Abstract
Kleptoplasty is a remarkable type of photosynthetic association, resulting from the maintenance of functional chloroplasts—the ‘kleptoplasts’—in the tissues of a non-photosynthetic host. It represents a biologically unique condition for chloroplast and photosynthesis functioning, occurring in different phylogenetic lineages, namely dinoflagellates, ciliates, foraminiferans and, most interestingly, a single taxon of metazoans, the sacoglossan sea slugs. In the case of sea slugs, chloroplasts from macroalgae are often maintained as intracellular organelles in cells of these marine gastropods, structurally intact and photosynthetically competent for extended periods of time. Kleptoplasty has long attracted interest owing to the longevity of functional kleptoplasts in the absence of the original algal nucleus and the limited number of proteins encoded by the chloroplast genome. This review updates the state-of-the-art on kleptoplast photophysiology, focusing on the comparative analysis of the responses to light of the chloroplasts when in their original, macroalgal cells, and when sequestered in animal cells and functioning as kleptoplasts. It covers fundamental but ecologically relevant aspects of kleptoplast light responses, such as the occurrence of photoacclimation in hospite, operation of photoprotective processes and susceptibility to photoinhibition. Emphasis is given to host-mediated processes unique to kleptoplastic associations, reviewing current hypotheses on behavioural photoprotection and host-mediated enhancement of photosynthetic performance, and identifying current gaps in sacoglossan kleptoplast photophysiology research.
Keywords: kleptoplasty, photoacclimation, photoprotection, photobehaviour, sacoglossan, macroalgae
1. Kleptoplasts
Present-day chloroplasts originated from an endosymbiotic process that started with the acquisition of a photosynthetic ancestral free-living cyanobacterium by a heterotrophic eukaryote, 1.2 billion years ago [1,2]. Despite this long and intimate coevolution, chloroplasts can be found today living intra-cellularly in organisms phylogenetically distant from the algal or plant cells in which they evolved. This is the case for chloroplasts from some macroalgal species that are ingested by animal herbivores but are not digested along with other cell components. The organelles resulting from this incomplete digestion, in this context termed ‘kleptoplasts’, remain structurally intact and photosynthetically competent for long periods, potentially for the entire lifespan of their new host [3,4]. Kleptoplasty is known to occur in a number of different phylogenetic lineages, such as dinoflagellates, ciliates, foraminiferans and metazoans [2,5]. The longest studied cases of kleptoplasty are those involving animals, all marine sacoglossan gastropod molluscs, most within the genus Elysia (figure 1). These sea slugs feed on macroalgae, either chlorophytes, rhodophytes or chromophytes, and sequester their chloroplasts in tubule cells of their digestive epithelium [3,6]. Despite the very small number of species known to participate in kleptoplasty, kleptoplastic organisms are not rare, and may even be cosmopolitan and form large populations, thus denoting a successful and well-adapted life form.
Figure 1.
The kleptoplast-bearing sacoglossan sea slugs (a) Elysia viridis, feeding on Codium tomentosum, and (b) Elysia chlorotica, feeding on Vaucheria litorea, and showing the opened parapodia (c,d, respectively). Courtesy of C. Brandão (a), A. Kharlamov (c), M. E. Rumpho and K. N. Pelletreau (b,d).
Kleptoplasty has raised considerable interest owing to the longevity of functional kleptoplasts in the absence of the original algal nucleus. In algae and plants, the processes required for chloroplasts to function are known to depend heavily on the continuous production, repair and replacement of plastid components, such as pigments, proteins and enzymes [7,8]. These processes involve intercompartmental crosstalk between the plastid and the nucleus, through both anterograde (nucleus-to-plastid) and retrograde (plastid-to-nucleus) signalling [9]. Furthermore, many of the photosynthetic proteins and enzymes required for general maintenance of the photosynthetic apparatus are nuclear-encoded and targeted to the chloroplast, with the expression of plastid-encoded genes also regulated by nuclear-encoded proteins [10]. Additionally, most of the metabolic machinery that equipped the ancestral cyanobacterium progenitor from which chloroplasts descended was lost during evolution inside their eukaryotic host, together with a large part of the original genome, which was transferred to the nucleus of the heterotrophic host cell [11]. Ironically, it is this ‘incomplete’ photosynthetic plastid that finds itself inside a new, unfamiliar host, for which evolution has not prepared it for—rather on the contrary. Modern chloroplasts may thus be expected to be largely unable to lead an autonomous life without the cell nucleus, making kleptoplasty in animal cells particularly intriguing as plastids and animal cells are phylogenetically unrelated and the latter were never photosynthetic [1].
Crosstalk between organelles is particularly important in the case of the chloroplast and nucleus, as frequent changes in the light environment require a continuous regulation of responses to maintain optimal photosynthetic function [10,12]. These include a range of processes involving short- and long-term photoacclimation and the efficient operation of photoprotective mechanisms against photoinhibition.
Here, we intend to update the state-of-the-art on kleptoplast photophysiology, centred on the comparative analysis of light responses of chloroplasts in their original macroalgal cells and when functioning as kleptoplasts, with emphasis on metazoan host-mediated processes affecting light exposure and photosynthetic performance.
2. Photoacclimation
Responses to changes in light environment primarily involve photoacclimation, phenotypic adjustments in various components of the photosynthetic apparatus to regulate excitation pressure, and maintaining carbon fixation while avoiding photo-oxidative stress. In eukaryotic algae, photoacclimation includes a range of processes such as changes in cellular content of photosynthetic reaction centres (RCI and RCII), photosynthetic and photoprotective pigments, photosynthetic unit number and size, photosystem II (PSII)/photosystem I stoichiometry, as well as protein degradation and de novo protein synthesis that regulate the amount of antennae proteins and the content and activity of RuBisCO and other enzymes [12].
The question of whether kleptoplasts are able to undergo photoacclimation in hospite is particularly relevant because several of the processes involved in photoacclimation require extensive expression of nuclear-encoded genes [2,4]. At present, available evidence is still based on a small number of associations but does not indicate the occurrence of photoacclimation in kleptoplasts. For the association Elysia viridis/Codium fragile, no differences in pigment content (normalized to chlorophyll a) were found between host and algal prey collected from the wild [13]. Also, for the case of E. viridis/Codium tomentosum, no changes in light-response curves (LC) of photosynthetic activity consistent with photoacclimation were observed when recently collected kleptoplast-bearing individuals were exposed to contrasting light regimes [14].
The absence of photoacclimation in kleptoplasts is also consistent with the strong association between LCs of relative electron transport rate (rETR) of algal food and corresponding kleptoplastic hosts. A compilation of all published results [13,15–17] and original data (S. Cruz, T. Santos, P. Cartaxana, R. Calado, J. Serôdio, unpublished; http://dx.doi.org/10.6084/m9.figshare.783888) showed that despite the large range in algal prey photoacclimation state (as denoted by the light-response curve photoacclimation parameter Ek, ranging from 28.7 to 356.0 µmol m−2 s−1), LC parameters of the macroalgae and kleptoplasts in their animal host cells were significantly correlated (figure 2). Significant correlations were found for parameters related to light-limited (α, given by the initial slope of the LC; figure 2a) and light-saturated photosynthesis (rETRm, the maximum rETR reached in the LC, figure 2b) as well as for Ek (figure 2c), indicating that a significant proportion of the variability of the light response of the kleptoplasts could be explained by the light response of the macroalgae's chloroplasts. The lowest Ek values were found for E. viridis and C. tomentosum, consistent with the low-light environment inside the macroalgae's dark fronds. By contrast, the highest Ek values were found for the light and optically thin Acetabularia acetabulum and its herbivore Elysia timida.
Figure 2.
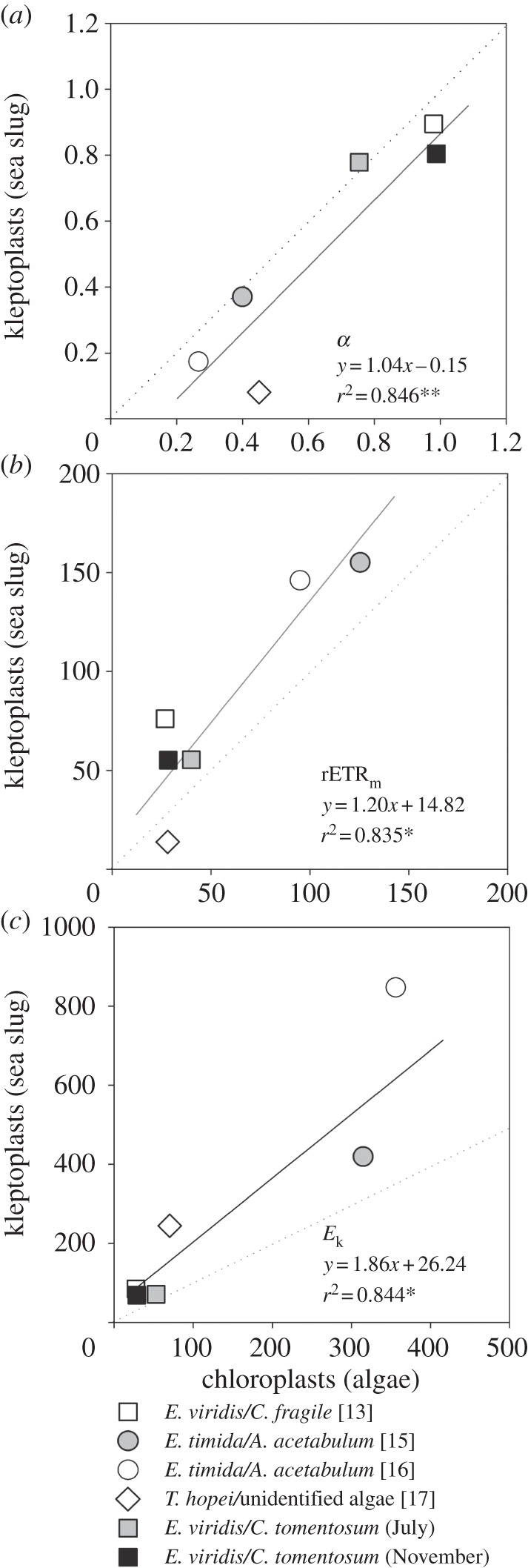
Linear relationship between published LC parameters (a) α, (b) rETRm and (c) Ek measured in chloroplasts (intact algae) and corresponding kleptoplasts (inside the animal host). Elysia viridis and E. timida are long-term retention species (functional kleptoplasts for more than 8 days), whereas Thuridilla hopei is a short-term retention species (kleptoplasts functional up to 8 days) [17]. The data for E. viridis/C. fragile [13] are the average of the values measured after the sea slugs filled their digestive cells with functional chloroplasts. Dotted lines indicate the 1 : 1 ratio. Specimens collected as in Cruz et al. [18], in July and November 2012. LCs measured and parameters estimated following Vieira et al. [14].
The association found between the LCs of the host and its algal food shown in this meta-analysis suggests that the kleptoplast may not undergo significant photoacclimation and that the kleptoplastic host's photoacclimation state essentially reflects the light environment of the ingested chloroplasts while inside the algal tissues. The absence of photoacclimation in kleptoplasts is consistent with their inability to synthesize crucial photosynthetic pigments, for example chlorophyll a, reported for various kleptoplastic associations [13,19]. However, in the case of Elysia chlorotica/Vaucheria litorea, and Elysia clarki/unidentified macroalgal species, kleptoplasts were shown to be capable of synthesizing chlorophylls and some chloroplast proteins [20–23]. While this remarkable capacity is likely to be relevant for the repair and replacement of pigments and proteins required for prolonging the functional status of kleptoplasts, it is uncertain whether this is enough to support changes in the photoacclimation state.
3. Host-mediated enhanced photosynthetic performance
Since its beginning, research on kleptoplast photosynthesis has predominantly focused on the provision of photosynthates to the host [24–26]. It seems that host animals receive a clear benefit from harbouring kleptoplasts, by obtaining a significant amount of carbon from photosynthesis (up to 60% of the carbon input was estimated to be derived from kleptoplast photosynthesis, for the Oxynoe viridis/Caulerpa longifolia association [25]). Furthermore, the assimilation of nitrogen was seen to be mediated by kleptoplasts, for E. viridis/C. fragile [27]. However, evidence is accumulating that the kleptoplastic association may also be transiently beneficial for the functioning of the sequestered plastid, despite their shortened lifespan. In fact, a number of recent studies have shown that the photosynthetic performance of kleptoplasts appears to be superior to corresponding chloroplasts within their original algal cells [13,15,16]. This agrees with the recognition that many non-photosynthetic hosts are able to enhance the photosynthetic release of non-kleptoplastic endosymbionts [5]. This tendency is also evident when analysing available datasets comparing fluorescence LC parameters measured in the host and the algae, summarized in figure 2. While no marked differences were found for light-limited photosynthesis (α; regression slope ≈ 1; figure 2a), most rETRm values (with the exception of Thuridilla hopei/unidentified algae) were significantly higher for the kleptoplast-bearing host than for the algal prey (slope > 1; figure 2b), supporting that the host somehow favours light-saturated photosynthesis of sequestered plastids.
In this context, it is important to note that the direct comparison of fluorescence data measured on sea slugs and macroalgae must be interpreted carefully. This is because fluorescence LCs measured on samples with such markedly different optical properties may be strongly affected by artefactual effects causing the overestimation of LC parameters independently of the inherent photophysiological response [28]. Depth-integration effects are illustrated in figure 3, comparing LCs measured on E. viridis and C. tomentosum when they are present (undisturbed specimens) and when they are minimized (‘inherent’ light response; macroalgae slurry and sea slug compressed in a thin concavity microscope slide). rETRm was found to reach considerably higher values (80.2%) for the sea slugs than for macroalgae. Of comparable relevance was the overestimation due to optical effects, with optically thin samples (i.e. inherent) displaying rETRm values of 32.7% and 27.5% lower for the macroalga and the sea slug, respectively.
Figure 3.
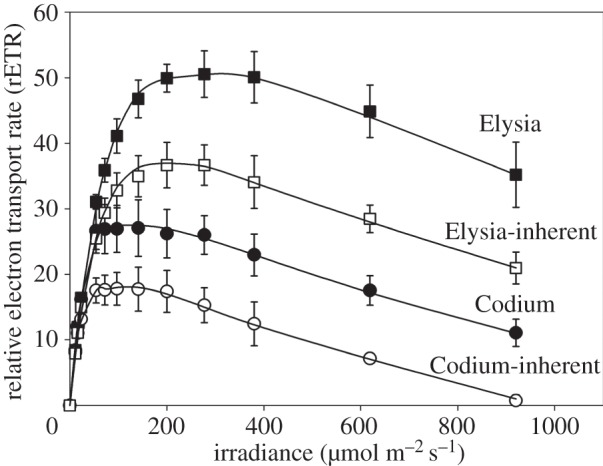
Host-mediated increase in photosynthetic performance in the association E. viridis/C. tomentosum and effects of depth integration of subsurface fluorescence. LCs of rETR as measured in intact (depth-integration effects present) E. viridis (‘Elysia’) and C. tomentosum (‘Codium’), and on corresponding optically thin samples (depth-integration effects minimized), E. viridis individuals placed in a 0.5 mm deep concavity microscope slide and covered by a coverslip, and a slurry of C. tomentosum (‘Elysia-inherent’ and ‘Codium-inherent’, respectively). Mean values of five replicates. Vertical bars represent 1 s.d. Specimens collected as in Cruz et al. [18]. LCs measured following Vieira et al. [14].
Higher photosynthetic performance of kleptoplasts has been tentatively attributed to their increased light exposure along the highly branched digestive gland of sacoglossan sea slugs [13] or to improved photoprotection provided by a combination of xanthophyll cycle operation and host behaviour [15]. Nonetheless, these hypotheses seem problematic: in the first case, because an increase in light availability can be expected to affect light-limited photosynthesis, but not, by definition, light-saturated photosynthetic rates; in the second case, because the activation of the xanthophyll cycle requires exposures to excess light in the time scale of tens of minutes [29,30], being thus largely irrelevant to explain changes in fluorescence LCs, as these record instantaneous photosynthetic activity, and during which actual exposure to high light is too short. On the other hand, no experimental evidence has thus far been provided to support that behavioural responses to high light have a photoprotective value (see below).
An alternative explanation for the enhanced photosynthetic performance of kleptoplasts is the likely intra-cellular supply of respired inorganic carbon and nutrients produced by the metabolism of the host. The importance of inorganic carbon and nutrient translocation from the heterotrophic host to the photosynthetic endosymbiont is well established in other marine animal–alga symbiotic associations, namely those between corals and dinoflagellates [31]. Enhancing effects on kleptoplast photosynthesis may thus be expected to occur in marine environments where inorganic carbon and nutrient availability is often limiting for algal photosynthesis [6]. This hypothesis is consistent with the observation that light-saturated (rETRm), not light-limited (α), photosynthesis shows the greatest increase in the kleptoplastic host, presumably owing to an insufficient supply of inorganic carbon, which is a main limiting factor of photosynthesis under light saturation. Another way the animal intra-cellular environment may enhance photosynthesis is through the inhibition of photorespiration. As the intensity of photorespiration depends on the CO2 to O2 ratio at the RuBisCO site [32], a decrease in photorespiration in kleptoplasts may be expected not only because more CO2 is made available but also owing to the higher consumption of O2 in the host animal cell through respiration.
4. Photoprotection and photoinhibition
Photoprotective mechanisms are necessary to deal with light energy absorbed in excess of that used by photochemical pathways and carbon fixation [29,30]. They are crucial in preventing the accumulation of reactive oxygen intermediates and minimizing photodamage to the photosynthetic apparatus [7]. For kleptoplasts, efficient photoprotection can be expected to be of major importance. On the one hand, plastids may be exposed to a new light environment, because of differing light attenuation properties of the host's tissues and its active motility in high light habitats, to which they may not be acclimated. On the other hand, damage caused by inefficient photoprotection may be harder to overcome and even aggravated in the absence of a fully functional PSII protein repair system. Recovery from photoinhibition relies heavily on repair and de novo synthesis of PSII proteins, for example the subunit D1, as damage from reactive oxygen species (ROS) occurs not only through direct inactivation of the PSII proteins, but mostly by inhibiting the repair of photodamaged components [7]. While synthesis of D1 and other PSII proteins were observed in kleptoplasts of the E. chlorotica/V. litorea association [21], this outstanding capacity does not seem to be widespread. For E. viridis/C. tomentosum, exposure to moderate light levels of only 140 µmol m−2 s−1 were found to cause a major decrease in kleptoplast longevity (on average, from 30.8 to 10.2 days), consistent with light dose-dependent photoinhibition [14]. Despite its importance, little is known on the operation of photoprotective mechanisms in kleptoplasts (reviewed in [28]). The xanthophyll cycle is the main photoprotective mechanism in many photoautotrophs, including algae of various groups [29], and should be a natural topic for kleptoplasty research. Although the presence of xanthophyll cycle pigments in kleptoplasts has been demonstrated [13,16,17], its operation as a response to high light exposure has, thus far, only been shown for the E. timida/A. acetabulum association [15].
5. Host-mediated photoprotection?
Kleptoplastic sea slugs have long been reported to display behavioural responses to high light with the apparent purpose of minimizing light exposure. These include the contraction of the parapodia, wing-like body extensions filled with a large number of kleptoplasts [33], rolling over on their side [34] or negative phototactic movement by crawling away from the light source [35]. Probably inspired by the parallelism with chloroplast light avoidance movements in plants [36], these behavioural responses have long been interpreted as a form of photoregulation, a process through which the animal could actively regulate the amount of light incident on, and ultimately absorbed by, the kleptoplasts [15]. In particular, by altering the surface area of the body exposed to light, the host could reduce the average light absorption by kleptoplasts and limit photodamage, ultimately to its own benefit.
For this ‘behavioural photoprotection’ hypothesis to be supported, the following conditions have to be verified: (i) the light levels that trigger the behavioural responses correspond to excess levels, from the viewpoint of kleptoplast photosynthesis; (ii) the behavioural responses cause a significant decrease in light availability inside the host, to levels low enough to reduce photoinhibition; and (iii) photodamage is actually lower when the animal has the chance to undergo conformational changes. Although behavioural photoprotection has been propagated in the literature, the fact is that none of these conditions has ever been experimentally demonstrated [28]. In fact, a number of arguments may be put forward against this hypothesis: (i) the possibility that the host movements are unrelated to photosynthetic activity; (ii) the doubtful photoprotective effectiveness of closing the parapodia, when kleptoplasts are often distributed all over the animal body surface; and (iii) insufficient light attenuation due to self-shading within the host tissue.
6. Currents gaps: oxidative stress and reactive oxygen species signalling
Kleptoplasty is an exciting research topic, as it represents a naturally occurring biologically unique condition for chloroplast and photosynthesis functioning. While the first studies on kleptoplasts date from more than 40 years ago (coincidentally, focused on photosynthesis [24]), this research field is still in a relatively early stage of development when compared with other animal–alga symbioses, namely corals. Recent research has centred on the molecular basis explaining the plastid long-term maintenance in the absence of algal nuclei, with the controversial hypothesis of horizontal transfer of genes from the alga nucleus to the host having received considerable attention (mostly using the association E. chlorotica/V. litorea as a biological model [37]). Most recent studies on kleptoplast photophysiology have focused on chloroplast longevity (reviewed in [28]), while fundamental photosynthetic processes that are well known in many other groups are still poorly understood in kleptoplasts.
An important topic that has been overlooked in kleptoplast research concerns ROS and photo-oxidative stress. ROS production in the chloroplast and associated photoinhibitory effects occur in all photosynthetic organisms and are very active fields of research in plant biology [38]. ROS are unavoidably generated by photosystem activity in the chloroplast thylakoids, having the PSII D1 protein as the immediate target [7]. Photo-oxidative damage builds up under high light when photoproduction of ROS exceeds the capacity of the scavenging/antioxidant network [39]. These processes appear especially relevant in the case of kleptoplasty as ROS may diffuse from the generation site and leach from the kleptoplast, coming in direct contact with animal cell cytoplasm and other organelles.
The translocation of ROS from the kleptoplast to the host cell opens up the possibility for the hypothesis of an ROS-mediated crosstalk between the algal plastid and the animal cell nucleus. ROS are known to play an important role as signalling molecules in plants and algae [30]. This is the case for H2O2, which is known to act as an intra-cellular and systemic (long-range, across cells or tissues) signalling molecule in response to changes in light exposure [40]. On the other hand, ROS are one of the factors involved in plastid signalling, a type of chloroplast-to-nucleus retrograde signalling by which molecules originating from the plastid affect nuclear gene expression [9]. It is conceivable that ROS induced by excess light can be sensed by the host and trigger protective countermeasures, such as the activation of the animal cell scavenging/antioxidant system or negative phototaxis.
Acknowledgements
We thank Mary E. Rumpho and Karen N. Pelletreau, Cláudio Brandão and Alexander Kharlamov for photographs of sacoglossa. We thank Tânia Santos for help in field and laboratory work. We thank two anonymous reviewers for their comments on the manuscript.
Funding statement
This work was supported by the Fundação para a Ciência e a Tecnologia, through grant no. SFRH/BPD/74531/2010 and by the FP7 Marie Curie Career Integration grant no. PCIG11-GA-2012-322349 (S.C.).
References
- 1.Raven JA, Beardall J, Flynn KJ, Maberly SC. 2009. Phagotrophy in the origins of photosynthesis in eukaryotes and as a complementary mode of nutrition in phototrophs: relation to Darwin's insectivorous plants. J. Exp. Bot. 60, 3975–3987. ( 10.1093/jxb/erp282) [DOI] [PubMed] [Google Scholar]
- 2.Dorell RG, Howe CJ. 2012. What makes a chloroplast? Reconstructing the establishment of photosynthetic symbioses. J. Cell Sci. 125, 1865–1875. ( 10.1242/jcs.102285) [DOI] [PubMed] [Google Scholar]
- 3.Rumpho ME, Dastoor FP, Manhart JR, Lee J. 2007. The kleptoplast. In Advances in photosynthesis and respiration: the structure and function of plastids (eds Wise RR, Hoober JK.), pp. 451–473. New York, NY: Springer. [Google Scholar]
- 4.Pierce SK, Curtis NE. 2012. Cell biology of the chloroplast symbiosis in sacoglossan sea slugs. Int. Rev. Cell Mol. Biol. 293, 123–148. ( 10.1016/B978-0-12-394304-0.00009-9) [DOI] [PubMed] [Google Scholar]
- 5.Johnson MD. 2011. The acquisition of phototrophy: adaptive strategies of hosting endosymbionts and organelles. Photosynth. Res. 107, 117–132. ( 10.1007/s11120-010-9546-8) [DOI] [PubMed] [Google Scholar]
- 6.Yellowlees D, Rees TAV, Leggat W. 2008. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 31, 679–694. ( 10.1111/j.1365-3040.2008.01802.x) [DOI] [PubMed] [Google Scholar]
- 7.Nishiyama Y, Allakhverdiev SI, Murata N. 2006. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 1757, 742–749. ( 10.1016/j.bbabio.2006.05.013) [DOI] [PubMed] [Google Scholar]
- 8.Eberhard S, Finazzi G, Wollman F-A. 2008. The dynamics of photosynthesis. Annu. Rev. Genet. 42, 463–515. ( 10.1146/annurev.genet.42.110807.091452) [DOI] [PubMed] [Google Scholar]
- 9.Kleine T, Voigt C, Leister D. 2009. Plastid signalling to the nucleus: messengers still lost in the mists? Trends Genet. 25, 185–192. ( 10.1016/j.tig.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 10.Toyoshima Y, Onda Y, Shiina T, Nakahira Y. 2005. Plastid transcription in higher plants. Crit. Rev. Plant Sci. 24, 59–81. ( 10.1080/07352680590910438) [DOI] [Google Scholar]
- 11.Martin W, Herrmann RG. 1998. Gene transfer from organelles to nucleus: how much, what happens, and why? Plant Physiol. 118, 9–17. ( 10.1104/pp.118.1.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suggett DJ, Le Floc'H E, Harris GN, Leonardos N, Geider RJ. 2007. Different strategies of photoacclimation by two strains of Emiliania huxleyi (Haptophyta). J. Phycol. 43, 1209–1222. ( 10.1111/j.1529-8817.2007.00406.x) [DOI] [Google Scholar]
- 13.Evertsen J, Johnsen G. 2009. In vivo and in vitro differences in chloroplast functionality in the two north Atlantic sacoglossans (Gastropoda, Opisthobranchia) Placida dendritica and Elysia viridis. Mar. Biol. 156, 847–859. ( 10.1007/s00227-009-1128-y) [DOI] [Google Scholar]
- 14.Vieira S, Calado R, Coelho H, Serôdio J. 2009. Effects of light exposure on the retention of kleptoplastic photosynthetic activity in the sacoglossan mollusc Elysia viridis. Mar. Biol. 156, 1007–1020. ( 10.1007/s00227-009-1144-y) [DOI] [Google Scholar]
- 15.Jesus B, Ventura P, Calado G. 2010. Behaviour and a functional xanthophyll cycle enhance photo-regulation mechanisms in the solar-powered sea slug Elysia timida (Risso, 1818). J. Exp. Mar. Biol. Ecol. 395, 98–105. ( 10.1016/j.jembe.2010.08.021) [DOI] [Google Scholar]
- 16.Costa J, Giménez-Casalduero F, Melo R, Jesus B. 2012. Colour morphotypes of Elysia timida (Sacoglossa, Gastropoda) are determined by light acclimation in food algae. Aquat. Biol. 17, 81–89. ( 10.3354/ab00446) [DOI] [Google Scholar]
- 17.Ventura P, Calado G, Jesus B. 2013. Photosynthetic efficiency and kleptoplast pigment diversity in the sea slug Thuridilla hopei (Vérany, 1853). J. Exp. Mar. Biol. Ecol. 441, 105–109. ( 10.1016/j.jembe.2013.01.022) [DOI] [Google Scholar]
- 18.Cruz S, Dionísio G, Rosa R, Calado R, Serôdio J. 2012. Anesthetizing solar-powered sea slugs for photobiological studies. Biol. Bull. 223, 328–336. [DOI] [PubMed] [Google Scholar]
- 19.Trench RK, Smith DC. 1970. Synthesis of pigment in symbiotic chloroplasts. Nature 227, 196–197. ( 10.1038/227196a0) [DOI] [PubMed] [Google Scholar]
- 20.Pierce SK, Biron RW, Rumpho ME. 1996. Endosymbiotic chloroplasts in molluscan cells contain proteins synthesized after plastid capture. J. Exp. Biol. 199, 2323–2330. [DOI] [PubMed] [Google Scholar]
- 21.Green BJ, Li W, Manhart JR, Fox TC, Summer EJ, Kennedy RA, Pierce SK, Rumpho ME. 2000. Mollusc-algal chloroplast endosymbiosis. Photosynthesis, thylakoid protein maintenance, and chloroplast gene expression continue for many months in the absence of the algal nucleus. Plant Physiol. 124, 331–342. ( 10.1104/pp.124.1.331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce SK, Curtis NE, Schwartz JA. 2009. Chlorophyll a synthesis by an animal using transferred algal nuclear genes. Symbiosis 49, 121–131. ( 10.1007/s13199-009-0044-8) [DOI] [Google Scholar]
- 23.Middlebrooks ML, Bell SS, Pierce SK. 2012. The kleptoplastic sea slug Elysia clarki prolongs photosynthesis by synthesizing chlorophyll a and b. Symbiosis 57, 127–132. ( 10.1007/s13199-012-0187-x) [DOI] [Google Scholar]
- 24.Trench RK, Boyle JE, Smith DC. 1973. The association between chloroplasts of Codium fragile and the mollusc Elysia viridis II: chloroplast ultrastructure and photosynthetic carbon fixation in Elysia viridis. Proc. R. Soc. Lond. B 184, 63–81. ( 10.1098/rspb.1973.0031) [DOI] [Google Scholar]
- 25.Raven JA, Walker DI, Jensen KR, Handley LL, Scrimgeour CM, Mcinroy SG. 2001. What fraction of the organic carbon in sacoglossans is obtained from photosynthesis by kleptoplastids? An investigation using the natural abundance of stable carbon isotopes and the values compared with those obtained previously. Mar. Biol. 138, 537–545. ( 10.1007/s002270000488) [DOI] [Google Scholar]
- 26.Maeda T, et al. 2012. Algivore or phototroph? Plakobranchus ocellatus (Gastropoda) continuously acquires kleptoplasts and nutrition from multiple algal species in nature. PLoS ONE 7, e42024 ( 10.1371/journal.pone.0042024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teugels B, Bouillon S, Veuger B, Middelburg JJ, Koedam N. 2008. Kleptoplasts mediate nitrogen acquisition in the sea slug Elysia viridis. Aquat. Biol. 4, 15–21. ( 10.3354/ab00092) [DOI] [Google Scholar]
- 28.Cruz S, Calado R, Serôdio J, Cartaxana P. 2013. Crawling leaves: photosynthesis in sacoglossan sea slugs. J. Exp. Bot. 64, 3999–4009. ( 10.1093/jxb/ert197) [DOI] [PubMed] [Google Scholar]
- 29.Goss R, Jakob T. 2010. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 106, 103–122. ( 10.1007/s11120-010-9536-x) [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Wakao S, Fischer BB, Niyogi KK. 2009. Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260. ( 10.1146/annurev.arplant.58.032806.103844) [DOI] [PubMed] [Google Scholar]
- 31.Furla P, Galgani I, Durand I, Allemand D. 2000. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203, 3445–3457. [DOI] [PubMed] [Google Scholar]
- 32.Beardall J, Quigg A, Raven JA. 2003. Oxygen consumption: photorespiration and chlororespiration. In Photosynthesis in algae (eds Larkum A, Douglas SE, Raven JA.), pp. 157–181. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 33.Schmitt V, Wägele H. 2011. Behavioral adaptations in relation to long-term retention of endosymbiotic chloroplasts in the sea slug Elysia timida (Opisthobranchia, Sacoglossa). Thalassas 27, 225–238. [Google Scholar]
- 34.Händeler K, Grzymbowski YP, Krug PJ, Wägele H. 2009. Functional chloroplasts in metazoan cells: a unique strategy in animal life. Front. Zool. 18, 1–18. ( 10.1186/1742-9994-6-28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallop A, Bartrop J, Smith DC. 1980. The biology of chloroplast acquisition by Elysia viridis. Proc. R. Soc. Lond. B 207, 335–349. ( 10.1098/rspb.1980.0027) [DOI] [Google Scholar]
- 36.Park Y-I, Chow WS, Anderson JM. 1996. Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol. 11, 867–875. ( 10.1104/pp.111.3.867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharya D, Pelletreau KN, Price DC, Sarver KE, Rumpho ME. 2013. Genome analysis of Elysia chlorotica egg DNA provides no evidence for horizontal gene transfer into the germ line of this kleptoplastic mollusc. Mol. Biol. Evol. 30, 1843–1852. ( 10.1093/molbev/mst084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foyer CH, Shigeoka S. 2011. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100. ( 10.1104/pp.110.166181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullineaux PM, Baker NR. 2010. Oxidative stress: antagonistic signaling for acclimation or cell death? Plant Physiol. 154, 521–525. ( 10.1104/pp.110.161406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullineaux PM, Karpinski S, Baker NR. 2006. Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol. 141, 346–350. ( 10.1104/pp.106.078162) [DOI] [PMC free article] [PubMed] [Google Scholar]



