a particular cellular response. To overcome these obstacles and dissect the complexity of signalling in plants under light stress, the conditional fluorescent (flu) mutant of Arabidopsis thaliana has been established as an experimental system to describe in molecular terms the biological role of one particular ROS, 1O2 [18,19], and determine whether or not it takes part in triggering stress acclimation in plants under light stress [20].
2. 1O2-mediated and EXECUTER-dependent signalling in the flu mutant
Mutations of the FLU gene interfere with the negative feedback control of tetrapyrrole biosynthesis in flu mutants and allow the accumulation of protochlorophyllide (Pchlide), the immediate precursor of chlorophyllide (Chlide), in the dark [18,21,22]. FLU is a nuclear-encoded protein that, after import and processing, becomes tightly associated with plastid membranes and forms part of a complex that comprises also the four enzymes catalyzing the final steps of chlorophyll synthesis [23]. It links chlorophyll synthesis and the target of feedback control, Glu tRNA reductase, the first enzyme committed to tetrapyrrole biosynthesis, thereby allowing the Mg2+ branch to control the initial steps of this pathway [24]. In contrast to dark-grown wild-type seedlings, flu seedlings are no longer able to restrict the accumulation of Pchlide [18]. When these seedlings are transferred from the dark to the light, they rapidly bleach and die because of the release of 1O2 [19] that is formed due to the photosensitizing activity of excess amounts of free Pchlide. The flu mutant remains viable though, when it is kept from the very beginning under continuous light. Under these conditions, Pchlide in the flu mutant is immediately photoreduced to Chlide and in this way does not reach critical levels that might lead to an enhanced production of 1O2. As long as flu plants are kept under continuous light, no obvious difference between mutant and wild-type can be observed [18,19]. These properties of the flu mutant have been exploited to study the physiological role of 1O2 by growing flu plants initially under continuous light and then transferring them to the dark and re-exposing them to light. The response of the flu mutant to 1O2 has been analysed in seedlings and in mature plants that are ready to bolt. Seedlings of flu bleach and die, when they are kept under repeated 16 h L : 8 h D cycles, whereas mature plants grown initially under continuous light develop necrotic lesions on their leaves and stop their growth immediately after being shifted to daily dark/light cycles [19]. By varying the length of the dark period, one can modulate non-invasively the level of the photosensitizer Pchlide, and define conditions that reduce the toxicity of 1O2 and reveal a genetic basis of its signalling role [25–27]. Under these optimized conditions, the nucleus-encoded and chloroplast-localized EXECUTER1 (EX1) and EXECUTER2 (EX2) proteins are essential for initiating 1O2-mediated responses. Inactivation of these proteins in an ex1/ex2/flu triple mutant abolishes 1O2-mediated cell death of seedlings and growth inhibition of mature plants [25,28]. As the triple mutant in the dark over-accumulates Pchlide and upon re-illumination generates 1O2 similar to the parental flu line but yet behaves like wild-type, 1O2-mediated responses in the flu mutant are not due to photo-oxidative damage but are the result of 1O2-mediated and EX-dependent signalling [28].
3. Control of 1O2-responsive nuclear gene expression in the flu mutant
After transferring flu seedlings from the dark to light, the release of 1O2 triggers an immediate induction of nuclear gene expression changes, with the number of affected genes rapidly increasing over time [19]. As these nuclear gene expression changes are suppressed in ex1/ex2/flu triple seedlings, they were initially attributed to a single retrograde plastid-to-nucleus signalling pathway that is activated within chloroplasts by the release of 1O2 and depends on the plastid proteins EX1 and EX2 [19,27]. Subsequent studies of the flu mutant revealed, however, that the regulation of 1O2-responsive genes in the flu mutant seems to be more complex than originally anticipated. One of the first cellular changes that occur within less than 30 min following the dark-to-light shift is a loss of chloroplast integrity and the rupture of the central vacuole [28]. These two events mark the beginning of a 1O2-mediated and EX-dependent programmed cell death response of the flu mutant and both precede the upregulation of most of the 1O2-responsive nuclear genes reported earlier [28,29]. The loss of cellular integrity is expected to impact nuclear gene expression. Hence, many of the 1O2-responsive genes of the flu mutant reported earlier are probably only indirectly affected by 1O2-mediated and EX-dependent signalling and their upregulation seems to mark activation of other signalling pathways closely associated with cellular damage [28,30]. This interpretation is supported by a comparison of nuclear gene expression changes in flu following a dark-to-light shift and in wild-type seedlings transferred from moderate light (90 µmol photons s−1 m−2, 20°C) to a combined low temperature/higher light stress (270 µmol photons s−1 m−2, 12°C) that previously had been shown to activate 1O2-mediated and EX-dependent signalling without triggering an immediate cell death response as seen in flu seedlings [28]. Most of the genes upregulated in flu following the release of 1O2 are not affected in wild-type exposed to the combined lower temperature/higher light stress, and vice versa only a minor fraction of the stress-induced and EX-dependent nuclear genes of wild-type is also affected in the flu mutant [29].
Oxidative damage in flu seedlings kept under optimized light/dark conditions is a consequence of 1O2-mediated and EX-dependent signalling that occurs after 1O2-mediated loss of chloroplast integrity had been initiated. Polyunsaturated fatty acids are a preferred target of ROS, and non-enzymatic peroxidation of these molecules has been used as a marker for oxidative damage [31,32]. At the beginning of re-illumination of pre-darkened flu seedlings, peroxidation of linolenic acid, the most prominent polyunsaturated fatty acid of chloroplast membranes [33], happens almost exclusively enzymatically by lipoxygenases and not through direct attack by 1O2 [19,26,28]. However, during the following 1O2-mediated programmed cell death response, non-enzymatic peroxidation of polyunsaturated fatty acids starts to prevail [28]. The signature of peroxidation products indicates that in flu seedlings during the progression of cell death, non-enzymatic peroxidation is caused primarily by 1O2 and not by other ROS [28,32]. Thus, in flu seedlings kept under conditions that favour 1O2-mediated and EX-dependent signalling, two different modes of 1O2 activity can be distinguished that occur sequentially during re-illumination of pre-darkened flu seedlings. First, 1O2 activates retrograde plastid-to-nucleus signalling without causing detectable cellular damage, and then, as a consequence of the 1O2-mediated and EX-dependent cell death response, 1O2 accelerates the loss of cellular integrity and lesion formation in flu seedlings by directly reacting with various cellular components, e.g. polyunsaturated fatty acids in chloroplast membranes. Initially, 1O2 seems to be exclusively generated by excitation of the photosensitizer Pchlide, but during the following loss of chloroplast integrity also chlorophyll seems to act as a photosensitizer that in a positive feedback loop enhances 1O2 production. As one would expect, this second phase of 1O2 formation is still part of the genetically controlled programmed cell death response initiated by 1O2- and EX-dependent signalling and is suppressed in flu/ex1/ex2 seedlings [28].
4. 1O2-mediated and EX-dependent signalling in wild-type
1O2-mediated and EX-dependent activation of a suicidal programme in flu seedlings or the arrest of bolting and flower formation during the transition from vegetative to generative growth in mature flu plants have not been found in wild-type plants, when these are exposed to environmental stress conditions that are known to promote 1O2 formation in chloroplasts. 1O2-mediated and EX-dependent signalling as seen in the flu mutant operates under conditions that are different from those to which the wild-type has adapted, and thus its physiological significance in wild-type may not be directly deduced from 1O2-mediated responses of the flu mutant. In the light-grown wild-type transferred to the dark, Pchlide does not over-accumulate as in flu but is barely detectable due to strict negative feedback control of tetrapyrrole biosynthesis [34]. Whereas in flu 1O2 signalling occurs without concomitant signalling induced by other ROS [19], in the wild-type 1O2 is released under stress conditions that also enhance the production of superoxide, H2O2 and other stress signalling molecules [28,30,35] that modulate 1O2-mediated responses shown by the flu mutant [20,36]. In wild-type seedlings transferred to the combined low temperature/higher light stress mentioned above, 1O2-mediated and EX-dependent signalling is activated as indicated by the rapid upregulation of the 1O2-responsive marker gene AAA-ATPase that is suppressed in ex1/ex2 seedlings [28]. However, unlike 1O2-mediated and EX-dependent signalling in flu seedlings, in wild-type seedlings this signalling pathway does not correlate with an immediate programmed cell death response and the subsequent collapse of seedlings. Only when seedlings have been kept for several days continuously under the combined low temperature/higher light stress, a 1O2-mediated and EX-dependent cell death response is initiated, which leads to the formation of microlesions in the wild-type without causing detectable oxidative damage or reducing the viability of seedlings [28].
5. Formation of 1O2 in wild-type under light stress
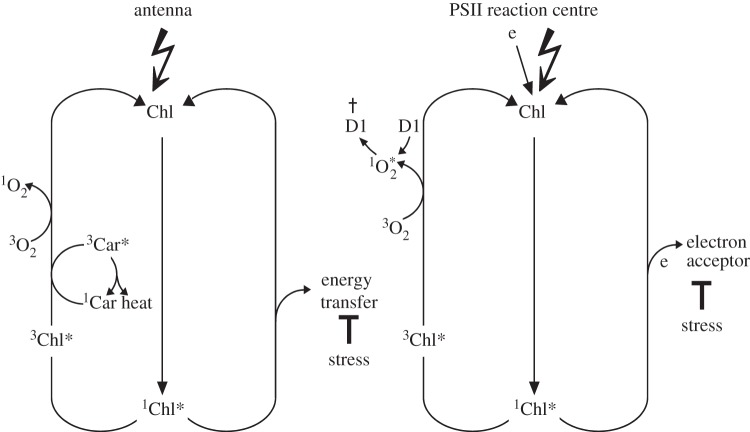
In the wild-type under light stress, chlorophyll acts as the photosensitizer that generates 1O2 [37]. Formation of 1O2 in chloroplasts may occur within either light-harvesting antennae complexes [38] or the reaction centre of photosystem II (PSII) [11,12]. Chlorophyll in light-harvesting antennae complexes is in close contact with carotenoids that efficiently quench excess light energy and suppress the release of 1O2 by scavenging directly this ROS whenever it is formed within their immediate vicinity [39] (figure 1). Only when the excitation energy absorbed by chlorophyll remains trapped inside the antennae complexes and exceeds the light-quenching capacity of carotenoids does the release of 1O2 occur, leading to non-enzymatic oxidation of lipids and carotenoids and causing oxidative damage [33,40] (figure 1). Some of the non-enzymatic oxidation products may act as signalling molecules [41,42]. Signalling under these conditions can be regarded as a consequence of oxidative damage and induces responses that are not dependent on EX1 and EX2 [28,42]. 1O2 production within the PSII reaction centre occurs whenever the electron acceptor site of PSII is reduced and unable to oxidize the excited reaction centre chlorophyll P680 [11,12]. This may happen even under low light intensities [43]. 1O2 generated by the reaction centre chlorophyll of PSII has been suggested to interact with the D1 protein of the PSII reaction centre that may act as a scavenger of 1O2 and, following its oxidation, needs to be replaced by a newly synthesized D1 polypeptide [12] (figure 1). 1O2 formed under mild light stress conditions does not seem to cause oxidative damage and its signalling activity depends on EX1 and EX2 as indicated by the absence of lesion formation in ex1/ex2 seedlings exposed to the combined low temperature/higher light stress [28].
Figure 1.
Production of 1O2 at two different sites in chloroplasts of Arabidopsis exposed to light stress. Under severe stress, excitation energy remains trapped inside the light-harvesting antenna pigment complexes shown on the left side and gives rise to excess amounts of 1O2 that exceed the light-quenching capacity of carotenoids closely connected to chlorophyll. 1O2 production under these conditions leads to non-enzymatic oxidation of lipids and carotenoids and photo-oxidative damage. 1O2 production within the PSII reaction centre shown on the right side occurs whenever the primary electron acceptor at the acceptor side of PSII remains reduced and is unable to oxidize the P680 chlorophyll of the PSII reaction centre. Carotenoids are not in contact with the reaction centre chlorophyll P680, and 1O2 formation may happen even under low light intensities. The PSII reaction centre protein D1 (D1) has been suggested to act as a 1O2 scavenger [12] and upon its oxidation to be degraded.
6. Light stress acclimation
In previous studies with Chlamydomonas reinhardtii [2] and a chlorophyll b-less light-sensitive mutant of Arabidopsis [44], it has been shown that 1O2 formed under light stress conditions may affect the susceptibility of cells to abiotic stress [2]. A transient or moderate elevation of 1O2 resulted in the activation of a subset of 1O2-responsive genes and protection against a subsequent more severe light stress. To assess the possible presence of a 1O2-mediated and EX-dependent stress acclimation in Arabidopsis thaliana wild-type plants, Arabidopsis seedlings grown under moderate light (90 µmol photons s−1 m−2, 20°C) were first exposed for 24 h to a brief combined low temperature/high light stress (270 µmol photons s−1 m−2, 12°C) [28]. These plants were then returned to the previous moderate light condition for 6 h to allow recovery from the pre-stress before they were re-exposed for various lengths of time to the combined low temperature/higher light stress (figure 2a). The induction of acclimation under these conditions was monitored by the effect of pre-stress on PSII activity. The functional state of PSII was determined by the maximum quantum efficiency of PSII expressed as the ratio of variable to maximum chlorophyll fluorescence (Fv/Fm) (figure 2b). During 24 h of combined low temperature/higher light treatment, wild-type seedlings suffered from photoinhibition of PSII as indicated by a drop of the Fv/Fm ratio from 0.86 to approximately 0.7. ex1/ex2 seedlings exposed to the same 24 h-pre-stress treatment showed a similar photoinhibition of PSII. However, when this stress treatment was continued for up to 96 h, PSII activity in ex1/ex2 seedlings was less inhibited than in the wild-type (figure 2b). After pre-stressed seedlings had been returned to the original moderate light conditions, they recovered from photoinhibition of PSII within the next 6 h and restored the original maximum quantum efficiency of PSII (figure 2b). The 24 h stress pre-treatment was sufficient to induce stress acclimation. When pre-stressed seedlings following the recovery phase were re-exposed to the same stress as used during the pre-stress treatment, they showed a strongly enhanced stress resistance as indicated by the reduced photoinhibition of pre-stressed seedlings relative to non-pre-stressed control seedlings (figure 2b). Upregulation of the 1O2-responsive marker gene AAA-ATPase in the wild-type and its suppression in ex1/ex2 reveals that 1O2-mediated and EX-dependent signalling was activated during the pre-stress treatment [28]. However, the protection against stress-induced photoinhibition of PSII in pre-stressed wild-type and ex1/ex2 seedlings was almost identical, suggesting that this acclimatory response was not dependent on EX1 and/or EX2 (figure 2b).
Figure 2.
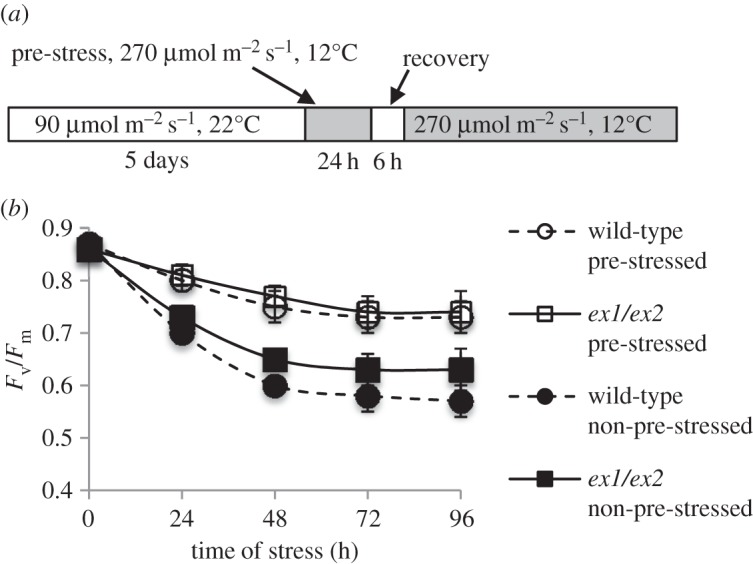
Stress acclimation of Arabidopsis seedlings. (a) Five-day-old seedlings initially grown at room temperature under moderate light are transferred for 24 h to a combined low temperature/higher light stress and moved back to the original moderate light conditions for 6 h, before they are exposed again for various lengths of time to the combined low temperature/higher light stress regime. (b) Following the 24 h pre-stress and 6 h of recovery, plants are acclimated as shown by the enhanced protection of PSII in pre-stressed seedlings relative to the non-pre-stressed controls. Results represent mean values of three independent experiments ±s.d.
7. Cross-protection of seedlings acclimated to light stress against other forms of stress
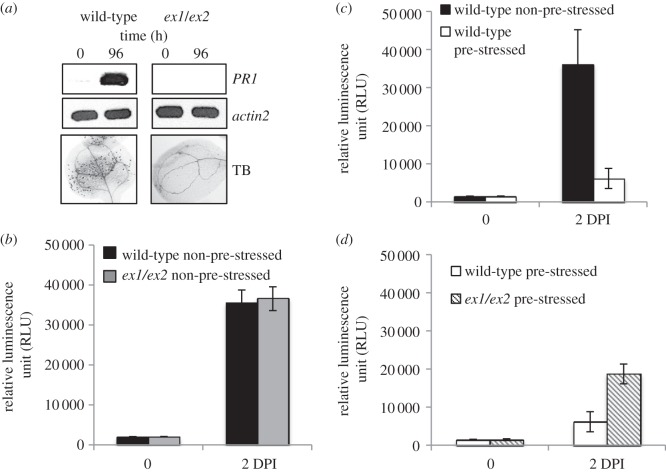
The possible contribution of 1O2-mediated and EX-dependent signalling to cross-protection against other forms of stress has been analysed. Several lines of evidence suggest that 1O2- and EX-dependent nuclear gene expression changes may enhance pathogen resistance. Among the 36 1O2-responsive transcription factor genes upregulated in the flu mutant shortly after the beginning of enhanced 1O2 production, 22 are also activated in response to pathogens (table 1; http://ausubellab.mgh.harvard.edu/nsf2010/). Microlesions similar to the ones formed in wild-type seedlings exposed to moderate light stress are closely associated with an enhanced pathogen resistance (figure 3a) [46] and the concomitant upregulation of the PATHOGEN-RELATED (PR) PROTEIN1 gene in light-stressed seedlings (figure 3a) resembles PR1 expression changes in response to various other abiotic and biotic stresses [47]. The light-stress-induced microlesion formation and PR1 expression were suppressed in ex1/ex2 seedlings (figure 3a). Collectively, these data suggest that 1O2-mediated and EX-dependent stress acclimation may reduce the susceptibility of seedlings to pathogens. To verify this suggestion, plants were spray-inoculated with virulent Pseudomonas syringae DC3000 that constitutively expresses a LUCIFERASE reporter gene [45]. Bacterial growth was determined after different lengths of incubation by measuring the luciferase activity in extracts of inoculated plants. In a first experiment, non-pre-stressed wild-type and ex1/ex2 seedlings grown under moderate light conditions were exposed to the pathogen and the number of bacteria in plants was determined after 2 days of incubation. Inactivation of the 1O2- and EX-dependent signalling pathway in ex1/ex2 seedlings did not seem to affect the bacterial growth (figure 3b). Both lines were equally susceptible to the pathogen. When non-pre-stressed wild-type seedlings and wild-type seedlings exposed for 24 h to the combined low temperature/higher light pre-stress and a subsequent 6 h recovery phase were compared, bacterial growth was strongly suppressed in pre-stressed seedlings, demonstrating that the light-stress-induced acclimation of the wild-type triggers cross-protection against the virulent Pseudomonas strain (figure 3c). Inactivation of 1O2-mediated and EX-dependent signalling diminished significantly the pathogen resistance of pre-acclimated seedlings but did not fully restore the pathogen susceptibility of non-pre-stressed wild-type seedlings that one would expect if cross-protection against pathogens in the pre-acclimated seedlings would be exclusively controlled by 1O2-mediated and EX-dependent signalling (figure 3d). These results confirm that 1O2− and EX-dependent signalling contributes to the light-stress-induced cross-protection against pathogens, but at the same time indicate that other signalling events must be considered to fully account for the enhanced pathogen resistance in light-stress-acclimated Arabidopsis seedlings. One of the features of the plant's reaction to a shift to high light is a rapid accumulation of H2O2 [5,28]. An increase in H2O2 is closely associated with stress acclimation and has been suggested to contribute also to an enhanced resistance of pre-acclimated Arabidopsis plants to pathogens [5,48]. Enhanced levels of 1O2 and H2O2 stimulate the production of hormones such as jasmonic acid, salicylic acid and ethylene that activate different sets of genes encoding antimicrobial proteins and enhance the resistance against distinct microbial pathogens [5,19,44,49]. Even though 1O2 and H2O2 operate via separate signalling pathways, they may interact with each other [36]. During light-stress-induced acclimation, both appear to undergo also extensive crosstalk with other stress-related signalling pathways [44,48,49]. One of the big challenges of future research will be to dissect this complex signalling network and to identify other signals that during light stress interact with 1O2 and H2O2 and control stress acclimation.
Table 1.
Identification of 1O2-responsive transcription factor genes of the flu mutant that are also activated in response to pathogens. Thirty-six transcription factor genes have been listed that are upregulated shortly after the dark-to-light shift of the flu mutant [19]. Genes in bold have also been reported to be activated in response to pathogens (http://ausubellab.mgh.harvard.edu/nsf2010/).
| common name | AGI number | |
|---|---|---|
| ERF/AP2 | ERF/AP2 | At1g21910 |
| ERF11 | At1g28370 | |
| ERF13 | At2g44840 | |
| ERF4 | At3g15210 | |
| ERF6 | At4g17490 | |
| ERF2 | At5g47220 | |
| ERF5 | At5g47230 | |
| ERF/AP2 | At5g51190 | |
| ERF/AP2 | At5g61590 | |
| ERF/AP2 | At5g61600 | |
| DREB2A | At5g05410 | |
| At1g25560 | ||
| RAV1 | At1g13260 | |
| RAV2 | At1g68840 | |
| MYB | MYB51 | At1g18570 |
| MYB15 | At3g23250 | |
| MYB77 | At3g50060 | |
| WRKY | WRKY40 | At4g31550 |
| WRKY33 | At2g38470 | |
| WRKY46 | At2g46400 | |
| WRKY22 | At4g01250 | |
| WRKY53 | At4g23810 | |
| WRKY11 | At4g31550 | |
| WRKY18 | At4g31800 | |
| Zinc finger | ZAT10/STZ | At1g27730 |
| ZAT11 | At2g37430 | |
| At5g04340 | ||
| ZAT12 | At5g59820 | |
| AZF1 | At5g67450 | |
| CZF1/ZFAR1 | At2g40140 | |
| At3g55980 | ||
| NAC | ANAC036 | At2g17040 |
| ANAC062 | At3g49530 | |
| ANAC102 | At5g63790 | |
| others | HSFA4A | At4g18880 |
| BT5 | At4g37610 |
Figure 3.
1O2-mediated and EX-dependent acclimation of Arabidopsis seedlings exposed to light stress confers cross-protection against the biotrophic pathogen P. syringae DC3000. (a) 1O2-mediated and EX-dependent signalling in wild-type seedlings exposed to the combined low temperature/higher light stress for 96 h as shown in figure 2a induces programmed cell death as revealed by trypan blue (TB) staining and upregulation of PR1 gene expression, both of which are suppressed in ex1/ex2 seedlings. (b–d) Plants were spray-inoculated with virulent P. syringae DC3000 that constitutively expresses a LUCIFERASE reporter gene [45]. Bacterial growth was determined after different lengths of incubation by measuring the luciferase activity in extracts of inoculated plants. (b) Non-pre-stressed wild-type and ex1/ex2 seedlings show a similar high susceptibility to the virulent pathogen. (c) Wild-type seedlings acclimated to light stress show an enhanced resistance against the pathogen as indicated by the strongly reduced number of bacteria grown on seedlings 2 days after infection (DPI). (d) Inactivation of the 1O2- and EX-dependent signalling pathway in ex1/ex2 seedlings only partially restores the susceptibility of non-acclimated control wild-type seedlings. Results in (b–d) represent mean values of three independent experiments ±s.d.
Funding statement
Work done in our laboratory has been supported by the Boyce Thompson Institute for Plant Research and the National Institutes of Health (grant no. R01-GM085036 to K.A.).
References
- 1.Bray EA, Bailey-Serres J, Weretilnyk E. 2000. Responses to abiotic stress. In Biochemistry and molecular biology of plants (eds Buchanan BB, Gruissem W, Jones RL.), pp. 1158–1203. Rockville, MD: American Society of Plant Physiologists. [Google Scholar]
- 2.Ledford HK, Chin BL, Niyogi KK. 2007. Acclimation to singlet oxygen stress in Chlamydomonas reinhardtii. Eukaryot. Cell 6, 919–930. ( 10.1128/EC.00207-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vierling E. 1991. The roles of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 579–620. ( 10.1146/annurev.pp.42.060191.003051) [DOI] [Google Scholar]
- 4.Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux PM. 1999. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284, 654–657. ( 10.1126/science.284.5414.654) [DOI] [PubMed] [Google Scholar]
- 5.Mateo A, Muhlenbock P, Rusterucci C, Chang CC-C, Miszalski Z, Karpinska B, Parker JE, Mullineaux PM, Karpinski S. 2004. LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol. 136, 2818–2830. ( 10.1104/pp.104.043646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowler C, Fluhr R. 2000. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 5, 241–246. ( 10.1016/S1360-1385(00)01628-9) [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa T, Morimoto Y, Madhusudhan R, Sawa Y, Shibata H, Yabuta Y, Nishizawa A, Shigeoka S. 2005. Acclimation to diverse environmental stresses caused by a suppression of cytosolic ascorbate peroxidase in tobacco BY-2 cells. Plant Cell Physiol. 46, 1264–1271. ( 10.1093/pcp/pci135) [DOI] [PubMed] [Google Scholar]
- 8.Stelling J, Sauer U, Szallasi Z, Doyle FJ, III, Doyle J. 2004. Robustness of cellular functions. Cell 118, 675–685. ( 10.1016/j.cell.2004.09.008) [DOI] [PubMed] [Google Scholar]
- 9.Mullineaux PM, Karpinski S. 2002. Signal transduction in response to excess light: getting out of the chloroplast. Curr. Opin. Plant Biol. 5, 43–48. ( 10.1016/S1369-5266(01)00226-6) [DOI] [PubMed] [Google Scholar]
- 10.Foyer CH, Noctor G. 2009. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid. Redox Signal. 11, 861–905. ( 10.1089/ars.2008.2177) [DOI] [PubMed] [Google Scholar]
- 11.Krieger-Liszkay A, Fufezan C, Trebst A. 2008. Singlet oxygen production in photosystem II and related protection mechanisms. Photosynth. Res. 98, 551–564. ( 10.1007/s11120-008-9349-3) [DOI] [PubMed] [Google Scholar]
- 12.Vass I, Cser K. 2009. Janus-faced charge recombination in photosystem II photoinhibition. Trends Plant Sci. 14, 200–205. ( 10.1016/j.tplants.2009.01.009) [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Wakao S, Fischer BB, Niyogi KK. 2009. Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260. ( 10.1146/annurev.arplant.58.032806.103844) [DOI] [PubMed] [Google Scholar]
- 14.Asada K. 1999. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639. ( 10.1146/annurev.arplant.50.1.601) [DOI] [PubMed] [Google Scholar]
- 15.Kozaki A, Takeba G. 1996. Photorespiration protects C3 plants from photooxidation. Nature 384, 557–560. ( 10.1038/384557a0) [DOI] [Google Scholar]
- 16.Halliwell B, Gutteridge JMC. 1989. Free radicals in biology and medicine, 2nd edn Oxford, UK: Clarendon. [Google Scholar]
- 17.Foote CS. 1968. Mechanisms of photosensitized oxidation. Science 162, 963–970. ( 10.1126/science.162.3857.963) [DOI] [PubMed] [Google Scholar]
- 18.Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K. 2001. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 98, 12 826–12 831. ( 10.1073/pnas.221252798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.op den Camp R, et al. 2003. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15, 2320–2332. ( 10.1105/tpc.014662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meskauskiene R, Würsch M, Laloi C, Vidi PA, Coll NS, Kessler F, Baruah A, Kim C, Apel K. 2009. A mutation in the Arabidopsis mTERF-related plastid protein SOLDAT10 activates retrograde signaling and suppresses 1O2-induced cell death. Plant J. 60, 399–410. ( 10.1111/j.1365-313X.2009.03965.x) [DOI] [PubMed] [Google Scholar]
- 21.von Wettstein D, Kahn A, Nielsen OF. 1974. Genetic regulation of chlorophyll synthesis analyzed with mutants of barley. Science 184, 800–802. ( 10.1126/science.184.4138.800) [DOI] [PubMed] [Google Scholar]
- 22.Lee KP, Kim C, Lee DW, Apel K. 2003. TIGRINA d, required for regulating the biosynthesis of tetrapyrroles in barley, is an ortholog of the FLU gene of Arabidopsis thaliana. FEBS Lett. 553, 119–124. ( 10.1016/S0014-5793(03)00983-9) [DOI] [PubMed] [Google Scholar]
- 23.Kauss D, Bischoff S, Steiner S, Apel K, Meskauskiene R. 2012. FLU, a negative feedback regulator of tetrapyrrole biosynthesis, is physically linked to the final steps of the Mg++-branch of this pathway. FEBS Lett. 586, 211–216. ( 10.1016/j.febslet.2011.12.029) [DOI] [PubMed] [Google Scholar]
- 24.Goslings D, Meskauskiene R, Kim C, Lee KP, Nater M, Apel K. 2004. Concurrent interactions of heme and FLU with GLU tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J. 40, 957–967. ( 10.1111/j.1365-313X.2004.02262.x) [DOI] [PubMed] [Google Scholar]
- 25.Wagner D, et al. 2004. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306, 1183–1185. ( 10.1126/science.1103178) [DOI] [PubMed] [Google Scholar]
- 26.Przybyla D, Göbel C, Imboden A, Feussner I, Hamberg M, Apel K. 2008. Enzymatic but not non-enzymatic peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J. 54, 236–248. ( 10.1111/j.1365-313X.2008.03409.x) [DOI] [PubMed] [Google Scholar]
- 27.Lee KP, Kim C, Landgraf F, Apel K. 2007. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 104, 10 270–10 275. ( 10.1073/pnas.0702061104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim C, Meskauskiene R, Zhang S, Lee KP, Ashok ML, Blajecka K, Herrfurth C, Feussner I, Apel K. 2012. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 24, 3026–3039. ( 10.1105/tpc.112.100479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim C, Apel K. 2013. Singlet oyxygen-mediated signaling in plants: moving from flu to wild type reveals an increasing complexity. Photosynth. Res. 116, 455–464. (doi:10.1007%2Fs11120-013-9876-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baruah A, Simkova K, Apel K, Laloi C. 2009. Arabidopsis mutants reveal multiple singlet oxygen signaling pathways involved in stress response and development. Plant Mol. Biol. 70, 547–563. ( 10.1007/s11103-009-9491-0) [DOI] [PubMed] [Google Scholar]
- 31.Berger S, Weichert H, Porzel A, Wasternack C, Kühn H, Feussner I. 2001. Enzymatic and non-enzymatic lipid peroxidation in leaf development. Biochim. Biophys. Acta 1533, 266–276. ( 10.1016/S1388-1981(01)00161-5) [DOI] [PubMed] [Google Scholar]
- 32.Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, van Breusegem F, Mueller MJ. 2008. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 148, 960–968. ( 10.1104/pp.108.125690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami J, Tsujama M, Kobayashi Y, Kodama H, Iba K. 2000. Trienoic fatty acids and plant tolerance of high temperature. Science 287, 476–479. ( 10.1126/science.287.5452.476) [DOI] [PubMed] [Google Scholar]
- 34.Tanaka R, Tanaka A. 2007. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 58, 321–326. ( 10.1146/annurev.arplant.57.032905.105448) [DOI] [PubMed] [Google Scholar]
- 35.Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. 2002. Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 53, 1249–1254. ( 10.1093/jexbot/53.372.1249) [DOI] [PubMed] [Google Scholar]
- 36.Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. 2007. Crosstalk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 104, 672–677. ( 10.1073/pnas.0609063103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hideg E, Kalai T, Hideg K, Vass I. 1998. Photoinhibition of photosynthesis in vivo results in singlet oxygen production: detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry 37, 11 405–11 411. ( 10.1021/bi972890+) [DOI] [PubMed] [Google Scholar]
- 38.Rinalducci S, Pedersen JZ, Zolla L. 2004. Formation of radicals from singlet oxygen produced during photoinhibition of isolated light-harvesting proteins of photosystem II. Biochim. Biophys. Acta 1608, 63–73. ( 10.1016/j.bbabio.2003.10.009) [DOI] [PubMed] [Google Scholar]
- 39.Codgell RJ, Frank HA. 1987. How carotenoids function in photosynthetic bacteria. Biochim. Biophys. Acta 895, 63–79. ( 10.1016/S0304-4173(87)80008-3) [DOI] [PubMed] [Google Scholar]
- 40.Ramel F, Birtic S, Cuiné S, Triantaphylidès C, Ravanat J-L, Havaux M. 2012. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 158, 1267–1278. ( 10.1104/pp.111.182394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller S, Hilbert B, Dueckershoff K, Roitsch T, Krischke M, Mueller MJ, Berger S. 2008. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 20, 768–785. ( 10.1105/tpc.107.054809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramel F, Birtic S, Ginies C, Soubgou-Taconnat L, Triantaphylidès C, Havaux M. 2012. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl Acad. Sci. USA 109, 5535–5540. ( 10.1073/pnas.1115982109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szilard A, Sass L, Hideg E, Vass I. 2005. Photoinactivation of photosystem II by flashing light. Photosynth. Res. 84, 15–20. ( 10.1007/s11120-004-7161-2) [DOI] [PubMed] [Google Scholar]
- 44.Ramel F, et al. 2013. Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen. Plant Cell 25, 1445–1462. ( 10.1105/tpc.113.109827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan J, Crooks C, Lamb C. 2007. High-throughput quantitative luminescence assay of the growth in planta of Pseudomonas syringae chromosomally tagged with Photorhabdus luminescence luxCDABE. Plant J. 53, 393–399. ( 10.1111/j.1365-313X.2007.03303.x) [DOI] [PubMed] [Google Scholar]
- 46.Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. 1998. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784. ( 10.1016/S0092-8674(00)81405-1) [DOI] [PubMed] [Google Scholar]
- 47.van Loon LC, van Strien EA. 1999. The families of pathogenesis-related proteins, their activities and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 55, 85–97. ( 10.1006/pmpp.1999.0213) [DOI] [Google Scholar]
- 48.Bechtold U, Karpinski S, Mullineaux PM. 2005. The influence of the light environment and photosynthesis on oxidative signaling responses in plant–biotrophic pathogen interactions. Plant Cell Environ. 28, 1046–1055. ( 10.1111/j.1365-3040.2005.01340.x) [DOI] [Google Scholar]
- 49.Danon A, Miersch O, Felix G, op den Camp R, Apel K. 2005. Concurrent activation of cell-death-regulating pathways by singlet oxygen in Arabidopsis thaliana. Plant J. 41, 68–80. ( 10.1111/j.1365-313X.2004.02276.x) [DOI] [PubMed] [Google Scholar]