Abstract
Providing an adequate quantity and quality of food for the escalating human population under changing climatic conditions is currently a great challenge. In outdoor cultures, sunlight provides energy (through photosynthesis) for photosynthetic organisms. They also use light quality to sense and respond to their environment. To increase the production capacity, controlled growing systems using artificial lighting have been taken into consideration. Recent development of light-emitting diode (LED) technologies presents an enormous potential for improving plant growth and making systems more sustainable. This review uses selected examples to show how LED can mimic natural light to ensure the growth and development of photosynthetic organisms, and how changes in intensity and wavelength can manipulate the plant metabolism with the aim to produce functionalized foods.
Keywords: controlled-environment agriculture, light-emitting diode, metabolism, microalgae, photosynthesis
1. Introduction
The rising population, climate changes, land use competition for food, feed, fuel and fibre production as well as the increasing demand for valuable natural compounds all reinforce the need for artificial growing systems such as greenhouses, soilless systems and vertical gardening, even in spacecrafts and space stations. Most of these growing systems require the application of additional, at least supplementary, light sources to ensure plant growth. Because these sources are heat dissipaters requiring cooling, artificial systems are frequently at odds with the demand for sustainability in industrial processes. In terms of both economics and sustainability, new lighting technologies such as light-emitting diodes (LEDs) thus were necessary to be developed [1,2]. Above all technological properties, LEDs should be compatible with the photosynthesis and light-signalling requirements of plants, which are tightly linked with the two main characteristics of light: wavelength and fluence.
Being mostly immobile, photosynthetic organisms must adapt to their biotic and abiotic environments that they sense through different types of receptors, including photoreceptors [3]. The pigment moiety of photoreceptors allows the receptor to extract from the incoming natural white light the specific information related to the intensity of the environmental light constraints. This information is used to develop the adequate response [3].
Photosynthesis is a photobiochemical process using light energy to produce ATP and NADPH, ultimately consumed in the assembly of carbon atoms in organic molecules. Functionally, photons are harvested by protein–chlorophyll (Chl)–carotenoid complexes (that form the light harvesting antenna of photosystems) and then transferred to the photosystem reaction centre, where electrons are generated; these processes take place in the chloroplast [4]. If lighting is too weak, photosynthesis cannot work efficiently and etiolation symptoms appear [5]. However, excessive light generates oxygen radicals and causes photoinhibition. Both phenomena strongly limit primary productivity [6].
Photosynthetic processes are often modified in plants grown under artificial lighting, because lamps do not usually mimic the spectrum and energy of sunlight. Agronomically, new lighting technologies such as LEDs have the potential to cover fluence and wavelength requirements of plants, while allowing specific wavelengths to be enriched, thus supplying the light quantity and quality essential for different phases of growth. The biomass and metabolic products of cultivated plants can therefore be modified.
This review gives a brief summary of the types of artificial lighting available for growing photosynthetic organisms. The capacity of LEDs to mimic the effects of natural light in terms of energy and information, thus ensuring the growth and development of photosynthetic organisms, and the potential for manipulating the plant metabolism to produce functionalized foods through changes in the intensity and wavelength are also reviewed here using selected examples.
2. Artificial light sources for photosynthesis
Artificial lighting should provide plants with energy and information required for development. For this purpose, fluorescent lamps, particularly those having enhanced blue and red spectra (i.e. cool fluorescent white lamps), are widely used in growth chambers, together with additional light sources to achieve the sustained photosynthetic photon fluence necessary for high productivity [1,7]. However, the spectrum and intensity of fluorescent lights are not stable over a long time (see the comparative information in the electronic supplementary material, table S1).
High intensity discharge (HID) lamps, such as metal halide and high-pressure sodium lamps, have relatively high fluence (max. 200 lumens per watt) and high photosynthetically active radiations (PARs) efficiency (max. 40%), and are typically used in greenhouses and plant growth rooms. The drawbacks including elevated arc to fire energy requirement, the high operational temperature preventing placement close to the canopy and the spectral distribution (high proportion of green–yellow region, significant ultraviolet radiation and altered red : far red ratio), which may shift according to the input power, strongly limit their use and innovation [8]. Among artificial lighting systems, LEDs present the maximum PAR efficiency (80–100%; see the electronic supplementary material, table S1). LEDs emitting blue, green, yellow, orange, red and far red are available and can be combined to provide either high fluence (over full sunlight, if desired), or special light wavelength characteristics, thanks to their narrow-bandwidth light spectrum [9]. The high efficiency, low operating temperature and small size enable LEDs to be used in pulsed lighting and be placed close to the leaves in interlighting and intracanopy irradiation [7]. Their long life expectancy and ease of control make them ideal for greenhouses in use all year round [7]. The LED technology is predicted to replace fluorescent and HID lamps in horticultural systems and to revolutionize controlled growth environments.
3. Changing light intensity and quality
From the biological point of view, the main questions about LEDs are related to their ability to mimic and enhance the beneficial effects of natural light while avoiding the adverse influence. Below, selected examples are used to provide a short review on useful properties of LED lights in these aspects.
(a). Light-emitting diode light(s) can sustain normal plant growth
Pioneer experiments on plant growth under red LEDs on lettuce were reported by Bula et al. [9]. Martineau et al. [8] calculated that the amounts of dry matter per mole of artificial lighting gained by lettuce grown using red (650 nm) LEDs or high-pressure sodium lamps were identical, and Chang et al. [10] calculated that the maximum photon utilization efficiency for growth of the green alga Chlamydomonas reinhardtii under red LEDs is centred at 674 nm. Lettuce grown under red LEDs presented hypocotyls and cotyledons that were elongated, a phenomenon known to be phytochrome-dependent. Under red LEDs illumination, phytochrome stimulation is especially high as far red light is not provided. Hypocotyl elongation could be prevented by adding at least 15 µmol m–2 s−1 of blue light [11]. Although a complete demonstration was not provided, one can hypothesize that the supplemented blue light activated cryptochrome, a blue-light photoreceptor that mediates reduction of hypocotyl length [12].
The efficiency of red (650–665 nm) LEDs on plant growth is easy to understand because these wavelengths perfectly fit with the absorption peak of chlorophylls [13] and phytochrome, while the supplemented blue light introduced the idea that growth under natural light could be mimicked using blue and red LEDs. In addition to providing a better excitation of the different types of photoreceptors, the blue + red combination allowed a higher photosynthetic activity than that under either monochromatic light [14]. Some authors attributed this effect to a higher nitrogen content of the blue-light-supplemented plants, whereas others suggested a better stomatal opening, thus providing more CO2 for photosynthesis. It is well established that stomata opening is controlled by blue-light photoreceptors [15]. This is possibly reflected in the increase of shoot dry matter with increasing levels of blue light [16]. The supplementation of blue + red LEDs could also be complemented with green LEDs. Illumination with more than 50% of green LED light causes a reduction in plant growth, whereas treatments containing up to 24% green light enhanced growth for some species [17]. Recently, LEDs have been successfully tested for their ability to allow the growth of agronomically important crops, fruit and flower plants, and even trees [14,18]. Table 1 shows the parameter changes in selected taxa exposed to different wavelengths of LEDs compared with the other light sources.
Table 1.
The effects of LEDs on plants' growth parameters and metabolism compared with conventional lights: selected examples. HPS, high-pressure sodium; CFL, compact fluorescent light; PPFD, photosynthetic photon flux density; DW, dry weight; FW, fresh weight.
| taxa | parameter | LEDs value (bold)/wavelength (nm)/intensity (PPFD) | conventional (HPS, CFL) value (bold)/type/intensity (PPFD) | references |
|---|---|---|---|---|
| Lactuca sativa var. capital | dry mass (gmol−1 m−2) wet mass (gmol−1 m−2) | 0.45/650/319 7.21 | 0.46/HPS, Na/642 8.18 | [8] |
| Raphanus sativus var. Saxa | productivity (gcm−2 day−1) | 0.14/455 + 640 + 660 + 735/9 + 120 + 9.4 + 3 | 0.9/HPS/250 | [19] |
|
Cucumis sativus L. ‘Bodega’ Lycopersicon esculentum ‘trust’ |
fruit FW (g) DW (g) fruit DW (g) plant DW (g) |
976/HPS + 445/400 + 16 47.5 54.8 113 |
735/HPS, Na/510 34 39.15 136 |
[20] |
|
Dendranthema grandiflorum Kitam ‘Cheonsu’ plantlets |
plantlet growth: FW (mg per plantlet) net photosynthesis (Pn, μmol CO2 m−2 s−1) |
/440;650;440 + 650; 650 + 720/50 361;446;750;498 0.75;1.95;4.6;2.2 |
/CFL/50 713 3.4 |
[21] |
|
Lactuca sativa cv. Grand rapids Petroselinum crispum cv. Moss curled Majorana hortensis Moench. |
metabolite (mg g−1 FW): carbohydrates nitrates C vit (mg %) carbohydrates nitrates C vit carbohydrates nitrates C vit |
/640; 455 + 640 + 735/200 8;10 0.8;1.0 7;5 42.5;23 non-evaluable 145;140 13;12 0.6;0,5 19;19 |
/HPS, Son-T Agro/200 2 1.4 10 35 non-evaluable 130 8 1.25 20 |
[22] |
|
Brassica oleracea cv. ‘Winterbor’ |
lutein (mg 100 g–1 FM) glucosinolate (mg 100 g–1 DM) |
/730;640;525;440;400/253, 6.9;11.2;7.8;9.8;8.1 21.7;32.0;0.8;ND;ND |
not used | [23] |
|
Petunia hybrid cv. Mitchell diploid Fragaria x ananassa cv. Strawberry festival |
volatile molecules (nmol kg−1): benzylalcohol 2-phenylethanol phenylacetaldehyde methyl butyrate ethyl caproate |
/660;755/50 0.23;0.2 0.25;0.17 4.5;4.0 /455;660;755/50 1.8;2.1;3.0 ND;0.5;0.2 |
CFL/50 0.015; 0.02; 2; 1.8; 1.9 |
[24] |
| Panax ginseng | metabolites phenolic acids (μg g−1 DW): vanilic acid coumaric acid ferulic acid |
/465;630/24 41;27 314;186 586;313 |
CFL/24 0.33 76 319 |
[25] |
|
Mentha sp. M. spicata M. piperita M longifolia |
essential oil (% of DW) | /660;470/500 4.34;5.03 7.00;3.11 4.37;3.19 |
/sunlight/1800 0.66 1.40 3.33 |
[14] |
(b). Chloroplast differentiation and de-differentiation
In the absence of light or under deep shade conditions, plants develop etiolation symptoms, such as the absence of Chl, reduced leaf size and hypocotyl elongation [5]. When the plants are exposed to light, chloroplast differentiation involves the accumulation of proteins, lipids and photosynthetic pigments [26]. The kinetics of Chl accumulation present a lag phase under white LED light, which is eliminated when plants are grown under blue LED (460–475 nm) but not in red LED light (650–665 nm) [27]. Interestingly, similar Chl amounts were reached, regardless of the LED colour. In contrast to Chl, red LED-irradiated pea leaves contained higher levels of β-carotene than those grown under blue or white LED light [27]. The light intensity is also important in Chl synthesis. For instance, Tripathy & Brown [28] showed that wheat seedlings accumulated Chl under red LED light at 100 µmol m–2 s–1, but not at 500 µmol m–2 s–1. This inhibition of Chl accumulation under high fluence red LED light could be avoided by the supplementation of blue light (30 µmol m–2 s–1). Although no demonstration of the effect was provided by the authors, the absence of Chl accumulation under high fluence red light could result from a fast photodestruction of the newly formed Chl molecules [29]. Interestingly, re-etiolation provides adequate conditions for the production of white asparagus, chicory or seakale [30]. In tea leaves, the re-etiolation increases the content of volatiles (aroma), especially volatile phenylpropanoids/benzenoids and several amino acids, including l-phenylalanine [31], suggesting the activation of a plastid-located shikimate pathway [32].
(c). High fluence light-emitting diode triggers production of secondary compounds
Photosynthetic organisms exposed to high light develop short- and long-term response mechanisms to reduce stress effects. Some of these mechanisms are the specific topic of other papers included in this special issue (xanthophyll cycle [33], non-photochemical quenching [34], re-oxidation of the reduction equivalents through photorespiration, the malate valve and the action of antioxidants [35]). This section is dedicated to the metabolic shifts triggered by high light stress. They are used in repairing mechanisms [36], shielding [37], reactive oxygen species (ROS) quenching [37] or the production of storage compounds [38]. The synthesis of the metabolites takes place in plastids (terpenoids [38]) or involves them (phenylpropanoids [32]). Typical examples are medicinal plants and herbs of pharmaceutical importance such as mint (Mentha sp.) [14] and jewel orchid (Anoectohilus sp.) [39]. However, a decrease in secondary metabolites, flavonoids and phenolics, was also observed with increasing irradiance in the medicinal plant cat's whiskers (Orthosiphon stamineus) [40], indicating that the light irradiance may have negative consequences on secondary metabolite production. In higher plants, it has been documented that depending on species and growing conditions, the secondary metabolites and pigments in the flavonoid family accumulate under photoinhibitory conditions at cell level [41], although the mechanistic aspects of LED light effects are not well understood.
The high fluence effect of LED light has been studied more in photosynthetic microorganisms, partly because they present huge biotechnological and economic potential (biofuels, pharmaceuticals, food additives and cosmetics) [42]. For instance, Wang et al. [43] assessed the economic efficiency of energy converted to biomass in microalga (Spirulina platensis) culture under different LED monochromatic lights as grams of biomass per litre per dollar. The data showed that at the light intensity of 1500–3000 µmol m−2 s−1, red LEDs consumed the least power and yielded the highest economic efficiency when emitted at the same intensity compared with blue LEDs (up to 110 versus lower than 10 g per litre per dollar, respectively). However, such a high fluence is not always requested. For instance, in the green microalga Dunaliella salina, light stress to drive the accumulation of β-carotene was within the range of 170–255 µmol m−2 s−1 using LEDs, whereas 1000 µmol m−2 s−1 photon flux was needed using conventional lights such as fluorescent lamps and high-pressure sodium lamps [44]. Additional red or blue (470 nm) LED light caused stress whereby the xanthophyll cycle was activated. The additional blue light was less stressful than the red light [45]. Katsuda et al. [46] reported that red LED light allowed the growth of the green alga Haematococcus pluvialis, whereas blue LED light enhanced astaxanthin production. More recently, Katsuda et al. [47] showed that in mixotrophic growing conditions, flashing LED light (8 µmol photon m−2 s−1) triggered similar astaxanthin concentration to continuous LED light (12 µmol photon m−2 s−1). Such low light requirement suggests the involvement of photoreceptors. A putative transduction mechanism of the blue light signal would involve major carotenoids in D. salina. Signalling of secondary carotenoid synthesis involves chloroplast-generated ROS [37]. Much more investigation is needed to understand the impact of LED light on primary and secondary metabolism of photosynthetic organisms.
(d). Modification of the metabolism through supplemental monochromatic lighting
The effect of supplemental blue and/or red LED light is not limited to growing and developmental properties. They also increase the antioxidant content of vegetables. For instance, red (658–660 nm) LED light increased the phenolics concentration in lettuce leaves [48] and the anthocyanin content of red cabbage leaves [27]. One can therefore imagine designing supplemental LED light treatments as pre- or post-harvesting processes to fashion raw materials. This would provide great commercial and production advantages. For instance, Colquhoun et al. [24] used LED treatment to modify the synthesis of volatile compounds in flowers and fruits. In tomato, a red LED treatment (668 nm, 50 µmol photon m−2 s−1) triggered a significant increase of 2-methyl-butanol and 3-methyl-1-butanol levels, whereas the amount of cis-3-hexanol was reduced when compared with the levels reached with white LED light. Because two of those three compounds are involved in the degree of tomato sweetness [49], one can hypothesize that the LED treatment will impact the taste of the fruit. The mechanism of action of the monochromatic light has not been studied as yet, but one can assume that the red light affects terpenoid production in the chloroplast through phytochrome. Alternatively, specific ROS production could have the same action as shown in the case of secondary carotenoid synthesis [37].
4. Photosynthesis in the light of future advances
Food production relies on photosynthesis. Providing sufficient quantity and quality of food for nine billion people as predicted in 2050 is especially challenging under the constraints of global climate change. Controlled-environment agriculture (CEA) technologies, including greenhouse, hydroponics, aquacultures and aeroponic systems, as well as the vertical farming possibilities, provide alternative and complementary sources for crop production, particularly in areas with limited daylight (in northern latitudes) or adverse environmental conditions (droughts, floods, storms and saline soils) or in areas with limited space, such as cities and space stations [1,7].
The advantages of CEA technologies, i.e. elevated crop yield per year (owing to shorter culture period under optimal environmental conditions and cultivation year round), greater growth area per m2 (large plant density, multi-tier cultivation shelves), efficient nutrient and water use, fewer crop losses and no pesticide application, make them efficient for crop production. In addition, these technologies may produce standard high-quality horticultural products. However, in contrast to outdoor agriculture, closed and indoor plant cultivations rely on novel light sources such as LEDs capable of stimulating plant growth while drastically reducing energy consumption.
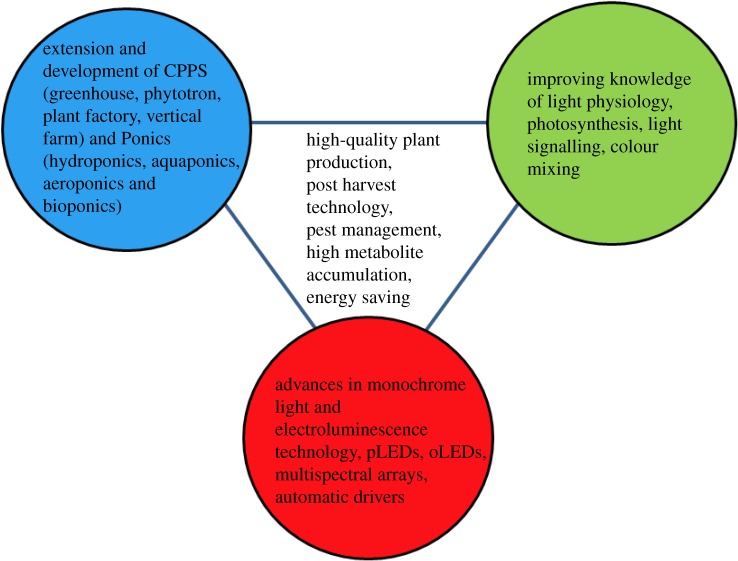
LEDs represent an innovative artificial lighting source for plants, both as supplemental or sole-source lighting, not only owing to their intensity, spectral and energy advances (see §2 and the electronic supplementary material, table S1), but also via the possibilities for targeted manipulation of metabolic responses in order to optimize plant productivity and quality. LEDs are now commercially applicable mainly for leafy greens, vegetables, herbs and pot flowers (table 1 and the electronic supplementary material, table S2). A more complete literature was also presented in the seventh International Symposium on Light in Horticultural Systems, held in Wageningen (http://www.actahort.org/books/956). The application of LEDs also has enormous potential for the processes that generate oxygen and purify water, in algal culture for producing feedstock, pharmaceuticals, fuels or dyes, and in plant tissue cultures for the micropropagation of, for example, strawberry or flowering plants [50,51]. Research on the effects of LEDs on primary and secondary metabolism of plants and on how the direction and mixing of LEDs influence plant responses, coupled with advances in the dynamic modification of light quantity and quality in different phases of growth may contribute to the efficient utilization of LED lighting technologies in plant cultivation in closed environments (figure 1).
Figure 1.
Trilateral connection of technological and physiological advances for improvement of plant production using LED lighting. CPPS, closed plant production systems; pLED, polymer light-emitting diode; oLED, organic light-emitting diode. (Online version in colour.)
The lighting industry needs to offer energy-efficient, ecologically sustainable lamps adapted to the changing requirements of consumers. LEDs equipped with driver chips could provide the additional benefits of operational flexibility, efficiency, reliability, controllability and intelligence for greenhouse lighting systems. However, the acceptance of solid-state LED lighting in niche applications in horticultural lighting will depend on improvements in conversion efficiency and light output per package of LED light and the cost of lumens per package. It is predicted that horticultural cultivation under controlled environmental conditions (horticulture industry) will expand in the near future, as was presented in the workshop on Challenges in Vertical Farming (http://challengesinverticalfarming.org/). The new technologies provide possibilities for economically efficient consumption of light energy for horticultural cultivation of crops both on Earth and in space in the near future, and may contribute to feeding the growing human population and maintaining outdoor (principally forest) ecosystems and thus to the protection of the Earth.
Acknowledgements
The authors acknowledge their institutes, Hungarian Academy of Sciences, University of Le Mans and Isfahan University of Technology. M.R.S. also thanks the Iranian National Elites Foundation for financial support of work on LED incubator construction and cultivation of horticultural and agronomic crops illuminated with LED lights.
Funding statement
E.D. appreciates partial financial support from TÁMOP (grant no. 4.2.2A-11/1/KONV-2012-0008).
References
- 1.Massa GD, Emmerich JC, Morrow RC, Bourget CM, Mitchell CA. 2006. Plant growth lighting for space life support: a review. Gravit. Space Biol. Bull. 19, 19–30. [Google Scholar]
- 2.Sheng CX, Singh S, Gambetta A, Drori T, Tong M, Tretiak S, Vardeny ZV. 2013. Ultrafast intersystem-crossing in platinum containing π-conjugated polymers with tunable spin–orbit coupling. Sci. Rep. 3, 2653 ( 10.1038/srep02653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng M, Chory J, Fankhauser C. 2004. Light signal transduction in higher plants. Annu. Rev. Genet. 38, 87–117. ( 10.1146/annurev.genet.38.072902.092259) [DOI] [PubMed] [Google Scholar]
- 4.Solymosi K, Keresztes A. 2012. Plastid structure, diversification and interconversions II. Land plants. Curr. Chem. Biol. 18, 187–204. ( 10.2174/2212796811206030003) [DOI] [Google Scholar]
- 5.Solymosi K, Schoefs B. 2010. Etioplast and etio-chloroplast formation under natural conditions: the dark side of chlorophyll biosynthesis in angiosperms. Photosynth. Res. 105, 143–166. ( 10.1007/s11120-010-9568-2) [DOI] [PubMed] [Google Scholar]
- 6.Barber J, Andersson B. 1992. Too much good thing: light can be bad for photosynthesis. Trends Biochem. Sci. 17, 61–66. ( 10.1016/0968-0004(92)90503-2) [DOI] [PubMed] [Google Scholar]
- 7.Yeh N, Chung JP. 2009. High-brightness LEDs: energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 13, 2175–2180. ( 10.1016/j.rser.2009.01.027) [DOI] [Google Scholar]
- 8.Martineau V, Lefsrud M, Naznin MT. 2012. Comparison of light-emitting diode and high-pressure sodium light treatments for hydroponics growth of Boston lettuce. HortScience 47, 477–482. [Google Scholar]
- 9.Bula RJ, Morrow RC, Tibbitts TW, Barta DJ, Ignatius RW, Martin TS. 1991. Light emitting diodes as a radiation source for plants. HortScience 26, 203–205. [PubMed] [Google Scholar]
- 10.Chang RL, et al. 2011. Metabolic network reconstruction of Chlamydomonas offers insight into light-driven algal metabolism. Mol. Syst. Biol. 7, 518 ( 10.1038/msb.2011.52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoenecke ME, Bula RJ, Tibbitts TW. 1992. Importance of ‘blue’ photon levels for lettuce seedlings grown under red-light-emitting diodes. HortScience 27, 427–430. [PubMed] [Google Scholar]
- 12.Ahmad M, Grancher N, Heil M, Blac RC, Giovani B, Galland P, Lardemer D. 2002. Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol. 129, 774–785. ( 10.1104/pp.010969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoefs B. 2002. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 13, 361–371. ( 10.1016/S0924-2244(02)00182-6) [DOI] [Google Scholar]
- 14.Sabzalian MR, Heydarizadeh P, Zahedi M, Boroomand A, Agharokh M, Sahba MR, Schoefs B. In press High performance of vegetables, flowers and medicinal plants in a red–blue LED incubator for indoor plant production. Agron. Sustain. Dev. ( 10.1007/s13593-014-0209-6) [DOI] [Google Scholar]
- 15.Schwartz A, Zeiger E. 1984. Metabolic energy for stomatal opening: roles of photophosphorylation and oxidative phosphorylation. Planta 161, 129–136. ( 10.1007/BF00395472) [DOI] [PubMed] [Google Scholar]
- 16.Goins GD, Yorio NC, Sanwo-Lewandowski MM, Brown CS. 1998. Life cycle experiments with Arabidopsis under red light-emitting diodes (LEDs). Life Support Biosph. Sci. 5, 143–149. [PubMed] [Google Scholar]
- 17.Kim HH, Wheeler RM, Sager JC, Goins GD, Norikane JH. 2006. Evaluation of lettuce growth using supplemental green light with red and blue light-emitting diodes in a controlled environment: a review of research at Kennedy Space Center. Acta Hort. 711, 111–119. [Google Scholar]
- 18.Astolfi S, Marianello C, Grego S, Bellarosa R. 2012. Preliminary investigation of LED lighting as growth light for seedlings from different tree species in growth chambers. Not. Bot. Horticult. Agrobot. 40, 31–38. [Google Scholar]
- 19.Tamulaitis G, Duchovskis P, Bliznikas Z, Breive K, Ulinskaite R, Brazaityte A, Novičkovas A, Žukauskas A. 2005. High-power light-emitting diode based facility for plant cultivation. J. Phys. D, Appl. Phys. 38, 3182–3187. ( 10.1088/0022-3727/38/17/020) [DOI] [Google Scholar]
- 20.Ménard C, Dorais M, Hovi T, Gosselin A. 2006. Developmental and physiological responses of tomato and cucumber to additional blue light. Acta Hort. (ISHS) 711, 291–296. [Google Scholar]
- 21.Kim SJ, Hahn EJ, Heo JW, Paek KY. 2004. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of Chrysanthemum plantlets in vitro. Sci. Horticult. 101, 143–151. ( 10.1016/j.scienta.2003.10.003) [DOI] [Google Scholar]
- 22.Urbonavičiuūtė A, Samuolienė G, Brazaitytė A, Ulinskaitė R, Jankauskienė J, Duchovskis P, Žukauskas A. 2008. The possibility to control the metabolism of green vegetables and sprouts using light emitting diode illumination. Sodininkyste ir Darz ininkyste 27, 83–92. [Google Scholar]
- 23.Lefsrud MG, Kopsell DA, Sams C. 2008. Irradiance from distinct wave-length light-emitting diodes affect secondary metabolites in kale. HortScience 43, 2243–2244. [Google Scholar]
- 24.Colquhoun TA, et al. 2013. Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biol. Tech. 86, 37–44. ( 10.1016/j.postharvbio.2013.06.013) [DOI] [Google Scholar]
- 25.Park SY, Lee JG, Cho HS, Seong ES, Kim HY, Yu CY, Kim JK. 2013. Metabolite profiling approach for assessing the effects of colored light-emitting diode lighting on the adventitious roots of ginseng (Panax ginseng C. A. Mayer). Plant Omics J. 6, 224–230. [Google Scholar]
- 26.Biswal UC, Biswal B, Raval MK. 2003. Chloroplast biogenesis: from proplastid to gerontoplast. Dordrecht, The Netherlands: Kluwer Academic Publisher (Springer). [Google Scholar]
- 27.Wua MC, Hou CY, Jiang CM, Wang YT, Wang CY, Chen HH, Chang HM. 2007. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 101, 1753–1758. ( 10.1016/j.foodchem.2006.02.010) [DOI] [Google Scholar]
- 28.Tripathy BC, Brown CS. 1995. Root–shoot interaction in the greening of wheat seedlings grown under red light. Plant Physiol. 107, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franck F, Schoefs B, Barthélemy X, Mysliwa-Kurdziel B, Strzalka K, Popovic R. 1995. Protection of native chlorophyll(ide) forms and of photosystem II against photodamage during early stages of chloroplast differentiation. Acta Physiol. Plant. 17, 123–132. [Google Scholar]
- 30.Péron JY. 1990. Seakale: a new vegetable produced as etiolated sprouts. In A dvances in new crops (eds Janick J, Simon JE.), pp. 419–422. Portland, OR: Timber Press. [Google Scholar]
- 31.Yang Z, et al. 2012. Characterization of volatile and non-volatile metabolites in etiolated leaves of tea (Camellia sinensis) plants in the dark. Food Chem. 135, 2268–2276. ( 10.1016/j.foodchem.2012.07.066) [DOI] [PubMed] [Google Scholar]
- 32.Brillouet JM, Romieu C, Schoefs B, Solymosi K, Cheynier V, Fulcrand H, Verdeil JL, Conéjéro G. 2013. The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta. Ann. Bot. 112, 1003–1014. ( 10.1093/aob/mct168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dall'Osto L, Cazzaniga S, Wada M, Bassi R. 2014. On the origin of a slowly reversible fluorescence decay component in the Arabidopsis npq4 mutant. Phil. Trans. R. Soc. B 369, 20130221 ( 10.1098/rstb.2013.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RohácŽek K, Bertrand M, Moreau B, Jacquette B, Caplat C, Morant-Manceau A, Schoefs B. 2014. Relaxation of the non-photochemical chlorophyll fluorescence quenching in diatoms: kinetics, components and mechanisms. Phil. Trans. R. Soc. B 369, 20130241 ( 10.1098/rstb.2013.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heyno E, Innocenti G, Lemaire SD, Issakidis-Bourguet E, Krieger-Liszkay A. 2014. Putative role of the malate valve enzyme NADP–malate dehydrogenase in H2O2 signalling in Arabidopsis. Phil. Trans. R. Soc. B 369, 20130228 ( 10.1098/rstb.2013.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long SP, Humphries S, Falkowski PG. 1994. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 633–662. ( 10.1146/annurev.pp.45.060194.003221) [DOI] [Google Scholar]
- 37.Lee DW, Gould KS. 2002. Why leaves turn red: pigments called anthocyanins probably protect leaves from light damage by direct shielding and by scavenging free radicals. Am. Sci. 90, 524–531. [Google Scholar]
- 38.Lemoine Y, Schoefs B. 2010. Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth. Res. 106, 155–177. ( 10.1007/s11120-010-9583-3) [DOI] [PubMed] [Google Scholar]
- 39.Ma Z, Sh L, Zhang M, Jiang S, Xiao Y. 2010. Light intensity affects growth, photosynthetic capacity, and total flavonoid accumulation of Anoectohilus plants. HortScience 45, 863–867. [Google Scholar]
- 40.Ibrahim MH, Jaafar HZ. 2012. Primary, secondary metabolites, H2O2, malondialdehyde and photosynthetic responses of Orthosiphon stimaneus Benth. to different irradiance levels. Molecules 17, 1159–1176. ( 10.3390/molecules17021159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan LK, Koay SS, Boey PL, Bhatt A. 2010. Effects of abiotic stress on biomass and anthocyanin production in cell cultures of Melastoma malabathricum. Biol. Res. 43, 127–135. ( 10.4067/S0716-97602010000100014) [DOI] [PubMed] [Google Scholar]
- 42.Mimouni V, Ulmann L, Pasquet V, Mathieu M, Picot L, Bougaran G, Cadoret J-P, Morant-Manceau A, Schoefs B. 2012. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 13, 2733–2750. ( 10.2174/138920112804724828) [DOI] [PubMed] [Google Scholar]
- 43.Wang CY, Fu CC, Liu YC. 2007. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 37, 21–25. ( 10.1016/j.bej.2007.03.004) [DOI] [Google Scholar]
- 44.Lamers PP, van de Laak CC, Kaasenbrood PS, Lorier J, Janssen M, De Vos RC, Bino RJ, Wijffels RH. 2010. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 106, 638–648. ( 10.1002/bit.22725) [DOI] [PubMed] [Google Scholar]
- 45.Fu W, Guðmundsson Ó, Paglia G, Herjólfsson G, ÓS Andrésson, Palsson B, Brynjólfsson S. 2013. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 97, 2395–2403. ( 10.1007/s00253-012-4502-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katsuda T, Lababpour A, Shimahara K, Katoh S. 2004. Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzyme Microb. Technol. 35, 81–86. ( 10.1016/j.enzmictec.2004.03.016) [DOI] [Google Scholar]
- 47.Katsuda T, Shiraishi H, Ishizu N, Ranjbar R, Katoh S. 2008. Effect of light intensity and frequency of flashing light from blue light emitting diodes on astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 105, 216–220. ( 10.1263/jbb.105.216) [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Kubota C. 2009. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Exp. Exp. Bot. 67, 59–64. ( 10.1016/j.envexpbot.2009.06.011) [DOI] [Google Scholar]
- 49.Tieman D, et al. 2012. The chemical interactions underlying tomato flavor preferences. Curr. Biol. 22, 1035–1039. ( 10.1016/j.cub.2012.04.016) [DOI] [PubMed] [Google Scholar]
- 50.Nhut DT, Takamura T, Watanabe H, Tanaka M. 2000. Light emitting diodes (LEDs) as a radiation source for micropropagation of strawberry. In Transplant production in the 21st century (eds Kubota C, Chun C.), pp. 114–118. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 51.Lian ML, Murthy HN, Paek KY. 2002. Effects of light emitting diodes (LEDs) on the in vitro induction and growth of bulblets of Lilium oriental hybrid ‘Pesaro’. Sci. Horticult. 94, 365–370. ( 10.1016/S0304-4238(01)00385-5) [DOI] [Google Scholar]