Abstract
Mitochondria and chloroplasts depend upon each other; photosynthesis provides substrates for mitochondrial respiration and mitochondrial metabolism is essential for sustaining photosynthetic carbon assimilation. In addition, mitochondrial respiration protects photosynthesis against photoinhibition by dissipating excess redox equivalents from the chloroplasts. Genetic defects in mitochondrial function result in an excessive reduction and energization of the chloroplast. Thus, it is clear that the activities of mitochondria and plastids need to be coordinated, but the manner by which the organelles communicate to coordinate their activities is unknown. The regulator of alternative oxidase (rao1) mutant was isolated as a mutant unable to induce AOX1a expression in response to the inhibitor of the mitochondrial cytochrome c reductase (complex III), antimycin A. RAO1 encodes the nuclear localized cyclin-dependent kinase E1 (CDKE1). Interestingly, the rao1 mutant demonstrates a genome uncoupled phenotype also in response to redox changes in the photosynthetic electron transport chain. Thus, CDKE1 was shown to regulate both LIGHT HARVESTING COMPLEX B (LHCB) and ALTERNATIVE OXIDASE 1 (AOX1a) expression in response to retrograde signals. Our results suggest that CDKE1 is a central nuclear component integrating mitochondrial and plastid retrograde signals and plays a role in regulating energy metabolism during the response to stress.
Keywords: redox, retrograde, chloroplast, mitochondria, kinase
1. Introduction
Plant cells harbour two distinct membrane enclosed organelles, mitochondria and chloroplasts. These organelles evolved from free-living prokaryotic organisms via independent endosymbiotic events. The organelles have retained their own distinct genomes but the gradual conversion from endosymbiont to organelle has been accompanied by a dramatic reduction in genome size as the organelles have either lost or transferred most of their genes to the nucleus. Genes that remain in the chloroplastic and mitochondrial genomes encode proteins involved in photosynthesis and respiration, respectively, or encode components of the organelle gene expression machinery (rRNA, tRNA and some ribosomal proteins). The majority of organellar proteins are encoded in the nucleus and both organelles are dependent on the nucleus to provide proteins required for the functions carried out in mitochondria and chloroplasts. The presence of genes encoding organellar proteins in different cellular compartments presents the complex problem of coordinating the activities of the different genomes of the plant cell. In order to achieve this coordination, mechanisms to orchestrate nuclear and organellar gene expression have evolved and these include both anterograde (nucleus-to-organelles) and retrograde (organelles-to-nucleus) controls [1]. Anterograde mechanisms coordinate gene expression in the organelle with cellular and environmental cues that are perceived and choreographed by genes in the nucleus. Retrograde (organelle-to-nucleus) signalling, on the other hand, coordinates the expression of nuclear genes encoding organellar proteins with the metabolic and developmental state of the chloroplasts and mitochondria.
It is clear that the organelles produce multiple signals at different times of their development and in response to changes in the environment, and these signals orchestrate major changes in nuclear gene expression [2]. The retrograde signals are not only essential for coordinating gene expression in the nucleus and in the organelles but they are also essential for balancing cellular energy metabolism and mediating plant stress responses. Many of the plastid signals identified so far can be linked to specific stress conditions such as changes in the redox state of the chloroplast, accumulation of reactive oxygen species (ROS) or accumulation of tetrapyrroles and phosphonucleotides [3,4]. Less is known about the mitochondria retrograde regulation (MRR) in plants. However, several studies indicate that, similar to the plastid signals, MRR is triggered by mitochondrial dysfunction such as disruption of the electron transport and accumulation of ROS [5].
The chloroplasts and mitochondria are functionally tightly linked and balancing the activities of the two energy organelles is essential to the plant. By using inhibitors of mitochondrial electron transport and mutants with impaired mitochondrial activity it was demonstrated that mitochondrial metabolism is essential for photosynthesis [6]. Photosynthesis provides substrates for mitochondrial respiration and translocators located on the envelope membranes of chloroplasts and mitochondria mediate the metabolite exchange which generates an important channel of communication between the organelles [6]. Possibly, a close association between plastid stromules and mitochondria reported by fluorescence microscopy with GFP-labelled plastids in tobacco might facilitate this communication [7]. Furthermore, a study of large-scale gene expression datasets related to perturbations of chloroplast and mitochondrial function showed a highly significant overlap between gene expression changes triggered by the chloroplast and mitochondrial perturbations [8]. This also suggests that the retrograde signals from each respective organelle are integrated to balance the activities of the organelles. However, the manner by which the organelles communicate and coordinate their activities is unknown but there are indications of interplay between plastid and mitochondrial retrograde signalling pathways. It is possible that the signals from each respective organelle are coordinated and mediated via the nucleus. The transcription factor abscisic acid insensitive 4 (ABI4) was shown to regulate LIGHT HARVESTING COMPLEX B (LHCB) expression in response to plastid signals and ALTERNATIVE OXIDASE 1 (AOX1a) expression in response to mitochondrial signals, supporting the model of interplay between retrograde signalling pathways [9,10]. Recently, another component involved in the regulation of AOX1a in response to MRR was identified from a genetic screen, REGULATOR OF ALTERNATIVE OXIDASE1 (RAO1). The rao1 mutant was isolated as a mutant unable to induce AOX1 expression in response to the inhibitor of the mitochondrial cytochrome c reductase (complex III), antimycin A (AA) [11]. RAO1 encodes the nuclear localized cyclin-dependent kinase E1 (CDKE1) and CDKE1 was described to be a central nuclear component integrating mitochondrial retrograde signals under various stress conditions, regulating a significant number of genes in the MRR regulon [11]. Here, we show that the rao1 mutant alleles also demonstrate a genome-uncoupled phenotype in response to redox changes to photosynthetic electron transport. Thus, CDKE1 responds both to plastid and mitochondrial signals. Our results suggest that CDKE1 is a central nuclear component integrating mitochondrial and plastid retrograde signals, and it plays an essential role in regulating cellular energy metabolism in response to changes in growth conditions and to stress.
2. Antimycin inhibits electron transport in both mitochondria and chloroplasts
The rao1 mutant showed impaired induction of AOX1 expression in response to 50 µM of the inhibitor AA [11]. AA inhibits mitochondrial electron transport by binding to the Qi site of the mitochondrial complex III [12]. In addition, AA has been demonstrated to inhibit photosynthetic electron transport by binding to the plastidic cytochrome b6f complex stromal-facing Qi pocket [12,13]. To quantify the effect of this inhibitor on plastid electron transport chain (PETC) activity, we evaluated the impact of increasing AA concentration using chlorophyll fluorescence. The concentration used to trigger AOX1a induction, 50 µM, resulted in a strong inhibition of plastid electron transport activity (figure 1a). Considering the shape of the fluorescence traces, the binding site of AA and the putative impact on cyclic electron flow [13], AA perturbs the initial photochemical electron flow during the transition from dark to light. The direct consequence of AA action is the interruption of cyclic electron transport flow around photosystem I (PSI) which results in a more reduced PSI reaction centre P700 [14]. This type of redox perturbation was described as a high light sensitivity in the pgr5 mutant (PROTON GRADIENT REGULATION 5) with impaired PSI cyclic electron transport [15]. A high sensitivity to fluctuating light intensities especially at the seedling stage was observed in Arabidopsis pgr5 [16], and a redox imbalance in the stroma was detected in the same mutant in rice [14]. Thus, the AA-induced plastid signal could be triggered by the impaired electron partitioning or acceptor side limitation affecting the redox poise of the PETC at PSI. The effect on plastid electron transport was also investigated for myxothiazol, another inhibitor of the complex III, but with a different binding site (Qo) [12]. The treatment with myxothiazol did not result in any alteration of the chlorophyll a fluorescence (figure 1b). Taken together, these results indicate that the AA-dependent mitochondrial retrograde signal used to select the rao mutants probably also contains a plastidic component with the potential to also stimulate induction of AOX1 expression.
Figure 1.
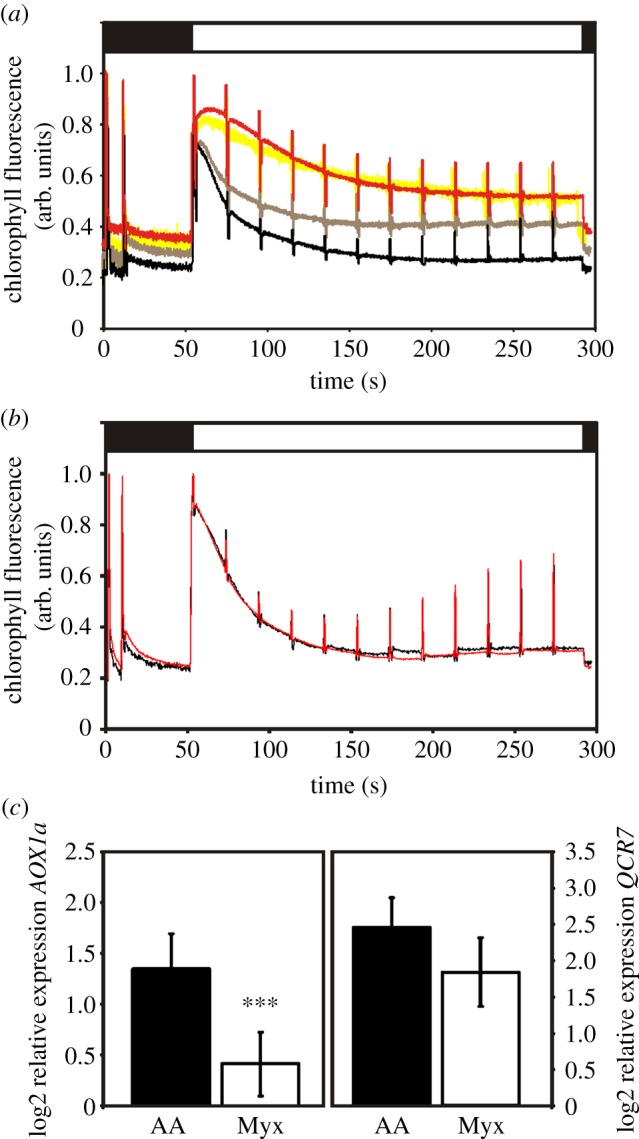
Effect on photosynthetic electron transport and nuclear gene expression of the mitochondrial complex III inhibitors AA and myxothiazol. Traces showing chlorophyll fluorescence in wild-type plants treated with increasing concentrations of AA (a) and myxothiazol (b). (a) No treatment (black trace) and treatments with 25 µM (grey trace), 50 µM (red trace) and 100 µM (yellow trace) of AA. (b) Untreated plants (black trace) and treatment with 50 µM myxothiazol (red trace). Black and white boxes above the curves represent absence or presence of actinic light (135 μmol m−2 s−1) during the measurements. (c) Log2 expression of AOX1a (At3g22370) (left panel) and QCR7 (At4g32470) (right panel) following treatment with 50 µM AA (black bars) or 50 µM myxothiazol (white bars) in 16-day-old seedlings. The expression was compared with untreated seedlings and the relative expression was calculated using PP2a (At1g13320) as a reference gene. Data represent mean (±s.d.) from at least three independent biological replicates. AOX1a expression was significantly different following AA and myxothiazol treatments as demonstrated by Student's t-test: ***p< 0.001.
The expression of AOX1a, the marker gene for MRR, is regulated by the transcription factor ABI4 [10]. ABI4 was shown to respond also to plastid signals to regulate LHCB expression, and possibly AOX1a expression is also regulated by signals originating in the plastids [9,10]. To test this, AOX1a expression was determined following treatment with the two inhibitors AA and myxothiazol (figure 1c). A stronger induction of AOX1 expression was observed following treatment with AA compared to the treatment with myxothiazol, indicating that a signal triggered by the inhibition of PETC also contributes to the induction of AOX1a. By contrast, expression of QCR7, a MRR marker gene responding to altered electron flow in the mitochondria [8], showed the same expression level for both inhibitors (figure 1c). Thus, AOX1a expression is sensitive to perturbations in the redox/energy status of both plastids and mitochondria.
3. CDKE1 integrates mitochondrial and plastid retrograde signals
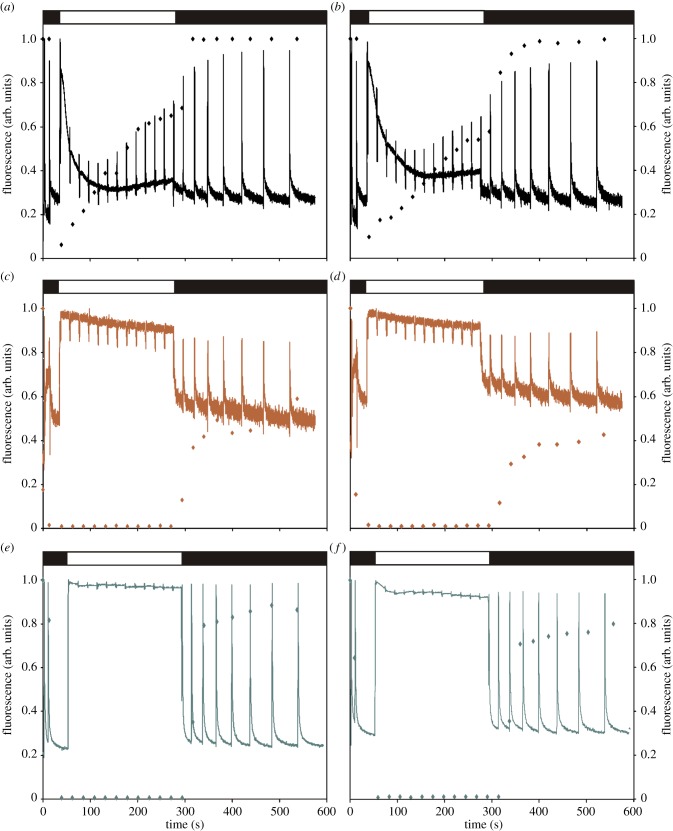
To investigate whether RAO1/CDKE1 responds to signals triggered by perturbations of the redox/energy status, not only in the mitochondria but also in plastids, we exposed the rao1 mutant alleles to conditions affecting exclusively the plastids. Two well-defined inhibitors of PETC, 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and 2,5-dibromo-3-methyl-6-isopropyl-benzoquinone (DBMIB) [17], were used to evaluate the response to plastid signals in the rao1 mutant. DCMU blocks the plastoquinone binding site at photosystem II (PSII) leaving the plastoquinone pool oxidized, and DBMIB inhibits electron transfer from plastoquinone to the cytochrome b6f complex, resulting in a more reduced intersystem. PETC activity was monitored by chlorophyll a fluorescence to confirm that inhibition of PETC was similar in wild-type and in the rao1.1 mutant (figure 2). Following treatment with the inhibitors an increase in chlorophyll fluorescence and the ‘closed’ status of the PSII reaction centres were determined by the qL parameter [18]. No difference in the response to DCMU or DBMIB between wild-type and the rao1.1 mutant could be observed (figure 2).
Figure 2.
Changes in electron flow measured through chlorophyll a fluorescence and the qL parameter following 3 h treatment with DCMU and DBMIB in wild-type (a,c,e) and in the rao1.1 mutant (b,d,f). Fluorescence traces (filled lines) and qL (symbols) in (a,b) untreated control plants, (c,d) in response to 50 µM DCMU and (e,f) in response to 100 µM DBMIB. Black and white boxes above the curves represent absence or presence of actinic light (135 μmol m−2 s−1) during the measurements. Data represent mean from at least three independent biological replicates.
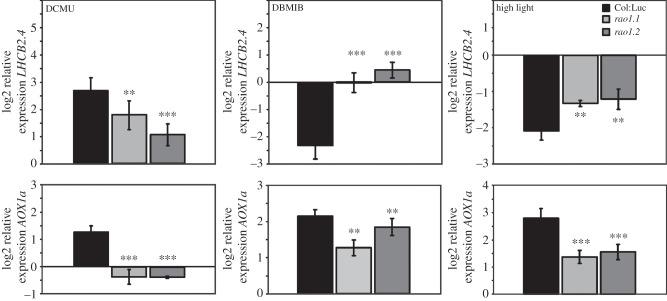
DCMU treatment results in induction of LHCB expression and DBMIB treatment in repression [19,20]. Thus, expression of LHCB2.4 (At3g27690) and AOX1a (At3g22370) was investigated in wild-type and in the rao1.1 and rao1.2 mutants following treatments with these inhibitors (figure 3). In addition to the pharmacological approach, we also evaluated gene expression in response to a 3 h high light exposure (1000 μmol m−2 s−1) (figure 3). The DCMU treatment inhibits the transfer of electrons from PSII, generating an almost completely oxidized plastoquinone pool. As a consequence, not only downstream components of the PETC remain oxidized, but there is also a higher NADP+/NADPH ratio and a more oxidized stroma. Thus, in addition to an inhibition of photosynthesis, DCMU generates an induced starvation scenario for the plant [21]. In wild-type a significant induction of LHCB2.4 expression was observed following DCMU treatment, whereas in the rao1 mutant alleles this induction was significantly impaired (figure 3). In wild-type, also AOX1a expression was induced following the DCMU treatment compared to the control conditions. However, this AOX1a induction was completely absent in the rao1 mutant plants (figure 3).
Figure 3.
Log2 expression of LHCB2.4 (At3g27690) and AOX1a (At3g22370) in 16-days-old seedlings of wild-type, Col:Luc (black bars), rao1.1 (pale grey) and rao1.2 (dark grey) following 3 h treatment with 50 µM DCMU, 100 µM DBMIB and exposure to high light intensity (1000 µmol m−2 s−1). The expression was compared with untreated seedlings for each genotype and the relative expression was calculated using PP2a (At1g13320) as a reference gene. Data represent mean (±s.d.) from at least five independent biological replicates. LHCB2.4 and AOX1a expression was significantly different in the rao1.1 and rao1.2 mutants compared with wild-type as demonstrated by Student's t-test: ***p < 0.001, **p < 0.005.
The antagonistic treatments using DBMIB and high light, both generating a highly reduced cue in the chloroplast, triggered the opposite response regarding LHCB2.4 expression. DBMIB treatment resulted in a very strong repression of LHCB2.4 expression in wild-type plants. This repression was completely absent in both rao1.1 and rao1.2 mutants (figure 3). Exposure to high light also resulted in a significant repression of LHCB2.4 expression. In the rao1 mutants the repression of LHCB2.4 was not as strong as it was for wild-type (figure 3). The discrepancy in the response between the DBMIB and high light treatments regarding LHCB expression in the rao1 mutants could be explained by the fact that exposure to excess light, in addition to triggering the redox-mediated plastid signal, also triggers the cry1-dependent pathway which is independent of the chloroplast status [22]. In contrast, the DBMIB treatment, in the short term, exclusively triggers the signal originating from the block in photosynthetic electron transfer and CDKE1/RAO1 most probably responds to pure redox/energy signals directly linked to PETC. Similarly to the DCMU treatment, AOX1a expression was significantly induced also by DBMIB and high light treatments. Thus, these results suggest that AOX1 expression is also regulated by signals originating in the plastids. In addition, the rao1 mutants demonstrated impaired regulation of AOX1a in response to all three treatments perturbing plastid redox and energy levels. Thus, CDKE1 responds both to plastid and mitochondrial signals and regulates both AOX1a and LHCB2.4 expression.
4. The rao1 mutants are sensitive to changes in light and redox conditions
The rao1 mutants showed impaired response to perturbations in the redox/energy status of both the plastids and the mitochondria, suggesting that CDKE1 plays a role in regulating energy metabolism and balancing the activities of the two organelles. Thus, we investigated the phenotype of the mutants in response to changing light conditions.
The initial characterization of rao1 mutants did not reveal any phenotype under control conditions. However, when the light intensity was shifted to 180 µmol photons m−2 s−1, the rao mutants experienced growth penalties during the initial stages of heterotrophic growth (figure 4a). To understand the underlying problem with the light shift in the rao1 mutants, we analysed the photosynthetic parameters using chlorophyll a fluorescence and measurements of PSI activity simultaneously. The data collected from rosette plants clearly indicated that the rao1 mutants do not suffer from photoinhibition, neither was any severe damage to the components of PSII that can be related to light sensitivity observed (see electronic supplementary material, table S1). However, a more detailed analysis of the parameters of PSI [23] showed a decrease in the operating efficiency of PSI as a consequence of a higher energy loss by acceptor side limitation. The higher values of Y(NA) (the quantum yield of non-photochemical energy dissipation in the PSI reaction centre) recorded for the rao1 mutants compared with wild-type indicate that these plants have an impaired capacity to remove electrons from PSI which is reflected by a more reduced P700 reaction centre. Similar condition, known as acceptor side limitation, was reported in mutants that lack the STN7 kinase. Under fluctuating light conditions the stn7 mutant demonstrated a remodelling of the PETC by a reduction in the amount of PSI [24,25]. Similar phenotype has been observed also in other mutants affected in proteins of the acceptor side of PSI [26]. Possibly, the limitation in the electron flow from PSI observed in the rao1 mutants could be explained by a more reduced stroma and lower availability of oxidized electron acceptors. In addition, this condition is prone to be exacerbated when dissipative mitochondrial mechanisms not efficiently consume the excess reducing equivalents produced in the plastids [27]. To test this assumption, the activities of PSII and PSI were evaluated in wild-type and mutant plants after a high light recovery experiment designed to challenge the mechanism behind the plasticity to react to fast redox changes [19]. Again the rao1 mutants did not show any permanent damage to PSII or associated LHC that could be linked to photoinhibition (parameter Fv/Fm) (figure 4b). However, the operating efficiency of both photosystems was affected by the high light treatment and the effect was especially strong in both rao1 alleles for PSI, which is in line with the hypothesis of an acceptor side limitation in the mutants (figure 4c,d). The mutants showed severely impaired ability to recover photosystem efficiency following the exposure to high light and probably a more reduced redox status of the chloroplast. This observation supports the phenotype triggered by exposure to higher light intensities during the initial stages of growth (figure 4a). Under such conditions when plants undergo the transition from heterotrophic to autotrophic growth, a delicate balance of the cellular energy metabolism is essential. Taken together, these results suggest that the rao1 mutants are unable to correctly balance the usage of energy owing to an impaired coordination of the metabolic activities of the energy producing organelles.
Figure 4.

Sensitivity to moderate and high light intensities of illumination in the rao1 mutants. The effect of different light intensities on plant growth in wild-type (Col:Luc), rao1.1 and rao1.2 (a). Photosynthetic parameters Fv/Fm (b), and quantum yields of PSII Y(PSII) (c) and PSI Y(PSI) (d) following exposure to high light (1000 µmol m−2 s−1) and with subsequent recovery to normal growth light intensity (150 µmol m−2 s−1). Filled symbols correspond to wild-type and open triangles correspond to rao1.1 and open boxes rao1.2. All photosynthetic measurements were conducted on rosette plants. Each point represents mean (±s.d.) of at least five independent measurements on individual plants. The YPSII and YPSI were significantly different following the recovery phase in the rao1.1 and rao1.2 mutants compared to wild-type as demonstrated by Student's t-test: ***p < 0.001.
5. The link between ABI4 and CDKE1
Our results suggest that CDKE1 integrates signals originating in both plastids and mitochondria. The activity of this kinase represents a switch that is needed to adapt nuclear gene expression to fluctuations in the energy production and consumption. The transcription factor ABI4 has also been shown to respond to signals originating in both the chloroplast and mitochondria regulating expression of LHCB and AOX1a, respectively [9,10]. To genetically test the interaction between ABI4 and CDKE1, double mutants were generated for rao1 and abi4.1. Expression of the two marker genes, LHCB2.4 and AOX1a was investigated in the rao1.1abi4.1 and rao1.2abi4.1 double mutants and compared with the respective single mutants (figure 5). The plants were again exposed to DCMU, DBMIB and high light and the expression was related to the control levels for each genotype. The abi4 single mutant demonstrated impaired response regarding LHCB expression compared to wild-type following the different treatments affecting the redox status of the chloroplasts as also has been described previously [9]. In the rao1.1abi4.1 and rao1.2abi4.1 double mutants the phenotype was significantly enhanced following the treatment with DCMU and the exposure to high light, resulting in a total insensitivity to redox changes and no change in LHCB2.4 expression compared to control conditions (figure 5). Regarding the DBMIB treatment, the effect on LHCB expression was completely abolished already in the rao1 single mutants so no further effect could be detected in the rao1.1abi4.1 and rao1.2abi4.1 double mutants following the treatment (figure 5). Thus, the analysis of the double mutants demonstrated an additive effect regarding the expression phenotype, suggesting that ABI4 and CDKE1 both respond to plastid signals and regulate LHCB expression but operate in two independent regulatory pathways.
Figure 5.
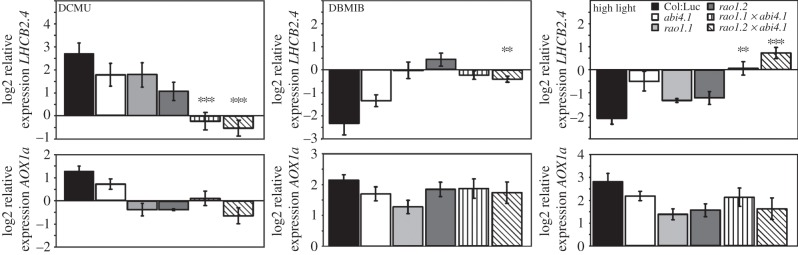
Log2 expression of AOX1a (At3g22370) and LHCB2.4 (At3g27690) in 16-days-old seedlings of Col:Luc (black bars), abi4 (open bar), rao1.1 (pale grey), rao1.2 (dark grey), abi4.1rao1.1 (vertical stripes) and abi4.1rao1.2 (diagonal stripes) following 3 h treatment with 50 µM DCMU, 100 µM DBMIB and exposure to high light intensity (1000 μmol m−2 s−1). The expression was compared with untreated seedlings for each genotype and the relative expression was calculated using PP2a (At1g13320) as a reference gene. Data represent mean (±s.d.) from at least three independent biological replicates. LHCB2.4 expression was significantly different in the abi4.1rao1.1 and abi4.1rao1.2 mutants compared with the abi4, rao1.1 and rao1.2 single mutants as demonstrated by Student's t-test: ***p < 0.001, **p < 0.005.
Interestingly the opposite result was found for AOX1 expression, where the expression levels were similar in the rao1.1abi4.1 and rao1.2abi4.1 double mutants compared to the respective single mutants (figure 5). Thus, no enhanced suppression of AOX1a induction could be found in the double mutant compared to the single mutants, supporting the suggestion that ABI4 and CDKE1 are genetically linked and act in concert to regulate AOX1a expression in response to plastid signals. However, no direct physical interaction was detected between ABI4 and CDKE1 in a co-immunoprecipitation experiment in Arabidopsis protoplasts (see electronic supplementary material, figure S1). Thus, another component(s) must be involved in the pathway regulating AOX1a expression. CDKE1 was identified as a clear activator of AOX1a expression [11], whereas ABI4 was defined as a convergent point of stress signals acting as a repressor of AOX1a in response to mitochondrial signals and abscisic acid [10]. However, the result from the abi4 single mutant suggests that in response to plastid signals, ABI4 might act as an activator of AOX1a expression similarly to CDKE1 since the induction of AOX1a expression in response to plastid redox changes was impaired compared to wild-type in the abi4 mutant (figure 5). There is also compelling evidence that suggests that ABI4 is able to both repress and induce transcription upon DNA binding [28]. Moreover, an additional developmental signal that varies between seedling and adult plant stages in combination with a regulation of transcription factors by CDKE1 as part of the mediator complex [29] are elements that cannot be ignored.
6. Concluding remarks
Mitochondria and chloroplasts depend upon each other for exchange of metabolites and energy equivalents. Photosynthetic carbon assimilation is essential for sustaining mitochondrial metabolism, and mitochondrial respiration protects photosynthesis against photoinhibition by dissipating excess redox equivalents from the chloroplasts [30,31]. AOX1a and LHCB2.4 are two components involved in energy metabolism in the plant cell. LHCB2.4 is involved in the photosynthetic generation of reducing power by harvesting solar energy whereas AOX1a is involved in the dissipation in the mitochondria of the same reducing power in response to cellular demands. We have shown that the kinase CDKE1 is involved in the regulation of both AOX1a and LHCB2.4 in response to signals originating in both the mitochondria and the chloroplasts. Analysis of the rao1.1abi4.1 and rao1.2abi4.1 double mutants showed that the regulation of LHCB2.4 by CDKE1 is not mediated via ABI4, and thus CDKE1 and ABI4 operate in two independent regulatory pathways in response to redox changes in the chloroplast (figure 5). ABI4 expression was shown to be activated by PHD type transcription factor with transmembrane domains (PTM), a transcription factor associated with the chloroplast envelope membrane. Furthermore, PTM was shown to be processed in response to chloroplast stress and an N-terminal fragment of the full length protein released to the nucleus where it activates ABI4 expression, necessary for the suppression of LHCB [32]. Our data suggest that CDKE1 represents a novel and independent pathway not linked to PTM and ABI4 [32]. Regarding the AOX1a regulation, analysis of the rao1.1abi4.1 and rao1.2abi4.1 double mutants suggests that ABI4 and CDKE1 are genetically linked and act in concert to regulate AOX1 expression in response to plastid signals (figure 5). Moreover, our data also suggest that ABI4 acts as positive regulator of AOX1a in response to retrograde signals originating in the plastids.
CDKE1 is a component of the kinase module of the plant mediator complex that relays regulatory signals between specific transcription factors bound to the promoter and RNA polymerase II [29]. CDKE1 is thereby in a perfect setting to integrate signals from both organelles and our results suggest that CDKE1 plays an essential role in regulating cellular energy metabolism in response to stress and changes in growth conditions. The rao1 mutants demonstrated severely impaired ability to recover photosystem I and II efficiency following exposure to high light and highly reduced redox status of the chloroplast (figure 4). The mutant alleles also showed a phenotype when exposed to higher light intensities during the initial stages of plant development (figure 4). Thus, the phenotype of the rao1 plants with impaired ability to integrate signals originating in the different organelles emphasizes the importance to balance or buffer redox imbalance during energy metabolism. The interplay between energy producing and consuming pathways is clearly achieved not only by the exchange of metabolites, e.g. malate [33], but also at the level of gene expression. The cellular ability to synchronize regulation of components targeted to the mitochondria and the chloroplasts presents an extra level of complexity to the concept of retrograde signalling.
7. Material and methods
(a). Plant material and growth conditions
All genotypes are in the Columbia ecotype. Seedlings of Arabidopsis thaliana were grown on 1 × B5 medium including 1% sucrose. All plant material was grown in long day conditions (16 L : 8 D). Seedlings were grown at 50–80 µmol photons m−2 s−1 and rosette plants were grown at 150 or 180 µmol photons m−2 s−1. High light exposure (3 h, 1000 µmol photons m−2 s−1) started in the middle of the light period and the control was sampled at the same time point. Inhibitor concentrations were used as follows: AA, three increasing concentrations (25 µM, 50 µM and 100 µM) and 50 µM myxothiazol [11]; 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), 50 µM for 3 h; 2,5-dibromo-3-methyl-6-isopropyl-benzoquinone (DBMIB), 100 µM for 3 h. DBMIB and AA were reapplied every hour to overcome possible degradation of these compounds. 16-days old seedlings of wild-type and mutant line were transferred from MS plates to plates with Whatman filters soaked with corresponding inhibitors or water for the control conditions.
(b). PSII and PSI photochemistry
In vivo chlorophyll fluorescence was measured using a modulation fluorometer DUAL-PAM-100 (Heinz Walz GmbH, Effeltrich, Germany) from the adaxial side of the attached leaf material. The nomenclature and interpretation of the results were conducted based on Baker [18] and Klughammer & Schreiber [23]. The maximal photochemical efficiency of PSII photochemistry in the dark acclimated state was evaluated as Fv/Fm = (Fm – Fo)/Fm after 30 min acclimation to darkness. In both the light and dark acclimated states, the minimal fluorescence intensity was measured by analytic modulated light, the maximal fluorescence intensity by saturating pulses (flash light intensity approx. 4000 µmol photons m–2 s–1) of 0.8 s duration.
(c). RNA isolation, cDNA synthesis and real-time PCR
Total RNA was isolated using the Plant RNA Mini kit (Omega). cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad). cDNA was used in a iQ SYBR Green Supermix reaction (Bio-Rad). At least three biological replicates were used for each data point and all reactions were performed in technical triplicates. RT PCR was run in CFX96 Real time system (Bio-Rad) and monitored by using the CFX Manager (Bio-Rad). Data were analysed by using LinRegPCR [34,35]. Primer sequences: PP2aFw, TAACGTG GCCAAAATGATGC; PP2aRv, GTTCTCCACAACCGCTTGGT; LHCB24Fw, GCCATCCAACGATCTCCTC; LHCB24Rv, TGGTCCGTACCAGATGCTC; AOX1aFw, AGCATCATGTTCCAACGACGTTTC; AOX1aRv, GCTCGACATCCATATCTCCTCTGG; QCR7Fw, TCCGCAGATACGGTCTTAGATACG; QCR7Rv,GCTGGTTCCGAGCATCAACAATC. The reference gene PP2a was chosen based on analysis conducted by [36]. No change in PP2a expression was detected for any of the treatments used.
Funding statement
This work was supported by grants from the Swedish Research Foundation, VR (ÅS), STINT and the Australian Research Council (JW).
References
- 1.Rodermel S. 2001. Pathways of plastid-to-nucleus signaling. Trends Plant Sci. 6, 471–478. ( 10.1016/S1360-1385(01)02085-4) [DOI] [PubMed] [Google Scholar]
- 2.Barajas-Lopez de JD, Blanco NE, Strand Å. 2013. Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim. Biophys. Acta 1833, 425–437. ( 10.1016/j.bbamcr.2012.06.020) [DOI] [PubMed] [Google Scholar]
- 3.Estavillo GM, et al. 2011. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23, 3992–4012. ( 10.1105/tpc.111.091033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez AP, Strand Å. 2008. Retrograde signaling and plant stress: plastid signals initiate cellular stress responses. Curr. Opin. Plant Biol. 11, 509–513. ( 10.1016/j.pbi.2008.06.002) [DOI] [PubMed] [Google Scholar]
- 5.Ho LH, Giraud E, Uggalla V, Lister R, Clifton R, Glen A, Thirkettle-Watts D, Van Aken O, Whelan J. 2008. Identification of regulatory pathways controlling gene expression of stress-responsive mitochondrial proteins in Arabidopsis. Plant Physiol. 147, 1858–1873. ( 10.1104/pp.108.121384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhoads DM. 2011. Plant mitochondrial retrogarde regulation. Adv. Plant Biol., Plant Mitochondria 1, 411–437. ( 10.1007/978-0-387-89781-3_16) [DOI] [Google Scholar]
- 7.Kwok EY, Hanson MR. 2004. Stromules and the dynamic nature of plastid morphology. J. Microsc. 214, 124–137. ( 10.1111/j.0022-2720.2004.01317.x) [DOI] [PubMed] [Google Scholar]
- 8.Van Aken O, Whelan J. 2012. Comparison of transcriptional changes to chloroplast and mitochondrial perturbations reveals common and specific responses in Arabidopsis. Front. Plant Sci. 3, 281 ( 10.3389/fpls.2012.00281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J. 2007. Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719. ( 10.1126/science%20;1140516) [DOI] [PubMed] [Google Scholar]
- 10.Giraud E, Van Aken O, Ho LH, Whelan J. 2009. The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol. 150, 1286–1296. ( 10.1104/pp.109.139782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng S, et al. 2013. Cyclin-dependent kinase E1 (CDKE1) provides a cellular switch in plants between growth and stress responses. J. Biol. Chem. 288, 3449–3459. ( 10.1074/jbc.M112.416727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cape JL, Bowman MK, Kramer DM. 2006. Understanding the cytochrome bc complexes by what they don't do. The Q-cycle at 30. Trends Plant Sci. 11, 46–55. ( 10.1016/j.tplants.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 13.Shikanai T. 2007. Cyclic electron transport around photosystem I: genetic approaches. Annu. Rev. Plant Biol. 58, 199–217. ( 10.1146/annurev.arplant.58.091406.110525) [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa Y, Yamamoto H, Okegawa Y, Wada S, Sato N, Taira Y, Sugimoto K, Makino A, Shikanai T. 2012. PGR5-dependent cyclic electron transport around PSI contributes to the redox homeostasis in chloroplasts rather than CO(2) fixation and biomass production in rice. Plant Cell Physiol. 53, 2117–2126. ( 10.1093/pcp/pcs153) [DOI] [PubMed] [Google Scholar]
- 15.Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T. 2002. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110, 361–371. ( 10.1016/S0092-8674(02)00867-X) [DOI] [PubMed] [Google Scholar]
- 16.Suorsa M, et al. 2012. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24, 2934–2948. ( 10.1105/tpc.112.097162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfannschmidt T, Schutze K, Brost M, Oelmuller R. 2001. A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J. Biol. Chem. 276, 36 125–36 130. ( 10.1074/jbc.M105701200) [DOI] [PubMed] [Google Scholar]
- 18.Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113. ( 10.1146/annurev.arplant.59.032607.092759) [DOI] [PubMed] [Google Scholar]
- 19.Kindgren P, et al. 2012. The plastid redox insensitive 2 mutant of Arabidopsis is impaired in PEP activity and high light-dependent plastid redox signalling to the nucleus. Plant J. 70, 279–291. ( 10.1111/j.1365-313X.2011.04865.x) [DOI] [PubMed] [Google Scholar]
- 20.Brautigam K, et al. 2009. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. Plant Cell 21, 2715–2732. ( 10.1105/tpc.108.062018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J. 2007. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942. ( 10.1038/nature06069) [DOI] [PubMed] [Google Scholar]
- 22.Kleine T, Kindgren P, Benedict C, Hendrickson L, Strand Å. 2007. Genome-wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol. 144, 1391–1406. ( 10.1104/pp.107.098293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klughammer C, Schreiber U. 2008. Saturation pulse method for assessment of energy conversion in PSI. PAM Appl. Notes 1, 11–14. [Google Scholar]
- 24.Grieco M, Tikkanen M, Paakkarinen V, Kangasjarvi S, Aro EM. 2012. Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol. 160, 1896–1910. ( 10.1104/pp.112.206466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tikkanen M, Grieco M, Kangasjarvi S, Aro EM. 2010. Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol. 152, 723–735. ( 10.1104/pp.109.150250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lintala M, Lehtimaki N, Benz JP, Jungfer A, Soll J, Aro E-M, Bolter B, Mulo P. 2012. Depletion of leaf-type ferredoxin-NADP+ oxidoreductase results in the permanent induction of photoprotective mechanisms in Arabidopsis chloroplasts. Plant J. 70, 809–817. ( 10.1111/j.1365-313X.2012.04930.x) [DOI] [PubMed] [Google Scholar]
- 27.Nunes-Nesi A, Araujo WL, Fernie AR. 2011. Targeting mitochondrial metabolism and machinery as a means to enhance photosynthesis. Plant Physiol. 155, 101–107. ( 10.1104/pp.110.163816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wind JJ, Peviani A, Snel B, Hanson J, Smeekens SC. 2013. ABI4: versatile activator and repressor. Trends Plant Sci 18, 125–132. ( 10.1016/j.tplants.2012.10.004) [DOI] [PubMed] [Google Scholar]
- 29.Mathur S, Vyas S, Kapoor S, Tyagi AK. 2011. The mediator complex in plants: structure, phylogeny, and expression profiling of representative genes in a dicot (Arabidopsis) and a monocot (rice) during reproduction and abiotic stress. Plant Physiol. 157, 1609–1627. ( 10.1104/pp.111.188300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niyogi KK. 1999. PHOTOPROTECTION REVISITED: genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 333–359. ( 10.1146/annurev.arplant.50.1.333) [DOI] [PubMed] [Google Scholar]
- 31.Niyogi KK. 2000. Safety valves for photosynthesis. Curr. Opin. Plant. Biol. 3, 455–460. ( 10.1016/S1369-5266(00)00113-8) [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Feng P, Xu X, Guo H, Ma J, Chi W, Lin R, Lu C, Zhang L. 2011. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2, 477 ( 10.1038/ncomms1486) [DOI] [PubMed] [Google Scholar]
- 33.Scheibe R. 2004. Malate valves to balance cellular energy supply. Physiol. Plant 120, 21–26. ( 10.1111/j.0031-9317.2004.0222.x) [DOI] [PubMed] [Google Scholar]
- 34.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 ( 10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66. ( 10.1016/S0304-3940(02)01423-4) [DOI] [PubMed] [Google Scholar]
- 36.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. 2005. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17. ( 10.1104/pp.105.063743) [DOI] [PMC free article] [PubMed] [Google Scholar]