Abstract
The rapid induction of the bundle sheath cell (BSC)-specific expression of ASCORBATE PEROXIDASE2 (APX2) in high light (HL)-exposed leaves of Arabidopsis thaliana is, in part, regulated by the hormone abscisic acid (ABA) produced by vascular parenchyma cells. In this study, we provide more details of the ABA signalling that regulates APX2 expression and consider its importance in the photosynthetic responses of BSCs and whole leaves. This was done using a combination of analyses of gene expression and chlorophyll a fluorescence of both leaves and individual BSCs and mesophyll cells. The regulation of APX2 expression occurs by the combination of the protein kinase SnRK2.6 (OST1):protein phosphatase 2C ABI2 and a Gα (GPA1)-regulated signalling pathway. The use of an ost1-1/gpa1-4 mutant established that these signalling pathways are distinct but interact to regulate APX2. In HL-exposed leaves, BSC chloroplasts were more susceptible to photoinhibition than those of mesophyll cells. The activity of the ABA-signalling network determined the degree of susceptibility of BSCs to photoinhibition by influencing non-photochemical quenching. By contrast, in HL-exposed whole leaves, ABA signalling did not have any major influence on their transcriptomes nor on their susceptibility to photoinhibition, except where guard cell responses were observed.
Keywords: abscisic acid, APX2, bundle sheath cells, high light, photoinhibition, reactive oxygen species (ROS)
1. Introduction
The ability of plants to respond and acclimate to changes in light intensity requires a complex signalling network, which is subjected to fine spatial and temporal control [1,2]. In Arabidopsis thaliana (Arabidopsis) leaves subjected for up to 60 min to moderate increases in light intensity, i.e. typically less than 10-fold of the growth photosynthetically active photon flux density (PPFD), hereafter referred to as high light (HL), photoinhibition is largely reversible [1,3]. Within 10 min of HL exposure, accumulation of the reactive oxygen species (ROS) hydrogen peroxide (H2O2) occurs in chloroplasts of bundle sheath cells (BSCs) [3,4]. Leaves subjected to HL and high humidity do not accumulate H2O2 [3]. This humidity dependency is also evident for the HL-mediated induction of BSC-specific ASCORBATE PEROXIDASE2 (APX2) [5] and led to the identification of abscisic acid (ABA) as a regulator of APX2 expression [3,4,6]. In HL-exposed leaves, vascular parenchyma, adjacent to BSCs in Arabidopsis, is the source of the ABA for the induction of APX2 [3,7]. APX2 induction in HL also requires signalling sourced from redox events around linear photosynthetic electron transport [6,8,9], H2O2 sourced from the chloroplast and plasma membrane [3,6] and a decreased cellular redox status determined by the thiol antioxidant glutathione [5].
The control of APX2 expression in BSCs involves at least one positive and one negative ABA-directed signalling pathway [3,4]. The pathway that positively regulates APX2 expression has been shown to involve the SUCROSE NONFERMENTING1 (SNF1)-related protein kinase SnRK2.6, also called OPEN STOMATA1 (OST1) [3,10,11]. The activity of OST1 and the other main foliar subclass III SnRK2 isoforms (SnRK2.2 and SNRK2.3) [10,11] is negatively regulated by the 2C class of protein phosphatases (PP2Cs) [12,13] of which there are five isoforms present in adult leaves [14]. Of the PP2Cs so far tested, ABI1 and ABI2 (ABI stands for ABA INSENSITIVE), as the dominant negative mutants abi1-1 and abi2-1 [15], impact upon APX2 expression [4]. This group of PP2C and SNRK2 isoforms along with their family of 14 cognate ABA START protein receptors are regarded as comprising a ‘core’ ABA-signalling pathway [13].
Heterotrimeric G protein signalling exerts a negative regulation of APX2 induction in HL [3] of which the Gα and Gβ subunits GPA1 and AGB1, respectively, play a prominent role. The rice (Oryza sativa) GPA1 homologue regulates the production of H2O2 sourced from superoxide anion, which is produced from O2 in a reaction catalysed by a RESPIRATORY BURST (NADPH) OXIDASE HOMOLOGUE (RBOH) at the plasma membrane [16]. In Arabidopsis, there are two major RBOH isoforms expressed in the leaf, RBOHD and RBOHF [17]. A double null mutant of these two genes shows inhibition of APX2 expression in HL [6]. In guard cells, ABA-induced stomatal closure is mediated by RBOHF and RBOHD [18] and may be linked to GPA1-mediated signalling [19,20]. It is not clear how the H2O2 from either the plasma membrane or the chloroplast in BSCs or guard cells can act as a signal to the nucleus by traversing a reducing cytosolic environment [21]. One mechanism in guard cells has been proposed in which oxidized GLUTATHIONE PEROXIDASE3 (GPX3) inhibits the activity of ABI2 and possibly ABI1 [22].
In this study, we set out to answer three questions regarding the role of ABA signalling in HL responses of Arabidopsis. First, could more detail be added to the core ABA signalling directing APX2 expression in BSCs? Second, is ABA signalling important for BSC responses to HL and not just the regulation of APX2? Third, is ABA signalling important for the immediate responses to HL of the leaf as a whole or is this confined to BSCs?
2. Material and methods
(a). Arabidopsis genotypes
All mutants used in this study have been described previously: abi1-1 and abi2-1 [15], abi1-2, abi2-2, abi2-2/abi1-2/hab1-1 (abi2-2T) and abi1-2/hab1-1/pp2ca1-1 (pp2ca1T) [14], hab1-1 and hab2-2 [23], ost1-1 [10], snrk2.2/snrk2.3 [11], gpx3-1 [22], gpa1-4 [24], rbohD, rbohF [17], ABA DEFICIENT2 (ABA2) over-expressing line 4-3 (ABA2OE) [25]), apx2-1 [26] and apx2-2 (SALK_057686), which was an independent isolate of an APX2 knockout mutant described previously [27]. Accession Col-0 was used as wild-type control in all cases, except for ost1-1, abi1-1 and abi2-1 for which accession Landsberg erecta (Ler) was used. The genotypes of all mutants were confirmed for this study.
A double mutant gpa1-4/ost1-1 was made by crossing gpa1-4 (Col-0; ♂) with ost1-1 (Ler, ♀). The cross was taken to be successful by confirming in F1 plants the presence of the heterozygous T-DNA insertion event of gpa1-4 using the polymerase chain reaction (PCR), conditions and primers on genomic DNA as previously described [24]. F2 progeny were first screened for a homozygous gpa1-4 mutation and among these a homozygous ost1-1 mutation was searched for by PCR of genomic DNA using primers ost1-1_F2 and ost1-1_R2 to SNRK2.6/OST1 (5′-CTGATTATAGATAGGGGAAACA-3′ and 5′-CTGATTATAGATAGGGGAAACA-3′ respectively) to generate a 800 bp amplicon. The amplicon was subjected to dideoxy sequencing using the BIG DYE Terminator v. 3.1 Ready Reaction Mix (Applied Biosystems) according to the manufacturer's instructions using the primer ost1-1_R3 (5′-TCACAAATAAATCAACAAATGC-3′) and the sequence generated on an ABI3100 DNA analyser (Applied Biosystems). The mutation G (wild-type) to A (ost1-1) [10] at location chr4:16273850 (in AT4G33950.2) was scored from the DNA sequence data to recover two gpa1-4 homozygous individuals that were also homozygous for the ost1-1 mutation. These plants were each self-crossed to generate F3 progeny, the homozygous ost1-1 mutation confirmed and homozygous gpa1-4 was confirmed as null by the absence of GPA1 transcript by reverse transcriptase (RT)-PCR as previously described [3] using primers: 5′-CATAGAACTGTCGGGGAAATTGTGAATCATCACCAGCC-3′ and 5′-GAAACAACAACGGCGAAGAGTTTTTTGCTTTCAGGGTTCT-3′. The F3 progeny were used in the experiments described here. From the same screening, two wild-type F2 individuals were chosen based on the absence of the above mutations and harbouring heterozygous loci for Ler and Col-0 using the cleaved amplified polymorphic sequence (CAPS) marker ATMYB3R (www.arabidopsis.org). These lines (F3) were used as wild-type controls for the APX2 expression analyses in this study on ost1-1/gpa1-4.
(b). Plant growth conditions
Plants were grown under 8 h photoperiods, 22 ± 1°C, 60% relative humidity and a PPFD of 150 (±15) µmol m−2 s−1 as described previously [28], hereafter termed low light (LL) conditions. Unless stated otherwise, all plants were used from 35 to 40 days post-germination.
(c). High light exposures and chlorophyll a fluorescence measurements and imaging
For APX2 gene expression experiments, plants were exposed to a PPFD of 1500 µmol m−2 s−1 from a white light emitting diode (LED) array (Isolight; Technologica Ltd, Colchester UK). Leaf surface temperature reached 27°C after 5 min exposure at 5 cm from the LED array and was constant thereafter for up to 6 h. After 6 h HL exposure using this LED source, the maximum photosystem II (PSII) quantum efficiency (Fv/Fm) [29] was 0.59 ± 0.03 (s.d., n = 8) from a LL value of 0.81 ± 0.01, measured using a PAM-2000 portable fluorometer (Walz GmbH, Effeltrich, Germany) according to the manufacturer's instructions. Exposure of plants to 27°C for 30 min under LL induced APX2 expression by 1.4-fold compared with plants at growth temperature (±0.4 s.d.; n = 3). By contrast, under the isolight the induction was 61.5-fold (±12.2 s.d.; n = 3). Thus, in this experimental system the predominant response studied was of Arabidopsis to HL and not the rise in temperature.
ABA-signalling mutants were examined for their whole leaf response to increasing PPFD from 200 to 1400 µmol m−2 s−1 in 200 µmol m−2 s−1 increments every 5 min. This was carried out on a chlorophyll a fluorescence (Cf) imaging system (Fluorimager, Technologica Ltd, Colchester, UK) in which the protocol had been pre-programmed into the instrument. Whole rosette Cf images were collected at each PPFD and the images were processed manually to collect numerical data from fully expanded leaves (see electronic supplementary material, figure S3a) for the Cf parameters Fq′/Fm′, Fv′/Fm′, Fq′/Fv′ and NPQ [29]. qL [29] was calculated post-measurement from the images of Fq′/Fm′, Fo′ and F′. The raw data from each leaf and each plant and treatment were fed via Excel into a program in R which calculated and plotted responses of the Cf parameters against PPFD and provided the data for statistical analysis. The R script is available upon request.
(d). High-resolution imaging
Three-week-old plants were subjected to HL for up to 1 h or kept in LL, then a single leaf was placed onto the microscopic slide, overlaid with a coverslip and Cf image data were collected under actinic light. Cf data from six mesophyll cells and six BSCs per leaf were analysed. In each experiment, three plants were analysed per treatment per cell type. Parameters were measured in a Micro-FluorCam (Photon System Instruments, Czech Republic). Calculations for Fq′/Fm′, Fv′/Fm′ and Fq′/Fv′ were done according to Baker [29].
For the study of apx2 mutants, 4-week-old plants were kept in LL or exposed to HL for 1 h. Then plants were kept in the dark for 30 min and a single leaf was placed in the measuring head of Imaging-PAM, version MINI equipped with Head IMAG-MIN/B (Walz GmbH) and Cf parameters collected over a range of PPFDs at 5 min intervals. Two areas from each leaf were taken for analysis, first from the mid-vein region and second from the leaf lamina. Three independent plants were taken for each treatment.
(e). Effect of a step change in photosynthetically active photon flux density on CO2 assimilation rate and stomatal conductance
Measurements were made using infrared gas exchange analysis on individual fully expanded leaves of 6-week-old plants following the methods of Lawson et al. [30]. Briefly, leaves were first equilibrated to a PPFD of 100 µmol m−2 s−1, ambient [CO2] of 400 µmol mol−1 and 50% humidity at 22°C. Following stabilization, light was increased to 1000 µmol m−2 s−1 and the responses of CO2 assimilation rate (A) and stomatal conductance (gs) were recorded every minute until the leaf had stabilized to the new environment. The rate of change in gs was determined during the first 15 min following a change in PPFD.
(f). RNA extraction, analysis of APX2 expression by quantitative real time polymerase chain reaction and microarray analysis
RNA extraction, cDNA synthesis and quantitative (q) real time PCR were carried out on fully expanded leaves as previously described [28] using a SYBR green kit (Bioline Reagents Ltd. London, UK) according to the manufacturer's instructions. APX2 primers used for qPCR were 5′ GATATTGCCGTTAGGCTTCTTGACCCT 3′ and 5′ GAAGAGCCTTGTCGGTTGGTAGTT 3′. CYCLOPHILIN (CYC; AT2G29960) was used as reference gene. The CYC primers were 5′ TCTTCCTCTTCGGAGCCATA 3′ and 5′ AAGCTGGGAATGATTCGATG 3′.
Microarray analysis was conducted using Agilent 4×44k arrays (G2519F-021169) exactly as previously described [28]. Comparisons were conducted between fully expanded leaves of mutant and wild-type plants exposed to HL. All microarray data were submitted to EMBL-EBI under the following codes: ABA2OE (E-MTAB-2048), abi2-2/abi1-2/hab1-1 (abi2-2T, E-MTAB-2047), rbohF (E-MTAB-2049), gpa1-4 (E-MTAB-2050), ost1-1 (E-MTAB-2051) and snrk2.2/snrk2.3 (snrk2D, E-MTAB-2052).
(g). Imaging H2O2 in leaves using Amplex Red Ultra
Infiltration of Aplex Red Ultra (ARU) into detached leaves followed by exposure to HL, its penetration properties, specific reaction with H2O2, imaging of the resulting resorufin fluorescence and digital processing of false-coloured images are described in detail by Galvez-Valdivieso et al. [3].
3. Results
(a). High light-induced APX2 expression is controlled by two separate ABA-directed signalling pathways
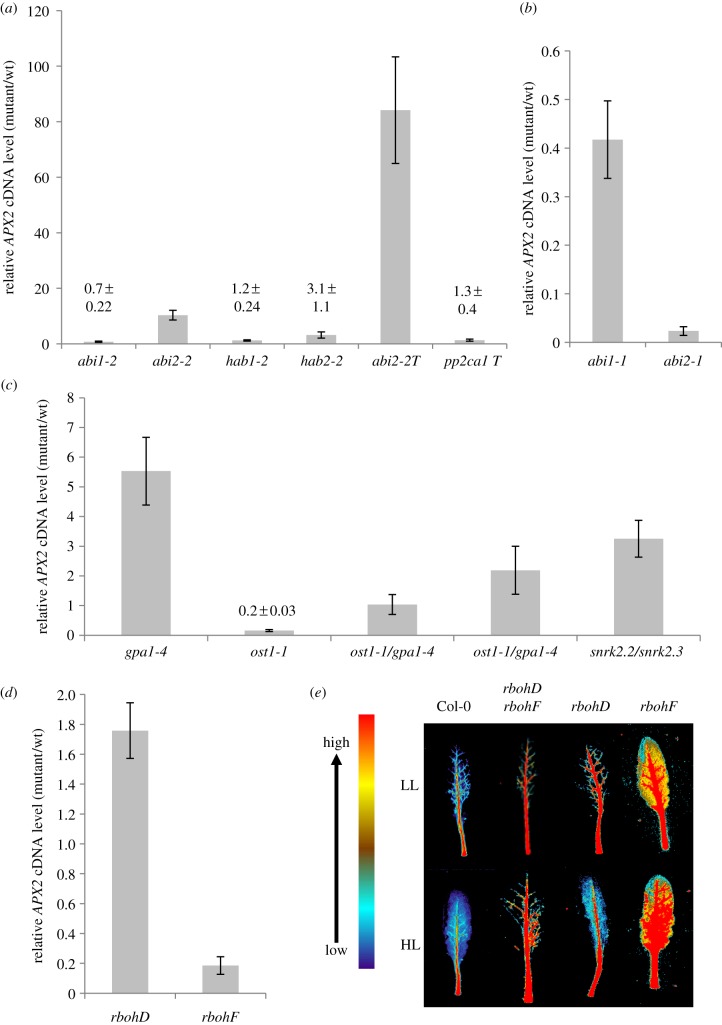
APX2 expression under HL was compared between mutant and wild-type controls for a range of ABA-signalling mutants (figure 1; see Material and methods) representing a more detailed analysis of the pathways described previously [3,4,6]. Exposure of abi2-2 to HL resulted in a ca 10-fold increased expression relative to Col-0 for APX2 (figure 1a), while in HL-exposed abi2-2/abi1-2/hab1-1 plants APX2 expression was increased ca 80-fold (figure 1a). No other PP2C mutant showed any major effect on HL-induced APX2 expression (figure 1a). The lack of effect on HL-responsive APX2 expression of abi1-2 or in abi1-2/hab1-1/pp2ca1-1 contrasts with earlier observations using the dominant negative alleles of ABI1 and ABI2, abi1-1 and abi2-1 [4,15]. These observations were confirmed here although the inhibition was the strongest with abi2-1 (figure 1b).
Figure 1.
Expression of APX2 and ARU staining for extracellular H2O2 in Arabidopsis leaves subjected to HL. (a) APX2 cDNA levels from HL-exposed single and triple PP2C mutants compared with HL exposed Col-0. HL exposures (1500 µmol m−2 s−1 PPFD for 30 min) were conducted on 5-week-old short day-grown rosettes and RNA extracted from fully expanded leaves. cDNA levels were determined by qPCR using a SYBR Green-based assay (see Material and methods) with CYC as the reference gene. Values are means (±s.e.) of two experiments each with six plants used (n = 12). (b) Relative APX2 cDNA levels in abi1-1 and abi2-1. Experimental conditions were as in (a), except that these are data (means±s.e.) from one experiment of six plants (n = 6). (c) Relative APX2 cDNA levels in ost1-1/gpa1-4, ost1-1, gpa1-4 and snrk2.2/snrk2.3. The HL conditions, sample size and qPCR were as in (a). Two individual lines of the double mutant were analysed. Wild-type controls were Ler for ost1-1, Col-0 for gpa1-4 and snrk2.2/snrk2.3 and an F3 Ler/Col-0 hybrid (see Material and methods). (d) APX2 cDNA levels in rbohD and rbohF relative to Col-0. The HL conditions, sample size and qPCR were as in (a). (e) Resorufin fluorescence in HL-exposed detached leaves of rbohD, rbohF and rbohD/rbohF. ARU (40 µM) was infiltrated into leaves (see Material and methods) and false-coloured image produced digitally against a scale of the fluorescence emission. (Online version in colour.)
Among the protein substrates for ABI2 and ABI1 are the major foliar SNRK2 isoforms, SNRK2.2, SNRK2.3 and SNRK2.6 (OST1) [12,31]. As previously shown [3], ost1-1 plants were strongly inhibited for HL-induced APX2 expression and gpa1-4 showed enhanced APX2 expression (figure 1c). HL-induced APX2 expression in ost1-1/gpa1-4 was in between the parental mutant values (figure 1c). As the expression phenotype of either single mutant was not evident in the double mutant, it was concluded that there are two separate signalling pathways, which nevertheless could act antagonistically on each other in controlling APX2 expression.
In Arabidopsis, a source of extracellular H2O2 for signalling for ABA and HL is from RBOHD and RBOHF isoforms (see Introduction). APX2 expression was inhibited only in rbohF plants (figure 1d). Examination of resorufin fluorescence to visualize H2O2 accumulation in HL-exposed leaves of rbohD and rbohF showed diminished and enhanced levels of veinal H2O2, respectively (figure 1f; electronic supplementary material, figure S1). Under the same conditions, the rbohD/rbohF double mutant accumulated less H2O2 (figure 1f; electronic supplementary material, figure S1).
(b). Mutants in ABA signalling that positively regulate APX2 expression are affected in photosynthetic efficiency in HL-exposed BSCs.
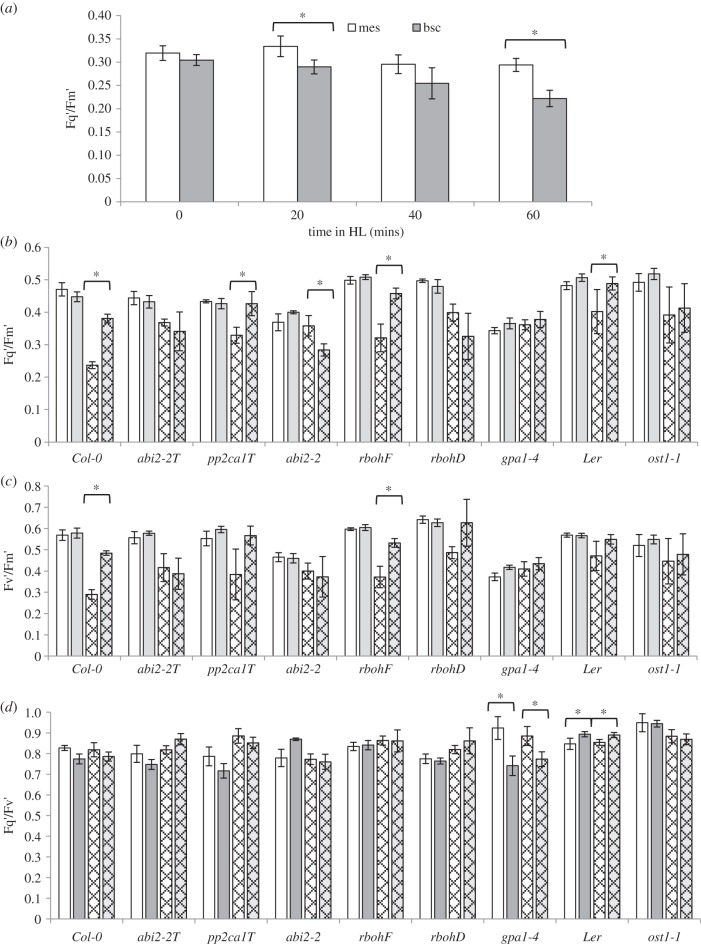
BSCs of Col-0 plants exposed to HL for up to 60 min suffered progressively more photoinhibition, here shown as a decline in the Cf parameter Fq′/Fm′, than neighbouring mesophyll cells (figure 2a). Therefore, the Cf parameters Fq′/Fm′, Fq′/Fv′ and Fv′/Fm′ were measured before and after 60 min HL in a set of ABA-signalling mutants primarily involved in regulating APX2 expression. In Col-0 and Ler plants, HL-exposed BSC chloroplasts again showed a larger decline in Fq′/Fm′and also Fv′/Fm′ than those from mesophyll cells (figure 2b,c). Fq′/Fv′ was no different between the two cell types (figure 2d). In comparison with their wild-type controls, abi2-2, abi2-2/abi1-2/hab1-1, ost1-1 and gpa1-4 did not show significantly lower Fq′/Fm′ and Fv′/Fm′ values in BSCs compared with mesophyll cells. It was concluded that ABA/ROS signalling, primarily associated with ABI2/OST1 [13] and GPA1, could influence the susceptibility of BSC chloroplasts to photoinhibition. By contrast, mesophyll cells were less affected in these mutants (figure 2b–d). In contrast, rbohF, in which HL-induced APX2 expression was inhibited (figure 1d), had no effect on BSC or mesophyll responses to HL compared with wild-type (figure 2b–d).
Figure 2.
Cell-specific chlorophyll fluorescence (Cf) parameters under HL and LL. (a) Plants were exposed to HL (1500 µmol m−2 s−1 PPFD) for 0, 20, 40 and 60 min, and then individual detached leaves were placed under a high-resolution Cf imaging system (see Material and methods) and Fq′/Fm′ determined for six different cells of BSC (grey bars) and mesophyll (white bars) per leaf from three plants (n = 18). (b–d) Fq′/Fm′ (b), Fv′/Fm′ (c) and Fq′/Fv′ (d) Cf parameters (mean ± s.e.) from HL-exposed bundle sheath and mesophyll cells (white checked bars and grey checked bars, respectively) and their corresponding LL controls (white bars and grey bars) of wild-type and ABA-signalling mutants. Plants were exposed to HL for 60 mins as in (a), and then individual detached leaves were imaged as in (a). Cf data were collected for six different cells of each type per leaf from three plants (n = 18). From these data, Fq′/Fm′ (b), Fv′/Fm′ (c) and Fq′/Fv′ (d) were calculated. In all panels a–d, pairs of columns marked with an asterisk (*) mean that the comparison is significant at p ≤ 0.001 (Student's t-test). The words abi2-2T and pp2ca1T are for the abi2-2/abi1-2/hab1-1 and pp2ca1-1/abi1-2/hab1-1 mutants, respectively.
From the above observations, it was reasoned that the degree of expression of APX2 might be a determinant of the susceptibility of BSCs to photoinhibition. To test this proposal, Fq′/Fm′, NPQ and qL were measured in the leaf lamina and mid-vein sections of two null alleles of APX2, apx2-1 and apx2-2 (see Material and methods) exposed to HL. No differences in response of these mutants to HL were observed compared with Col-0 (see electronic supplementary material, figure S2).
(c). Abscisic acid signalling in whole leaf high light responses
ABA-signalling mutants were examined for their whole leaf response to HL (see Material and methods). Whole rosette Cf images were collected and the images were processed to collect data from fully expanded leaves for Fq′/Fm′, Fv′/Fm′, Fq′/Fv′, NPQ and qL (Material and methods). An example is shown in the electronic supplementary material, figure S3a and the type of data plots obtained is shown in the electronic supplementary material, figure S3b. The combined data from all analyses of 13 ABA-signalling mutants can be found in the electronic supplementary material, figure S4. This large dataset was condensed by carrying a multivariate statistical analysis using principal component analysis (PCA) to look for patterns or trends within groups of mutants and wild-type accessions (see electronic supplementary material, figure S3c,d). PCA showed that only minor effects occurred at the whole leaf level in mutants after variation among wild-type controls was taken into account. Some of these whole leaf effects could be a consequence of increased stomatal conductance (gs), which have been observed during the first approximately 15 min of HL exposure [3,4]. Stomatal conductance, gs, started and attained different values in many of the mutants and thus had altered carbon assimilation rates (Asat; table 1). The rates of change in gs in response to 15 min HL were in many cases affected by the mutations (table 1).
Table 1.
Stomatal conductance (gs), rates of stomatal closure and maximum rates of photosynthesis (Asat) in response to 15 min at a PPFD of 1000 µmol m−2 s−1. Values are the means ± s.d. (n = 3). Those values marked * are significantly different from their control genotypes at p ≤ 0.05 (Student's t-test).
| genotype | gs (0 min) (mmol m−2 s−1) | gs (15 min) (mmol m−2 s−1) | rate of change of gs (mmol m−2 s−1) | Asat (15 min) (µmol m−2 s−1) |
|---|---|---|---|---|
| Col-0 | 207.5 ± 77.0 | 308.0 ± 92.2 | 6.7 ± 1.1 | 11.8 ± 0.4 |
| Ler | 309.1 ± 64.2 | 431.5 ± 96.6 | 8.2 ± 2.7 | 15.3 ± 1.5 |
| abi2-1 | 75.4 ± 15.0* | 102.3 ± 23.2* | 1.8 ± 0.5* | 7.1 ± 1.1* |
| abi2-2 | 153.6 ± 22.3 | 209.3 ± 38.8 | 3.7 ± 1.4* | 10 ± 1.4 |
| abi1-1 | 202.2 ± 86.0 | 288.7 ± 136.2 | 5.8 ± 3.6 | 12.1 ± 3.1 |
| abi1-2 | 533 ± 251.6 | 642.9 ± 253.9 | 7.3 ± 0.5 | 21.1 ± 4.5 |
| hab1-1 | 91.7 ± 26.8 | 109 ± 88.8 | 1.2 ± 4.2 | 8.7 ± 1.5 |
| abi2-2/abi1-2/ hab1-1 | 32.2 ± 4.4 | 44.0 ± 2.8* | 0.8 ± 0.1* | 4.5 ± 2.2* |
| pp2ca1/abi1-2/ hab1-1 | 29.8 ± 4.8 | 43.7 ± 8.9* | 0.9 ± 0.4* | 5.3 ± 1.6* |
| ost1-1 | 214.8 ± 43.0 | 243.9 ± 38.3 | 1.9 ± 0.6* | 10.1 ± 1.3* |
| rbohD/rbohF | 60.8 ± 14.6* | 125.2 ± 33.8* | 4.3 ± 1.3* | 7.3 ± 0.2* |
| gpa1-4 | 82.1 ± 15.9 | 111.8 ± 13.4 | 2.0 ± 0.9* | 11.1 ± 2.4 |
Microarray analyses were conducted on whole leaf RNA prepared from HL-exposed wild-type leaves compared with the following ABA-signalling mutants: ost1-1, snrk2.2/snrk2.3, gpa1-4, abi2-2/abi1-2/hab1-1 and rbohF. Apart from effects on the transcript levels of genes in the null mutants, no significant differences were detected in the mutants' transcriptomes compared with their wild-type controls. The data have been lodged with the European Bioinformatics Institute ArrayExpress (see Material and methods). It was concluded that HL-responsive genes expressed in major leaf tissues, for example the mesophyll, were not influenced by the same ABA-signalling pathways shown to affect APX2 expression in BSCs.
(d). Does abscisic acid have any role to play in the immediate response of leaves to high light?
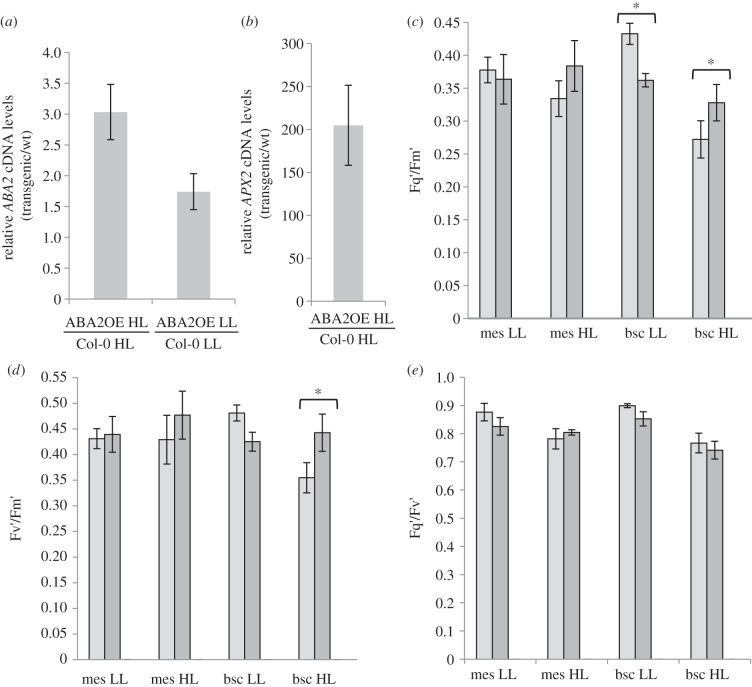
The above lack of any major effect of HL-associated ABA-signalling pathways on whole leaf responses contrasts with 816 genes identified from published data as commonly responsive to ABA and HL treatments [3]. However, these data were from plants treated with 10–100 µM ABA. We reasoned that this may have overestimated the number of ABA-regulated genes in HL-exposed leaves in which the levels only double [3]. Plants that overexpress the short-chain dehydrogenase gene ABA2, which codes for a key enzyme of ABA biosynthesis, show twofold increased levels of ABA [25]. The plants overexpressed ABA2 under LL and HL conditions (figure 3a). Under HL conditions, APX2 was 200-fold more expressed than in Col-0 (figure 3b). BSCs of HL-exposed ABA2OE plants showed less inhibition of Fq′/Fm′ compared with neighbouring mesophyll cells (figure 3c; p ≤ 0.001) acting through Fv′/Fm′ (p ≤ 0.001; figure 3d) and no effect on Fq′/Fv′ (p > 0.001; figure 3e). In contrast, the exposure of leaves to HL from 200 to 1400 µmol m−2 s−1 revealed that the ABA2OE had no differences in any Cf parameter compared with Col-0 (see electronic supplementary material, figure S4). Microarray analysis of total leaf RNA from HL-exposed ABA2OE plants showed no significant differences in gene expression compared with HL wild-type plants. The data are available for inspection from ArrayExpress (see Material and methods). It was concluded that changes in ABA levels associated with HL exposure [3] do not impact significantly on the transcriptomes of major leaf tissues.
Figure 3.
APX2 and ABA2 expression and response of BSCs and mesophyll cells to HL in ABA2OE and Col-0 plants. (a) ABA2 cDNA levels in ABA2OE plants relative to Col-0 leaves from HL-exposed or LL plants normalized with respect to CYC expression (see legend figure 1a and Material and methods). Values are means (±s.e.) from four plants from two experiments (n = 8). (b) APX2 cDNA levels in ABA2OE plants relative to Col-0 normalized to CYC expression. Values are means (±s.e.) from four plants from three experiments (n = 12). (c) Fq′/Fm′ values (mean ± s.e.; n = 18) for mesophyll (mes) and BSCs (bsc) of Col-0 (light grey bars) and ABA2OE (dark grey bars) plants subjected HL and LL as in the legend of figure 2. (d), as for (c), but Fv′/Fm′ values. (e), as for (c), but Fq′/Fv′ values. Pairs of columns marked with an asterisk (*) denote a significant difference (p ≤ 0.001) as in figure 2.
4. Discussion
(a). APX2 is controlled by two distinct but interacting ABA-signalling routes
Positive regulation of APX2 expression by ABA is achieved by signalling primarily involving OST1 (SnRK2.6) and the PP2C isoform ABI2 (figure 1a–c). Of the remaining four foliar PP2C isoforms, HAB1, HAB2 and PP2CA were ruled out as regulators of APX2 expression (figure 1a). However, the role of ABI1 is more ambiguous. The abi1-1 and abi2-1 mutations are in the phosphatase catalytic site (G180D and G168D, respectively), which causes reduced enzyme activity with a standard heterologous substrate [15]. However, genetically this causes a hypermorphic phenotype [12,32] often resulting in quantitatively indistinguishable characteristics associated with abi1-1 and abi2-1, and consequently many functions of ABA they affect [15], including APX2 expression (figure 1b) [5]. This degree of redundancy in the phenotype of abi1-1 and abi2-1 was not reflected in the null mutant abi1-2 and abi2-2 alleles in which there would have been an absence of the respective PP2C isoform (figure 1a and table 1) [14]. Thus from considering APX2 expression in abi2-1 and abi2-2 (figure 1a,b), ABI2 can clearly be determined to regulate the expression of this gene. However, abi2-2/abi1-2/hab1-1 showed markedly more stimulation of HL-responsive APX2 expression than abi2-2 (figure 1a), which suggests that ABI1 acts in a secondary role to, or cooperates with, ABI2 to regulate APX2 expression in BSCs.
In a number of different experimental systems probing the role of ABA, the interaction of class A PP2Cs with SnRK2 class III isoforms shows a high degree of overlap [13,31]. By contrast, OST1 (SnRK2.6) retains a dominant role in HL-induced positive control of APX2 expression and may be primarily confined to an interaction with ABI2 in this context. This could be because the ABI2:OST1 interaction is BSC-specific in HL-exposed leaves and has to respond to small changes in ABA levels [3]. That an ABI2:OST1 combination may dominate the positive regulation of APX2 may also reflect a more prominent role of H2O2 in modulating ABA signalling in BSCs than in other leaf tissues. ABI2 reversibly reacts, via redox-active cysteine residues, with H2O2, inhibiting its activity [33]. In vivo, this redox regulation of ABI2 may be conveyed by oxidized GPX3 [22]. However, no effect of gpx3-1 on APX2 expression was observed (data not shown). Nevertheless, it remains possible that another BSC-specific GPX isoform could fulfil a redox transduction role to ABI2 in this tissue. A putative reversible oxidation of ABI2 in BSCs is attractive because it would augment or amplify the regulation by ABA [13], thus enhancing a signalling response to HL despite a possible small increase in basal ABA levels [3]. In support of this suggestion, treatment of LL-grown leaves with 10 µM ABA, in the absence of any increase in H2O2, takes several hours to induce APX2 expression [4].
There was a clear loss of APX2 induction in HL-exposed rbohF leaves (figure 1d). One substrate of OST1 is RBOHF [34], which might connect plasma membrane-sourced ROS production to the OST1:ABI2 signalling pathway in BSCs. In rbohD and rbhohD/rbohF leaves, extracellular H2O2 production was diminished (figure 1e). This suggests that the increased ROS production may arise from RBOHD and that in wild-type plants the enzyme is negatively regulated by RBOHF in veinal tissue. This suggestion is consistent with a recent model showing that RBOHF negatively regulates RBOHD through salicylic acid [35]. Furthermore, the connection of G-protein signalling into NADPH oxidase-sourced ROS production [16,19,20] suggests a way in which the GPA1- and OST1-mediated signalling pathways can be distinct but interact to achieve a response of BSCs to HL (figure 1c).
(b). Bundle sheath cell chloroplasts are more susceptible to photoinhibition than mesophyll chloroplasts under high light conditions
HL-exposed BSCs have lower PSII operating efficiency (Fq′/Fm′) than mesophyll cells (figure 2a,b; p < 0.001; Student's t-test). The lowered maximum PSII operating efficiency (Fv′/Fm′) of BSC chloroplasts in HL compared with LL conditions (figure 2c) suggests that increased non-photochemical quenching [29] but not photochemical quenching capacity (Fq′/Fv′; figure 2e) occurred. Nevertheless, these changes in non-photochemical quenching were not sufficient to prevent photoinhibition in HL-exposed BSCs compared with mesophyll cells (figure 2a,b). This suggests that BSC chloroplasts suffered photoinhibition in HL, whereas mesophyll chloroplasts did not. Arabidopsis leaves are classified as ‘moderately heterobaric’ [36] i.e. have some limitations to the lateral diffusion of CO2 through to BSCs, which may also offer some resistance to inward CO2 diffusion [36]. Therefore, under HL conditions restricted photosynthetic capacity in BSCs could promote photoinhibition caused by increased singlet oxygen (1O2) production in PSII antennae, while still not being sufficient to promote a significant alteration in photochemical quenching. This situation would quickly lead to the production of other ROS. For example, the reaction of 1O2 with the high amount of ascorbate in the chloroplast produces H2O2 under physiologically relevant conditions [37]. This would explain the increased H2O2 observed specifically in HL-exposed BSC chloroplasts [3,4], despite this HL exposure not promoting substantial photoinhibition in the rest of the leaf [3,4,6].
(c). Abscisic acid signalling modulates photoinhibition in high light-exposed bundle sheath cells
The reduction in PSII operating efficiency in the BSC chloroplasts of some ABA-signalling mutants in HL was less, such that the distinction between mesophyll and BSCs was lost (figure 2a; p > 0.001; Student's t-test). There was an association of altered PSII operating efficiency of BSCs in HL (figure 2a) to stomatal responses to HL in the mutants (table 1). However, this was not as evident in mesophyll cells of the same mutants (figure 2b). Thus in HL, guard cells are influenced by ABA signalling but it is difficult to discern how this might influence BSC responses. It has been proposed that a transitory drop in leaf turgor in HL caused by increased stomatal conductance would induce ABA biosynthesis in the vascular parenchyma [3,4]. In which case, mutants with restricted stomatal opening in response to HL (table 1) would result in less induction of APX2 and less protection from photoinhibition. This did not occur (figures 1–3). Alternatively, lateral CO2 diffusion to BSCs would be even more restricted in some of these mutants and ABA2OE plants, which should have increased susceptibility to photoinhibition in their BSCs. Manifestly this situation did not arise (figures 2a and 3c). Thus, these factors are unlikely to explain the mutants' reduced susceptibility to photoinhibition of BSCs or the effects upon APX2 expression.
Those ABA-signalling mutants that show reduced differences in photoinhibition between mesophyll and BSCs (figures 2b and 3c) were associated with altered APX2 expression (figures 1a–c, 3b). However, the loss of APX2 expression in apx2-1 and apx2-2 did not affect responses to HL (see electronic supplementary material, figure S2). This may reflect that APX2 is a single component of an extensive BSC antioxidant network, which ABA and H2O2 may regulate at multiple points. It should be noted that, to our knowledge, the antioxidant network of BSCs of C3 plants, for example Arabidopsis, is unknown, with APX2 being the only known specific component [3,5].
Finally, it should be noted that the ABA signalling primarily influenced non-photochemical quenching (figures 2c and 3d), although how this was achieved is not clear from this study. An impact on antioxidant processes that decreased susceptibility of BSCs to photoinhibition may have been expected to be reflected in altered photochemical quenching [3,29]. This may explain the effect of gpa1-4 on this parameter (figure 1d) but no other ABA-signalling mutant was affected (figures 1d and 3e). Therefore, the impact on non-photochemical quenching suggests strongly that ABA signalling in HL-exposed BSCs plays roles beyond direct regulation of an antioxidant network and supports a more extensive response to HL than has hitherto been envisaged.
Acknowledgements
The authors are grateful to Drs Pedro Rodriguez, Julian Schroeder, Wan-Hsing Cheng, Jian-Kang Zhu, Miguel Torres, Jeffrey Leung, Alan Jones and Nottingham Arabidopsis Stock Centre for providing the mutants used in this study.
Funding statement
K.S. and L.M. acknowledge the support of a BBSRC and NERC research studentship, respectively. S.K. and M.G. acknowledge the support of the Welcome2008/1 programme operated within the framework of the Foundation for Polish Science, co-financed by the European Regional Development Fund and by the REGPOT project FP7-286093 WULS-PLANT HEALTH.
References
- 1.Mullineaux PM, Baker NR. 2010. Oxidative stress: antagonistic signaling for acclimation or cell death? Plant Physiol. 154, 521–525 (doi:10.1104/pp.110.161406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kangasjärvi S, Nurmi M, Tikkanen M, Aro E-M. 2009. Cell-specific mechanisms and systemic signaling as emerging themes in light acclimation of C3 plants. Plant Cell Environ. 32, 1230–1240 (doi:10.1111/j.1365-3040.2009.01982.x) [DOI] [PubMed] [Google Scholar]
- 3.Galvez-Valdivieso G, et al. 2009. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell. 21, 2143–2162 (doi:10.1105/tpc.108.061507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR. 2003. Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 33, 691–705 (doi:10.1046/j.1365-313X.2003.01656.x) [DOI] [PubMed] [Google Scholar]
- 5.Ball L, et al. 2004. Evidence for a direct link between glutathione biosynthesis and stress defence gene expression in Arabidopsis. Plant Cell 16, 2448–2462 (doi:10.1105/tpc.104.022608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bechtold U, Richard O, Zamboni A, Gapper C, Geisler M, Pogson B, Karpinski S, Mullineaux PM. 2008. Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis. J. Exp. Bot. 59, 121–133 (doi:10.1093/jxb/erm289) [DOI] [PubMed] [Google Scholar]
- 7.Endo A, et al. 2008. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 147, 1984–1993 (doi:10.1104/pp.108.116632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux PM. 1999. Systemic signalling and acclimation in response to excess excitation energy in Arabidopsis. Science 284, 654–657 (doi:10.1126/science.284.5414.654) [DOI] [PubMed] [Google Scholar]
- 9.Jung H-S, Crisp PA, Estavillo GM, Cole B, Hong F, Mockler TC, Pogson BJ, Chory J. 2013. Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc. Natl Acad. Sci. USA 110, 14 474–14 479 (doi/10.1073/pnas.1311632110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. 2002. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14, 3089–3099 (doi:10.1105/tpc.007906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii H, Zhu J-K. 2009. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl Acad. Sci. USA 106, 8380–8385 (doi:10.1073/pnas.0903144106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlad F, et al. 2008. Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21, 3170–3184 (doi:10.1105/tpc.109.069179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. 2010. Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61, 651–679 (doi:10.1146/annurev-arplant-042809-112122) [DOI] [PubMed] [Google Scholar]
- 14.Rubio S, et al. 2009. Triple loss of function protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiol. 150, 1345–1355 (doi:10.1104/pp.109.137174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung J, Merlot S, Giraudat J. 1997. The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9, 759–771 (doi:10.1105/tpc.9.5.759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K. 2002. The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc. Natl Acad. Sci. USA 99, 13 307–13 312 (doi:10.1073/pnas.192244099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres MA, Dangl JL, Jones JDG. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl Acad. Sci. USA 99, 517–522 (doi:10.1073/pnas.012452499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak JM, et al. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633 (doi:10.1093/emboj/cdg277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra G, Zhang W, Deng F, Zhao J, Wang X. 2006. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312, 264–266 (doi:10.1126/science.1123769) [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, et al. 2009. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis . Plant Cell. 21, 2357–2377 (doi:10.1105/tpc.108.062992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullineaux PM, Karpinski S, Baker NR. 2006. Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol. 141, 346–350 (doi:10.1104/pp.106.078162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao Y, Lv D, Wang P, Wang X-C, Chen J, Miao C, Song C-P. 2006. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 18, 2749–2766 (doi:10.1105/tpc.106.044230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saez A, Apostolova N, Gonzalez-Guzman M, Gonzalez-Garcia MP, Nicola C, Lorenzo O, Rodriguez PL. 2004. Gain-of-function and loss-of-function phenotypes of the protein phosphatase 2C HAB1 reveal its role as a negative regulator of abscisic acid signalling. Plant J. 37, 354–369 (doi:10.1046/j.1365-313X.2003.01966.x) [DOI] [PubMed] [Google Scholar]
- 24.Jones AM, Ecker JR, Chen JG. 2003. A re-evaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol. 131, 1623–1627 (doi:10.1104/pp.102.017624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin P-C, Hwang S-G, Endo A, Okamoto M, Koshiba T, Cheng W-H. 2007. Ectopic expression of ABSCISIC ACID 2/GLUCOSE INSENSITIVE 1 in Arabidopsis promotes seed dormancy and stress tolerance. Plant Physiol. 143, 745–758 (doi:10.1104/pp.106.084103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szechynska-Hebda M, Kruk J, Gorecka M, Karpinska B, Karpinski S. 2010. Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell. 22, 2201–2218 (doi:10.1105/tpc.109.069302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larkindale J, Vierling E. 2007. Core genome responses involved in acclimation to high temperature. Plant Physiol. 146, 748–761 (doi:10.1104/pp.107.112060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bechtold U, Lawson T, Mejia-Carranza J, Meyer RC, Brown IR, Altmann T, Ton J, Mullineaux PM. 2010. Constitutive salicylic acid defences do not compromise seed yield, drought tolerance and water productivity in the Arabidopsis accession C24. Plant Cell Environ. 33, 1959–1973 (doi:10.1111/j.1365-3040.2010.02198.x) [DOI] [PubMed] [Google Scholar]
- 29.Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113 (doi:10.1146/annurev.arplant.59.032607.092759) [DOI] [PubMed] [Google Scholar]
- 30.Lawson T, Lefebvre S, Baker NR, Morison JIL, Raines CA. 2008. Reductions in mesophyll and guard cell photosynthesis impact on the control of stomatal responses to light and CO2. J. Exp. Bot. 59, 3609–3619 (doi:10.1093/jxb/ern211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Sinozaki K. 2009. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 106, 17 588–17 593 (doi:10.1073/pnas.0907095106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moes D, Himmelbach A, Korte A, Haberer G, Grill E. 2008. Nuclear localization of the mutant protein phosphatase abi1 is required for insensitivity towards ABA responses in Arabidopsis. Plant J. 54, 806–819 (doi:10.1111/j.1365-313X.2008.03454.x) [DOI] [PubMed] [Google Scholar]
- 33.Meinhard M, Rodriguez PL, Grill E. 2002. The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 214, 775–782 (doi:10.1007/s00425-001-0675-3) [DOI] [PubMed] [Google Scholar]
- 34.Sirichandra C, et al. 2009. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583, 2982–2986 (doi:10.1016/j.febslet.2009.08.033) [DOI] [PubMed] [Google Scholar]
- 35.Chaouch S, Queval G, Noctor G. 2012. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 69, 613–627 (doi:10.1111/j.1365-313X.2011.04816.x) [DOI] [PubMed] [Google Scholar]
- 36.Morison JIL, Lawson T. 2007. Does lateral gas diffusion in leaves matter? Plant Cell Environ. 30, 1072–1085 (doi:10.1111/j.1365-3040.2007.01685.x) [DOI] [PubMed] [Google Scholar]
- 37.Kramarenko GG, Hummel SG, Martin SM, Buettner GR. 2006. Ascorbate reacts with singlet oxygen to produce hydrogen peroxide. Photochem. Photobiol. 82, 1634–1637 (doi:10.1562/2006-01-12-RN-774) [DOI] [PMC free article] [PubMed] [Google Scholar]