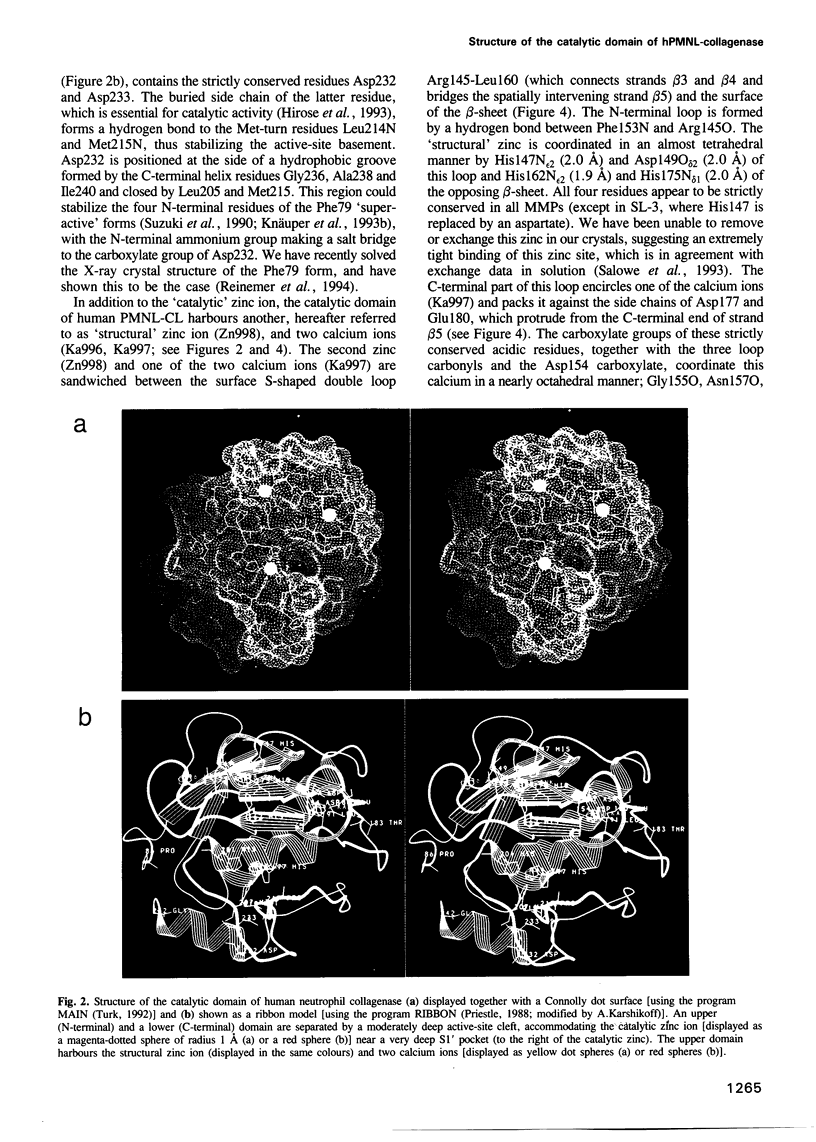
Abstract
Matrix metalloproteinases are a family of zinc endopeptidases involved in tissue remodelling. They have been implicated in various disease processes including tumour invasion and joint destruction. These enzymes consist of several domains, which are responsible for latency, catalysis and substrate recognition. Human neutrophil collagenase (PMNL-CL, MMP-8) represents one of the two 'interstitial' collagenases that cleave triple helical collagens types I, II and III. Its 163 residue catalytic domain (Met80 to Gly242) has been expressed in Escherichia coli and crystallized as a non-covalent complex with the inhibitor Pro-Leu-Gly-hydroxylamine. The 2.0 A crystal structure reveals a spherical molecule with a shallow active-site cleft separating a smaller C-terminal subdomain from a bigger N-terminal domain, composed of a five-stranded beta-sheet, two alpha-helices, and bridging loops. The inhibitor mimics the unprimed (P1-P3) residues of a substrate; primed (P1'-P3') peptide substrate residues should bind in an extended conformation, with the bulky P1' side-chain fitting into the deep hydrophobic S1' subsite. Modelling experiments with collagen show that the scissile strand of triple-helical collagen must be freed to fit the subsites. The catalytic zinc ion is situated at the bottom of the active-site cleft and is penta-coordinated by three histidines and by both hydroxamic acid oxygens of the inhibitor. In addition to the catalytic zinc, the catalytic domain harbours a second, non-exchangeable zinc ion and two calcium ions, which are packed against the top of the beta-sheet and presumably function to stabilize the catalytic domain.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann U., Wu S., Flaherty K. M., McKay D. B. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993 Sep;12(9):3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bläser J., Knäuper V., Osthues A., Reinke H., Tschesche H. Mercurial activation of human polymorphonuclear leucocyte procollagenase. Eur J Biochem. 1991 Dec 18;202(3):1223–1230. doi: 10.1111/j.1432-1033.1991.tb16494.x. [DOI] [PubMed] [Google Scholar]
- Bode W., Gomis-Rüth F. X., Huber R., Zwilling R., Stöcker W. Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. Nature. 1992 Jul 9;358(6382):164–167. doi: 10.1038/358164a0. [DOI] [PubMed] [Google Scholar]
- Bode W., Gomis-Rüth F. X., Stöckler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the 'metzincins'. FEBS Lett. 1993 Sep 27;331(1-2):134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- Fraser R. D., MacRae T. P., Suzuki E. Chain conformation in the collagen molecule. J Mol Biol. 1979 Apr 15;129(3):463–481. doi: 10.1016/0022-2836(79)90507-2. [DOI] [PubMed] [Google Scholar]
- Gomis-Rüth F. X., Kress L. F., Bode W. First structure of a snake venom metalloproteinase: a prototype for matrix metalloproteinases/collagenases. EMBO J. 1993 Nov;12(11):4151–4157. doi: 10.1002/j.1460-2075.1993.tb06099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Rüth F. X., Stöcker W., Huber R., Zwilling R., Bode W. Refined 1.8 A X-ray crystal structure of astacin, a zinc-endopeptidase from the crayfish Astacus astacus L. Structure determination, refinement, molecular structure and comparison with thermolysin. J Mol Biol. 1993 Feb 20;229(4):945–968. doi: 10.1006/jmbi.1993.1098. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Secreted forms of human neutrophil collagenase. J Biol Chem. 1986 Apr 25;261(12):5645–5650. [PubMed] [Google Scholar]
- Hasty K. A., Jeffrey J. J., Hibbs M. S., Welgus H. G. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987 Jul 25;262(21):10048–10052. [PubMed] [Google Scholar]
- Hasty K. A., Pourmotabbed T. F., Goldberg G. I., Thompson J. P., Spinella D. G., Stevens R. M., Mainardi C. L. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem. 1990 Jul 15;265(20):11421–11424. [PubMed] [Google Scholar]
- Hirose T., Patterson C., Pourmotabbed T., Mainardi C. L., Hasty K. A. Structure-function relationship of human neutrophil collagenase: identification of regions responsible for substrate specificity and general proteinase activity. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2569–2573. doi: 10.1073/pnas.90.7.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. A., Matthews B. W. Binding of hydroxamic acid inhibitors to crystalline thermolysin suggests a pentacoordinate zinc intermediate in catalysis. Biochemistry. 1981 Nov 24;20(24):6912–6920. doi: 10.1021/bi00527a026. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Knäuper V., Krämer S., Reinke H., Tschesche H. Characterization and activation of procollagenase from human polymorphonuclear leucocytes. N-terminal sequence determination of the proenzyme and various proteolytically activated forms. Eur J Biochem. 1990 Apr 30;189(2):295–300. doi: 10.1111/j.1432-1033.1990.tb15489.x. [DOI] [PubMed] [Google Scholar]
- Knäuper V., Osthues A., DeClerck Y. A., Langley K. E., Bläser J., Tschesche H. Fragmentation of human polymorphonuclear-leucocyte collagenase. Biochem J. 1993 May 1;291(Pt 3):847–854. doi: 10.1042/bj2910847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knäuper V., Wilhelm S. M., Seperack P. K., DeClerck Y. A., Langley K. E., Osthues A., Tschesche H. Direct activation of human neutrophil procollagenase by recombinant stromelysin. Biochem J. 1993 Oct 15;295(Pt 2):581–586. doi: 10.1042/bj2950581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallya S. K., Mookhtiar K. A., Gao Y., Brew K., Dioszegi M., Birkedal-Hansen H., Van Wart H. E. Characterization of 58-kilodalton human neutrophil collagenase: comparison with human fibroblast collagenase. Biochemistry. 1990 Nov 27;29(47):10628–10634. doi: 10.1021/bi00499a008. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Moore W. M., Spilburg C. A. Purification of human collagenases with a hydroxamic acid affinity column. Biochemistry. 1986 Sep 9;25(18):5189–5195. doi: 10.1021/bi00366a031. [DOI] [PubMed] [Google Scholar]
- Murphy G., Allan J. A., Willenbrock F., Cockett M. I., O'Connell J. P., Docherty A. J. The role of the C-terminal domain in collagenase and stromelysin specificity. J Biol Chem. 1992 May 15;267(14):9612–9618. [PubMed] [Google Scholar]
- Murphy G., Docherty A. J. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992 Aug;7(2):120–125. doi: 10.1165/ajrcmb/7.2.120. [DOI] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Bretz U., Baggiolini M. Collagenase is a component of the specific granules of human neutrophil leucocytes. Biochem J. 1977 Jan 15;162(1):195–197. doi: 10.1042/bj1620195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Enghild J. J., Suzuki K., Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl)mercuric acetate. Biochemistry. 1990 Jun 19;29(24):5783–5789. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S., Fields G. B., Birkedal-Hansen H., Van Wart H. E., Fields G. Sequence specificities of human fibroblast and neutrophil collagenases. J Biol Chem. 1991 Apr 15;266(11):6747–6755. [PubMed] [Google Scholar]
- Odake S., Okayama T., Obata M., Morikawa T., Hattori S., Hori H., Nagai Y. Vertebrate collagenase inhibitor. II. Tetrapeptidyl hydroxamic acids. Chem Pharm Bull (Tokyo) 1991 Jun;39(6):1489–1494. doi: 10.1248/cpb.39.1489. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Grams F., Huber R., Kleine T., Schnierer S., Piper M., Tschesche H., Bode W. Structural implications for the role of the N terminus in the 'superactivation' of collagenases. A crystallographic study. FEBS Lett. 1994 Jan 31;338(2):227–233. doi: 10.1016/0014-5793(94)80370-6. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. A comparison of the heme binding pocket in globins and cytochrome b5. J Biol Chem. 1975 Sep 25;250(18):7525–7532. [PubMed] [Google Scholar]
- Salowe S. P., Marcy A. I., Cuca G. C., Smith C. K., Kopka I. E., Hagmann W. K., Hermes J. D. Characterization of zinc-binding sites in human stromelysin-1: stoichiometry of the catalytic domain and identification of a cysteine ligand in the proenzyme. Biochemistry. 1992 May 19;31(19):4535–4540. doi: 10.1021/bi00134a001. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez R., Alexander C. M., Behrendtsen O., Breathnach R., Werb Z. Role of zinc-binding- and hemopexin domain-encoded sequences in the substrate specificity of collagenase and stromelysin-2 as revealed by chimeric proteins. J Biol Chem. 1993 Apr 5;268(10):7238–7247. [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schnierer S., Kleine T., Gote T., Hillemann A., Knäuper V., Tschesche H. The recombinant catalytic domain of human neutrophil collagenase lacks type I collagen substrate specificity. Biochem Biophys Res Commun. 1993 Mar 15;191(2):319–326. doi: 10.1006/bbrc.1993.1220. [DOI] [PubMed] [Google Scholar]
- Springman E. B., Angleton E. L., Birkedal-Hansen H., Van Wart H. E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a "cysteine switch" mechanism for activation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Enghild J. J., Morodomi T., Salvesen G., Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry. 1990 Nov 6;29(44):10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Wang D., Bode W., Huber R. Bovine chymotrypsinogen A X-ray crystal structure analysis and refinement of a new crystal form at 1.8 A resolution. J Mol Biol. 1985 Oct 5;185(3):595–624. doi: 10.1016/0022-2836(85)90074-9. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Wu H., Byrne M. H., Stacey A., Goldring M. B., Birkhead J. R., Jaenisch R., Krane S. M. Generation of collagenase-resistant collagen by site-directed mutagenesis of murine pro alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5888–5892. doi: 10.1073/pnas.87.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonath A., Traub W. Polymers of tripeptides as collagen models. IV. Structure analysis of poly(L-proly-glycyl-L-proline). J Mol Biol. 1969 Aug 14;43(3):461–477. doi: 10.1016/0022-2836(69)90352-0. [DOI] [PubMed] [Google Scholar]