Abstract
This review focuses on feedback pathways that serve to match plant energy acquisition with plant energy utilization, and thereby aid in the optimization of chloroplast and whole-plant function in a given environment. First, the role of source–sink signalling in adjusting photosynthetic capacity (light harvesting, photochemistry and carbon fixation) to meet whole-plant carbohydrate demand is briefly reviewed. Contrasting overall outcomes, i.e. increased plant growth versus plant growth arrest, are described and related to respective contrasting environments that either do or do not present opportunities for plant growth. Next, new insights into chloroplast-generated oxidative signals, and their modulation by specific components of the chloroplast's photoprotective network, are reviewed with respect to their ability to block foliar phloem-loading complexes, and, thereby, affect both plant growth and plant biotic defences. Lastly, carbon export capacity is described as a newly identified tuning point that has been subjected to the evolution of differential responses in plant varieties (ecotypes) and species from different geographical origins with contrasting environmental challenges.
Keywords: phloem loading, photosynthesis, PsbS, source–sink, tocopherol, zeaxanthin
1. Introduction
Multiple pathways of communication, over both short and long distances, between the various functional parts of the plant are being elucidated. It appears self-evident that such communication pathways should enable the plant to respond appropriately to opportunities as well as challenges in its physical and biological environment. We place the communication between chloroplast and the whole plant into a context of the adjustment in form and function via physiological acclimation of individual plants to their growth environment as well as genetic adaptation of species and varieties (ecotypes) to the climate and other features of the habitats in which the respective lines evolved.
2. Matching energy acquisition with energy utilization: established and emerging signalling pathways serving to optimize chloroplast and whole-plant function in a given environment
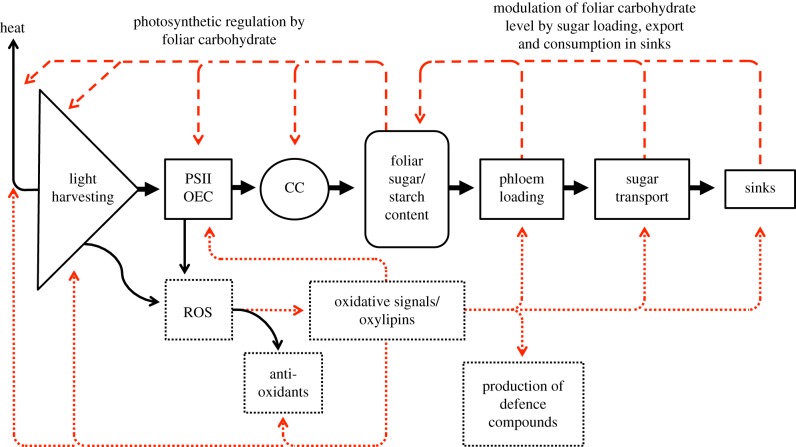
Here, we review the signalling pathways serving to match light availability, and energy acquisition in photosynthesis, with energy utilization for plant growth, development and defences—in the context of plant genetic make-up, developmental status and a wide range of environmental conditions. Figure 1 presents the principal functional sequence from solar energy acquisition and sugar production to sugar distribution throughout the plant (solid arrows), starting with light absorption in the light-harvesting complexes (light harvesting) and primary photochemistry (in photosystem II (PSII) and the oxygen-evolving complex (OEC)) supporting photosynthetic electron transport and CO2 fixation (in the Calvin cycle (CC)), followed by the loading of sugars into the phloem conduits and the transport of sugars to the plant's sinks (sugar-consuming and sugar-storing tissues). In addition, figure 1 features a host of feedback loops falling into two principal categories of either (i) (dashed arrows along the top) providing feedback from the plant's sugar-transport system and its sinks, via modulation of foliar sugar/starch content, back to light harvesting, photochemistry and CO2 fixation or (ii) (dotted arrows along the bottom) providing both feed-forward and feedback loops (dotted arrows along the bottom) associated with chloroplast redox balance (determined by the ratio, and action of, oxidants and antioxidants) as influenced by the environment and by the chloroplast's photoprotective network. In the following, we will examine examples of source- and sink-driven signalling networks, and will argue that both source- and sink-driven signals modulate phloem loading/transport, and thereby impact plant response to environmental stresses.
Figure 1.
Schematic depiction of principal steps involved in photosynthesis (light harvesting, photochemistry, involving photosystem II (PSII) and the oxygen-evolving complex (OEC) and carbon fixation in the Calvin cycle (CC)) and in sugar export (phloem loading and sugar transport) to the plant's sinks (solid arrows) as well as (dashed arrows) feedback loops from sugar export and the plant's sinks to photosynthesis and (dotted arrows) feed-forward and feedback loops related to the generation of reactive oxygen species (ROS) in light harvesting and photochemistry. Heat = heat released via thermal dissipation of excess excitation energy in light-harvesting antennae. (Online version in colour.)
(a). Sink-to-source signalling in photosynthetic adjustment
It has long been recognized that the leaf's capacity for photosynthesis is adjusted in response to the level of demand generated by the plant's sinks for the products of photosynthesis [1–3]. A plant's inherent growth rate, developmental status and the opportunity (or lack thereof) for plant growth presented in the environment all contribute to setting this ‘sink demand’ as the level of plant sink strength. For example, (i) short-lived annual species tend to have higher inherent growth rates than slowly growing evergreens, (ii) a plant in its reproductive state tends to have greater sink strength than a vegetative plant and (iii) soil conditions (e.g. water and nutrient availability, temperature and soil texture/structure), together with climatic features (e.g. light availability, temperature, humidity), cumulatively generate either plant growth-promoting or plant growth-impeding environmental conditions. It has been shown that regulation of foliar photosynthetic capacity occurs via modulation of the expression of photosynthetic genes involved in e.g. light harvesting, photochemistry and other thylakoid processes, and CO2 fixation (e.g. [4–6]). The negative feedback via limiting carbon export was established by studying photosynthetic repression in response to inhibition of carbon export from leaves via e.g. girdling of branches or trees (e.g. [7–9]) or cold-girdling of stems [1,5]; the negative feedback from limiting sink strength was demonstrated e.g. via photosynthetic repression in response to removal of fruit and other sinks (e.g. [7,10–12]). Foliar sugar and/or starch accumulation are typically observed under either limiting carbon export at the leaf level or limiting plant sink strength [13].
(b). Contrasting overall outcomes in environments with contrasting opportunities for plant growth: increased plant growth versus plant growth arrest
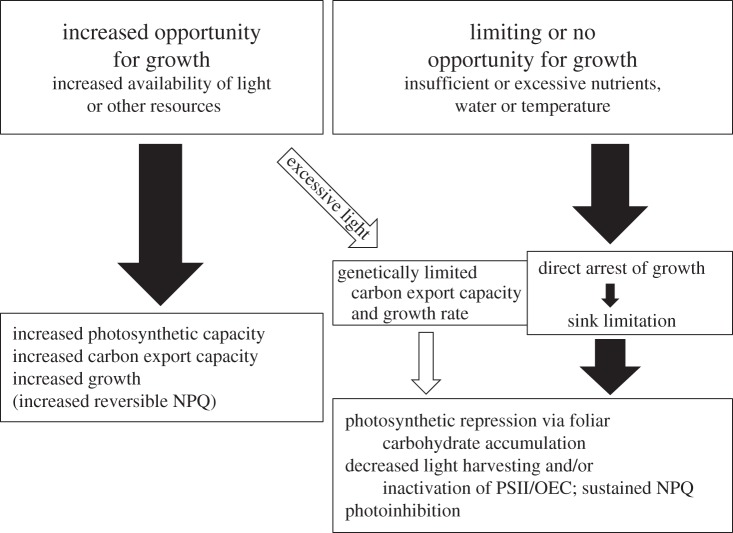
Figure 2 illustrates that two principal scenarios with opposite outcomes can be distinguished by examining the level of ‘opportunity’ for plant growth presented by the environment. We will illustrate these scenarios for the example of overall plant growth response to resource availability in the environment, while acknowledging that the same principles also apply to other more complex features, including temporal dynamics in the environment and in plant-organ-specific growth patterns. Plant response to opportunity presented by the environment presumably involves the feedback loops detailed in §2a and some of those discussed in §2c. On the one hand (figure 2, left-hand side), there is the scenario of an increase in the opportunity for plant growth presented by the environment. Such a scenario can arise via increased availability of previously limiting resources such as light, water and/or nutrients, a shift to a more favourable temperature regime, etc., experienced by a species with the appropriate genetic adaptations and developmental state allowing it to upregulate photosynthetic capacity, carbon export capacity (see §3) and growth rate. As an additional response to increased light availability, any plant not increasing its growth and photosynthesis rates enough to use all of the additional available light will probably upregulate its capacity for thermal dissipation of any excess of absorbed light [14,15] and/or employ a variety of mechanisms to decrease light interception, e.g. via changes in leaf angle or leaf reflectance [16–19], or decrease light absorption, via decreases in chlorophyll content and light-harvesting capacity (e.g. [20,21]).
Figure 2.
Schematic depiction of contrasting scenarios, representing increased, limiting or no opportunity for growth in the environment, and their respective effects on photosynthesis, carbon export, plant growth and the flexibility of thermal dissipation. NPQ, non-photochemical quenching on chlorophyll fluorescence, as an indicator for thermal dissipation of excess excitation energy; OEC, oxygen-evolving complex; PSII, photosystem II.
On the other hand (figure 2, right-hand side), limiting or no opportunity for plant growth is presented in environments with either limiting or excessive resources (like water, nutrients) or unfavourable temperatures. Such environmental conditions typically result in reduced growth or even growth arrest and a resulting decrease in plant sink strength [13]. Furthermore, in the latter environments, photosynthetic depression, with decreased photosynthetic capacity and light-harvesting capacity and/or inactivation of photochemistry (‘photoinhibition’), is seen [13,22,23].
While decreased photosynthetic capacity and light-harvesting capacity are features that have long been associated with photosynthetic repression under sink limitation (see above), the phenomenon of the ‘photoinhibition’ of photosynthesis—involving an inactivation of PSII and/or OEC—is commonly instead interpreted as a sign of ‘damage’ (e.g. [24–31]). We have recently summarized evidence showing that, in all cases in which photoinhibitory inactivation of photochemistry and foliar carbohydrate levels were both assessed, photoinhibition was found to be associated with greater accumulation of foliar sugar and/or starch [13]. Viewing such responses in the context of the whole plant in its environment thus offers opportunities for a more holistic interpretation of responses like the phenomenon of ‘photoinhibition’. The invariable association of photoinhibition with foliar carbohydrate accumulation in plants under field conditions suggests that the tacit assumption that photoinhibition limits plant productivity (e.g. [32–40]) should be re-assessed for the possibility that limiting plant productivity instead triggers photoinhibition [13]. Based on the above-described data, the possibility that inactivation of PSII and/or OEC is, in fact, a principal component (together with decreased light harvesting and CO2 fixation) of photosynthetic repression under certain sink limiting conditions should be seriously considered.
There is a third scenario (figure 2, middle), where light availability is suddenly increased for a plant that is not genetically adapted for, and/or finds itself in an environment not suitable for, increasing carbon export and/or plant sink strength. Such a scenario is not common in natural settings, but is a frequently used experimental approach to study photoinhibition in response to sudden transfer of shade-grown plants to high light. We have shown that sudden transfer of evergreens grown under non-fluctuating low light levels to highly excessive light results in photoinhibition of PSII and of photosynthetic capacity accompanied by foliar carbohydrate accumulation [13,41]. Especially in evergreens, which, as their name implies, do not commonly lower their chlorophyll content (or their light-harvesting capacity), photoinhibition is associated with a conversion of the otherwise rapidly reversible, flexible thermal dissipation of excess light (assessed from non-photochemical quenching (NPQ) of chlorophyll fluorescence) to a less flexible form of thermal dissipation (sustained NPQ) remaining continuously engaged for prolonged time periods [14,15,22,41–43]. An analogous scenario for annuals, as species presumably possessing sufficient carbon export capacity and sink strength to respond to increased light availability, is a sudden transfer from low to high growth light environment under limiting soil nitrogen supply, which results in photoinhibition as well [44].
(c). Oxidative signals and phloem blocking/occlusion as modulated by chloroplast photoprotection
In the lower portion of figure 1 (dotted arrows), selected examples are featured for the generation of oxidative signals associated with light-harvesting and primary photochemistry. In plants adapted and acclimated to environments where leaves absorb a greater level of light than they are able to use through electron transport, a large fraction of the resulting excess excitation energy is safely dissipated (as heat; figure 1) via thermal dissipation of excess excitation energy [23]. The low remaining levels of excitation energy in excess of what is used in electron transport lead to the formation of reactive oxygen species (ROS; figure 1). At low concentrations, ROS trigger formation of oxidative signals (figure 1), while massive ROS formation presumably leads to cellular damage [45,46]. In light-harvesting complexes, excess excitation energy promotes the formation of (electronically excited) singlet oxygen as a form of ROS, while charge separation in primary photochemistry can give rise to the formation of singly reduced superoxide as the second major form of ROS arising in the light reactions [47]. Chloroplast antioxidant defences (figure 1) de-excite, and thereby detoxify, both singlet oxygen and superoxide, but highly excessive light levels presumably allow remaining ROS to trigger multiple redox-associated signalling pathways. For selected reviews of various examples of the many different components of the redox-signalling network originating in the chloroplast, see the studies of Munné-Bosch et al. [48], Baginsky & Link [49], Dietz et al. [50], Foyer et al. [51] and Mullineaux et al. [52]. We will highlight a selected example of one such signalling pathway, leading to the lipid-peroxidation-derived oxylipin messengers (figure 1), that had previously been shown to be involved in the regulation of the biosynthesis of chloroplast antioxidants and facilitators of thermal dissipation [53–55] and are now emerging as themselves being subjected to modulation by some of these same chloroplast antioxidants and facilitators of thermal dissipation [56] (table 1). We are highlighting this specific example among the many redox-signalling networks because of the connections that have been made between this particular signalling pathway and phloem loading/transport.
Table 1.
Summary of evidence for a role of the antioxidant tocopherol, the photosystem II protein PsbS and the xanthophyll zeaxanthin in modulating cell wall ingrowths in phloem-loading complexes, herbivore resistance and oxylipin levels. While the npq1 mutant exhibited a trend for increased cell wall ingrowths that was not significant, double mutant npq1 lut2 exhibited significantly greater wall ingrowths than wild-type when transferred from low to high growth light. The PsbS-deficient npq4 mutant exhibited significantly greater oxylipin levels than wild-type under herbivore attack in the field but not in the absence of herbivores.
| mutant or treatment | resulting effect | sources |
|---|---|---|
| vte mutant (tocopherol-/‘vitamin E’-deficient) | callose deposition resulting in enhanced wall ingrowths in phloem-loading complexes | [57] |
| oxylipin (methyl jasmonate) treatment | enhanced wall ingrowths in phloem-loading complexes | [58] |
| npq1 lut2 mutants (zeaxanthin- and lutein-deficient) | enhanced wall ingrowths in phloem-loading complexes | [56] |
| npq4 mutant (PsbS-deficient) | enhanced herbivore resistance | [59] |
| vte mutant (tocopherol-/‘vitamin E’-deficient) | enhanced oxylipin levels and enhanced ROS levels | [60–63] |
| npq1 mutant (zeaxanthin-deficient) | enhanced oxylipin levels | [56] |
| npq4 mutant (PsbS-deficient) | enhanced oxylipin levels and enhanced ROS levels | [59,64] |
Table 1 summarizes evidence for modulation of the formation of oxylipin messengers by the antioxidant vitamin E (tocopherols) and two factors directly associated with thermal energy dissipation in the light-harvesting complexes, the xanthophylls zeaxanthin and lutein and the PSII protein PsbS. Mutants deficient in the antioxidant vitamin E, in the xanthophylls zeaxanthin and lutein or in the PsbS protein generated greater levels of ROS [62,64] and greater levels of oxylipin messengers compared with wild-type (table 1). The key oxylipin studied in this work (figure 1 and table 1) was the stress hormone jasmonic acid, known to regulate plant growth and development as well as (biotic) defences against pathogens and pests (e.g. [65]). In addition, the latter studies also gave attention to actual evidence for altered biotic defences; it was found that the PsbS-deficient mutant (with lower levels of flexible NPQ and enhanced production of ROS and jasmonic acid) was more resistant to herbivore attack than the wild-type under field conditions (table 1). Similarly, evidence was also accumulated for an alteration of plant responses associated with defences against pathogens. An occlusion of the leaf's phloem-loading complexes and its long-distance phloem conduits presumably blocks the internal spreading of pathogens (like fungi and viruses that use these transport channels) within the plant [66–68]. Mutants deficient in the antioxidant vitamin E, and thus forming increased levels of jasmonic acid (table 1), were shown to be impaired in phloem loading, carbon export, and plant growth and accumulated foliar carbohydrates (for a review, see [48] and also [57,63,69–72]). Similarly, mutants deficient in zeaxanthin exhibited not only enhanced oxylipin production but also increased cell wall deposition in phloem-loading cells (table 1).
It has been recognized that cell wall architecture is a component of plant biotic defence: ‘A rigid cell wall can fend off pathogen attack by forming an impenetrable, physical barrier’ [73, p. 549]. The specific occlusion of the gateways involved in phloem loading and carbon export from leaves is a defence mechanism that presumably comes at a cost to plant growth and thus has far-reaching implications. It is increasingly recognized that combinations of abiotic and biotic stresses in a plant's environment can either exacerbate or ameliorate each other [74]. Likewise, multiple plant-hormone-based signalling pathways exhibit both antagonistic and cooperative interactions [75–77]. It is feasible that trade-offs may exist not only between plant growth versus defence and thus plant abiotic and biotic stress resistance, but also between plant resistance to pests versus pathogens, and/or between additional specific features. For example, greater vitamin E content of certain pepper varieties was associated with inhibition of the development of an insect pest of pepper [78]—whereas decreased vitamin E content of Arabidopsis mutants was associated with enhanced putative pathogen defence via phloem occlusion (table 1). Future research should examine combinations of individual plant species and specific pests or pathogens in specific abiotic environments.
3. Adaptation to contrasting environmental challenges with respect to carbon export-related features
In addition to being the site of the above-described occlusion (table 1 and figure 1) in the possible service of defence against pathogens that spread through the phloem, phloem-loading complexes were recently proposed to be the target point for overall adjustment of foliar carbon export capacity in response to environmental change [79,80]. Differences in the adjustability of phloem loading and carbon export were, furthermore, shown to be excellent predictors for differences in photosynthetic acclimation to the environment between plant varieties (ecotypes) and species from different geographical regions [81]. The latter work indicates that control points and within-plant signalling networks are subjected to genetic adaptation of plants to their environment over evolutionary time. These responses are featured in figures 1 and 2 as (increased, decreased or genetically limited) carbon export capacity (phloem loading/sugar transport) and photosynthetic capacity.
Firstly, it was shown that number and size of the cells loading sugars into the phloem's sugar-transporting sieve tubes as well as number and size of the sieve tubes are adjusted to growth temperature in the winter annual species Arabidopsis and spinach [79–81]. The characterized winter annuals typically germinate in the autumn, overwinter as rosettes, and complete their life cycle in the following spring. These winter annuals exhibited larger and more numerous phloem cells in foliar minor veins when grown under cool temperatures, which was suggested to serve to maintain a high level of sugar export—and a high photosynthetic capacity—at cool growth temperatures despite an increased viscosity of the phloem sap (and other limitations to sugar transport) under these cool temperatures. The latter concomitant upregulation of photosynthetic capacity and phloem-loading capacity also co-occurs with upregulation of other leaf anatomical features, e.g. increased leaf thickness and, especially, an increase the number of the chloroplast-packed palisade layers of the leaf [82]. Future research should elucidate which signalling networks are involved in this concerted upregulation. Transcription factors, such as CBF/DREB (C-repeat binding factor/dehydration-responsive-element-binding protein), involved in plant adaptation/acclimation to cold stress [83,84], are attractive candidates as key components of such networks.
Furthermore, local varieties (ecotypes) of Arabidopsis adapted to the latitudinal extremes (Sweden versus Italy) of this species' geographical range exhibited different sensitivities to environmental cues. Only the Swedish ecotype responded to a decrease in growth temperature (without an increase in growth light intensity) with an increase in the size of the sieve tubes of the leaves’ minor veins (figure 3; see also [80,81]). A combination of both low temperature and high light during plant growth was required to elicit a similar response in the Italian ecotype (figure 3; see also [80,81]). These findings suggest that the two ecotypes of this winter annual species do not differ in the principal ability to adjust leaf anatomy to cool growth temperature, but do differ in the intensity of cues required to elicit such a response. The greater responsiveness of the Swedish ecotype to cool temperature makes intuitive sense. These responses are consistent with putative genetic differences in the operation of the signalling networks involved.
Figure 3.
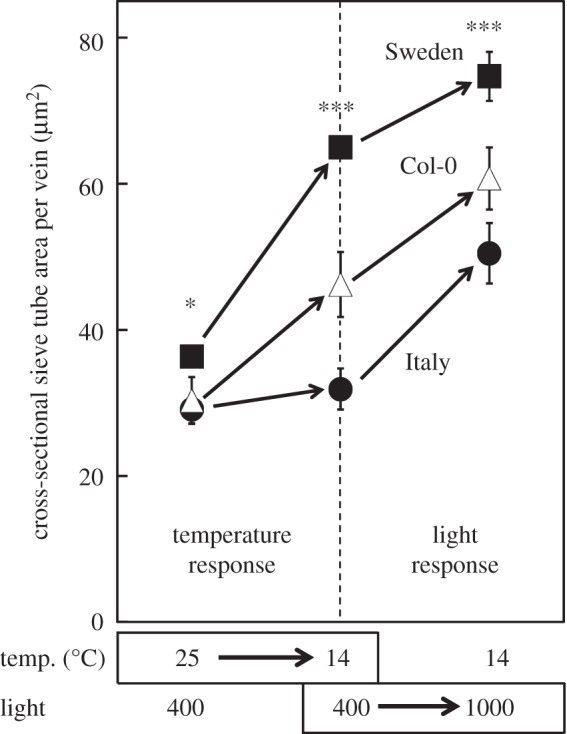
Differences in total cross-sectional sieve tube area per foliar sugar-loading vein induced by growth temperature (temp.) and growth light environment in Arabidopsis ecotypes from Sweden (filled squares) and Italy (filled circles) (as well as in the model line Col-0 (open triangles)). Sieve tubes are also referred to as sieve elements in the literature on plant vasculature. *p < 0.05; ***p < 0.001; light intensity is given in µmol photon m−2 s−1.
In addition to differential adaptations to the environment between members (ecotypes) of a given winter annual species, different strategies are also seen between winter annual and summer annual species. In contrast to winter annuals, summer annuals typically germinate in the spring, grow over the summer and complete their life cycle in the autumn. Figure 4 shows similar photosynthetic capacities in both the winter annual Arabidopsis and the summer annual squash grown under warm temperatures and high light. Anatomical adjustments to phloem-loading cells are made by plants using either of the two major mechanisms for active sugar loading into the sugar-transporting phloem (sieve) tubes (see [85]), i.e. apoplastic loading via membrane transporters (as the phloem-loading mode used by Arabidopsis) and symplastic loading through plasmodesmatal openings in the cell wall (as the phloem-loading mode used by squash). However, while carbon export in Arabidopsis apparently relies on a few large veins per area of leaf (large number of sieve tubes per given sugar-loading vein and a low vein density per leaf area), carbon export in squash instead apparently relies on many small veins per area of leaf (small number of sieve tubes per given sugar-loading vein and a high vein density per leaf area; figure 4). Future research should address the implications of this difference in number (density) and size of sugar-loading and -exporting leaf veins between winter and summer annuals (for data on additional species, see [79]) for sugar export and phloem loading in source leaves (as well as, possibly, for sugar import and phloem unloading in sink tissues), and e.g. tolerance of cold versus hot temperatures as well as for abiotic versus biotic stress tolerance.
Figure 4.
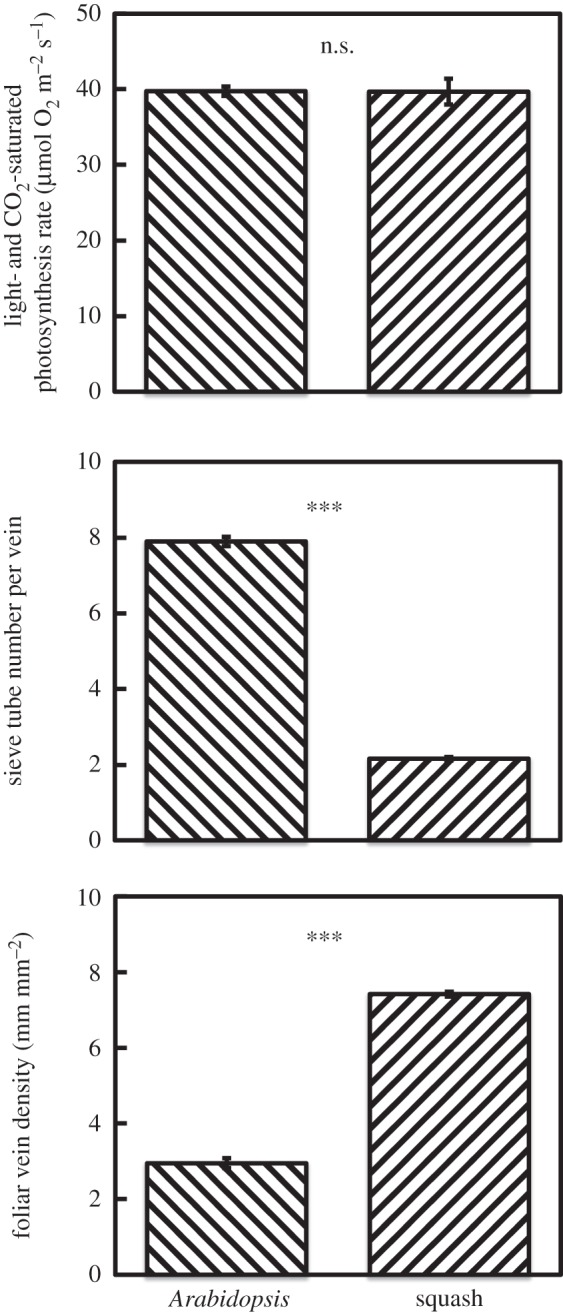
(a) Light- and CO2-saturated photosynthetic rate (measured at 25°C), (b) sieve element number per foliar minor vein and (c) foliar vein density, in the winter annual Arabidopsis thaliana and the summer annual squash, Cucurbita pepo L. cv. Italian Zucchini Romanesco, both grown at 25°C under a 9-h photoperiod of 1000 µmol photon m−2 s−1. ***p < 0.001. For further details on methods and growing conditions, see Cohu et al. [80,81].
Acknowledgements
We thank Onno Muller for the characterization of squash.
Funding statement
This work was supported by the National Science Foundation (Award nos. IOS-0841546 and DEB-1022236 to B.D.-A. and W.W.A.) and the University of Colorado at Boulder.
References
- 1.Krapp A, Stitt M. 1995. An evaluation of direct and indirect mechanisms for the ‘sink-regulation’ of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195, 313–323. ( 10.1007/BF00202587) [DOI] [Google Scholar]
- 2.Paul MJ, Driscoll SP. 1997. Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ. 20, 110–116. ( 10.1046/j.1365-3040.1997.d01-17.x) [DOI] [Google Scholar]
- 3.Paul MJ, Foyer CH. 2001. Sink regulation of photosynthesis. J. Exp. Bot. 52, 1383–1400. ( 10.1093/jexbot/52.360.1383) [DOI] [PubMed] [Google Scholar]
- 4.Krapp A, Quick WP, Stitt M. 1991. Ribulose-1,5-bisphosphate carboxylase-oxygenase, other Calvin-cycle enzymes, and chlorophyll decrease when glucose is supplied to mature spinach leaves via the transpiration stream. Planta 186, 58–69. ( 10.1007/BF00201498) [DOI] [PubMed] [Google Scholar]
- 5.Krapp A, Hofmann B, Schäfer C, Stitt M. 1993. Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the ‘sink regulation’ of photosynthesis? Plant J. 3, 817–828. ( 10.1111/j.1365-313X.1993.00817.x) [DOI] [Google Scholar]
- 6.Kilb B, Wietoska H, Godde D. 1996. Changes in the expression of photosynthetic genes precede loss of photosynthetic activities and chlorophyll when glucose is supplied to mature spinach leaves. Plant Sci. 115, 225–235. ( 10.1016/0168-9452(96)04362-2) [DOI] [Google Scholar]
- 7.Myers DA, Thomas RB, DeLucia EH. 1999. Photosynthetic responses of loblolly pine (Pinus taeda) needles to experimental reduction in sink demand. Tree Physiol. 19, 235–242. ( 10.1093/treephys/19.4-5.235) [DOI] [PubMed] [Google Scholar]
- 8.Rivas F, Gravina A, Agusti M. 2007. Girdling effects on fruit set and quantum yield efficiency of PSII in two Citrus cultivars. Tree Physiol. 27, 527–535. ( 10.1093/treephys/27.4.527) [DOI] [PubMed] [Google Scholar]
- 9.Urban L, Alphonsout L. 2007. Girdling decreases photosynthetic electron fluxes and induces sustained photoprotection in mango leaves. Tree Physiol. 27, 345–352. ( 10.1093/treephys/27.3.345) [DOI] [PubMed] [Google Scholar]
- 10.Mondal MH, Brun WA, Brenner ML. 1978. Effects of sink removal on photosysnthesis and senescence in leaves of soybean (Glycine max L.) plants. Plant Physiol. 61, 394–397. ( 10.1104/pp.61.3.394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wünsche JN, Greer DH, Laing WA, Palmer JW. 2005. Physiological and biochemical leaf and tree responses to crop load in apple. Tree Physiol. 25, 1253–1263. ( 10.1093/treephys/25.10.1253) [DOI] [PubMed] [Google Scholar]
- 12.Duan W, Fan PG, Wan LJ, Li WD, Yan ST, Li SH. 2008. Photosynthetic response to low sink demand after fruit removal in relation to photoinhibition and photoprotection in peach trees. Tree Physiol. 28, 123–132. ( 10.1093/treephys/28.1.123) [DOI] [PubMed] [Google Scholar]
- 13.Adams WW, III, Muller O, Cohu CM, Demmig-Adams B. 2013. May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth. Res. 117, 31–44. ( 10.1007/s11120-013-9849-7) [DOI] [PubMed] [Google Scholar]
- 14.Demmig-Adams B, Moeller DL, Logan BA, Adams WW., III 1998. Positive correlation between levels of retained zeaxanthin+antheraxanthin and degree of photoinhibition in shade leaves of Schefflera arboricola. Planta 205, 367–374. ( 10.1007/s004250050332) [DOI] [Google Scholar]
- 15.Demmig-Adams B, et al. 2006. Modulation of PsbS and flexible versus sustained energy dissipation by light environment in different species. Physiol. Plant. 127, 670–680. ( 10.1111/j.1399-3054.2006.00698.x) [DOI] [Google Scholar]
- 16.Mooney HA, Ehleringer J, Björkman O. 1977. The leaf energy balance of leaves of the evergreen desert shrub Atriplex hymenelytra. Oecologia 29, 301–310. ( 10.1007/BF00345804) [DOI] [PubMed] [Google Scholar]
- 17.Ehleringer JR, Björkman O. 1978. Comparison of photosynthetic characteristics of Encelia species possessing glabrous and pubescent leaves. Plant Physiol. 62, 185–190. ( 10.1104/pp.62.2.185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMillen GG, McClendon JH. 1979. Leaf angle: an adaptive feature of sun and shade leaves. Bot. Gaz. 140, 437–442. ( 10.1086/337110) [DOI] [Google Scholar]
- 19.Ludlow MM, Björkman O. 1984. Paraheliotropic leaf movement in Siratro as a protective mechanism against drought-induced damage to primary photosynthetic reactions: damage by excessive light and heat. Planta 161, 505–518. ( 10.1007/BF00407082) [DOI] [PubMed] [Google Scholar]
- 20.Adams WW, III, Barker DH. 1998. Seasonal changes in xanthophyll cycle-dependent energy dissipation in Yucca glauca Nuttall. Plant Cell Environ. 21, 501–511. ( 10.1046/j.1365-3040.1998.00283.x) [DOI] [Google Scholar]
- 21.Verhoeven AS, Demmig-Adams B, Adams WW., III 1997. Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and nitrogen stress. Plant Physiol. 113, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demmig-Adams B, Adams WW., III 2006. Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol. 172, 11–21. ( 10.1111/j.1469-8137.2006.01835.x) [DOI] [PubMed] [Google Scholar]
- 23.Demmig-Adams B, Cohu CM, Muller O, Adams WW., III 2012. Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth. Res. 113, 75–88. ( 10.1007/s11120-012-9761-6) [DOI] [PubMed] [Google Scholar]
- 24.Adir N, Zer H, Shochat S, Ohad I. 2003. Photoinhibition—a historical perspective. Photosynth. Res. 76, 343–370. ( 10.1023/A:1024969518145) [DOI] [PubMed] [Google Scholar]
- 25.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. 2007. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 1767, 414–421. ( 10.1016/j.bbabio.2006.11.019) [DOI] [PubMed] [Google Scholar]
- 26.Murata N, Allakhverdiev SI, Nishiyama Y. 2012. The mechanism of photoinhibition in vivo: Re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochim. Biophys. Acta 1817, 1127–1133. ( 10.1016/j.bbabio.2012.02.020) [DOI] [PubMed] [Google Scholar]
- 27.Tyystjärvi E. 2008. Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 252, 361–376. ( 10.1016/j.ccr.2007.08.021) [DOI] [Google Scholar]
- 28.Tyystjärvi E. 2013. Photoinhibition of photosystem II. Intl. Rev. Cell Mol. Biol. 300, 243–303. [DOI] [PubMed] [Google Scholar]
- 29.Keren N, Krieger-Liszkay A. 2011. Photoinhibition: molecular mechanisms and physiological significance. Physiol. Plant. 142, 1–5. ( 10.1111/j.1399-3054.2011.01467.x) [DOI] [PubMed] [Google Scholar]
- 30.Takahashi S, Badger MR. 2011. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 16, 53–60. ( 10.1016/j.tplants.2010.10.001) [DOI] [PubMed] [Google Scholar]
- 31.Vass I. 2012. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta 1817, 209–217. ( 10.1016/j.bbabio.2011.04.014) [DOI] [PubMed] [Google Scholar]
- 32.Farage PR, Long SP. 1991. The occurrence of photoinhibition in an over-wintering crop of oil-seed rape (Brassica napus L.) and its correlation with changes in crop growth. Planta 185, 279–286. ( 10.1007/BF00194071) [DOI] [PubMed] [Google Scholar]
- 33.Baker NR, Farage PK, Stirling CM, Long SP. 1994. Photoinhibition of crop photosynthesis in the field at low temperatures. In Photoinhibition of photosynthesis; from molecular mechanisms to the field (eds Baker NR, Bowyer JR.), pp. 349–363. Oxford, UK: Bios Scientific Publishers. [Google Scholar]
- 34.Long SP, Humphries S, Falkowski PG. 1994. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 45, 633–662. ( 10.1146/annurev.pp.45.060194.003221) [DOI] [Google Scholar]
- 35.Raven JA. 1994. The cost of photoinhibition to plant communities. In Photoinhibition of photosynthesis; from molecular mechanisms to the field (eds Baker NR, Bowyer JR.), pp. 449–464. Oxford, UK: Bios Scientific Publishers. [Google Scholar]
- 36.Raven JA. 2011. The cost of photoinhibition. Physiol. Plant. 142, 87–104. ( 10.1111/j.1399-3054.2011.01465.x) [DOI] [PubMed] [Google Scholar]
- 37.Losciale P, Chow WS, Grappadelli LC. 2010. Modulating the light environment with the peach ‘asymmetric orchard’: effects on gas exchange performances, photoprotection, and photoinhibition. J. Exp. Bot. 61, 1177–1192. ( 10.1093/jxb/erp387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G. 2012. Achieving yield gains in wheat. Plant Cell Environ. 35, 1799–1823. ( 10.1111/j.1365-3040.2012.02588.x) [DOI] [PubMed] [Google Scholar]
- 39.Zhu XG, Ort DR, Whitmarsh J, Long SP. 2004. The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J. Exp. Bot. 55, 1167–1175. ( 10.1093/jxb/erh141) [DOI] [PubMed] [Google Scholar]
- 40.Zhu X-G, Long SP, Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261. ( 10.1146/annurev-arplant-042809-112206) [DOI] [PubMed] [Google Scholar]
- 41.Adams WW, III, Watson AM, Mueh KE, Amiard V, Turgeon R, Ebbert V, Logan BA, Combs AF, Demmig-Adams B. 2007. Photosynthetic acclimation in the context of structural constraints to carbon export from leaves. Photosynth. Res. 94, 455–466. ( 10.1007/s11120-006-9123-3) [DOI] [PubMed] [Google Scholar]
- 42.Adams WW, III, Demmig-Adams B, Verhoeven AS, Barker DH. 1995. ‘Photoinhibition’ during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust. J. Plant Physiol. 22, 261–276. ( 10.1071/PP9950261) [DOI] [Google Scholar]
- 43.Adams WW, III, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B. 2006. Energy dissipation and photoinhibition: a continuum of photoprotection. In Photoprotection, photoinhibition, gene regulation, and environment; adavances in photosynthesis and respiration, vol. 21 (eds Demmig-Adams B, Adams WW, III, Mattoo AK.), pp. 49–64. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 44.Ferrar PJ, Osmond CB. 1986. Nitrogen supply as a factor influencing photoinhibition and photosynthetic acclimation after transfer of shade-grown Solanum dulcamara to bright light. Planta 168, 563–570. ( 10.1007/BF00392277) [DOI] [PubMed] [Google Scholar]
- 45.Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F. 2000. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 57, 779–795. ( 10.1007/s000180050041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nanda AK, Andrio E, Marino D, Pauly N, Dunand C. 2010. Reactive oxygen species during plant–microorganism early interactions. J. Integr. Plant Biol. 52, 195–204. ( 10.1111/j.1744-7909.2010.00933.x) [DOI] [PubMed] [Google Scholar]
- 47.Ledford HK, Niyogi KK. 2005. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ. 28, 1037–1045. ( 10.1111/j.1365-3040.2005.01374.x) [DOI] [Google Scholar]
- 48.Munné-Bosch S, Queval G, Foyer CH. 2013. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol. 161, 5–19. ( 10.1104/pp.112.205690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baginsky S, Link G. 2006. Redox regulation of chloroplast gene expression. In Photoprotection, photoinhibition, gene regulation, and environment. Advances in photosynthesis and respiration, vol. 21 (eds Demmig-Adams B, Adams WW, III, Mattoo AK.), pp. 269–287. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 50.Dietz K-J, Stork T, Finkemeier I, Lamkemeyer P, Li W-X, El-Tayeb MA, Michel K-P, Pistorius E, Baier M. 2006. The role of peroxiredoxins in oxygenic photosynthesis of cyanobacteria and higher plants: Peroxide detoxification or redox sensing? In Photoprotection, photoinhibition, gene regulation, and environment. Advances in photosynthesis and respiration, vol. 21 (eds Demmig-Adams B, Adams WW, III, Mattoo AK.), pp. 303–319. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 51.Foyer CH, Trebst A, Noctor G. 2006. Signaling and integration of defense functions of tocopherol, ascorbate and glutathione. In Photoprotection, photoinhibition, gene regulation, and environment. Advances in photosynthesis and respiration, vol. 21 (eds Demmig-Adams B, Adams WW, III, Mattoo AK.), pp. 241–268. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 52.Mullineaux PM, Karpinski S, Creissen GP. 2006. Integration of signaling in antioxidant defenses. In Photoprotection, photoinhibition, gene regulation, and environment. Advances in photosynthesis and respiration, vol. 21 (eds Demmig-Adams B, Adams WW, III, Mattoo AK.), pp. 223–239. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 53.Pérez AG, Sanz C, Richardson DG, Olías JM. 1993. Methyl jasmonate vapor promotes β-carotene synthesis and chlorophyll degradation in Golden Delicious apple peel. J. Plant Growth Regul. 12, 163–167. ( 10.1007/BF00189648) [DOI] [Google Scholar]
- 54.Sandorf I, Holländer-Czytko H. 2002. Jasmonate is involved in the induction of tyrosine aminotransferase and tocopherol biosynthesis in Arabidopsis thaliana. Planta 216, 173–179. ( 10.1007/s00425-002-0888-0) [DOI] [PubMed] [Google Scholar]
- 55.Wolucka BA, Goossens A, Inzé D. 2005. Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J. Exp. Bot. 56, 2527–2538. ( 10.1093/jxb/eri246) [DOI] [PubMed] [Google Scholar]
- 56.Demmig-Adams B, Cohu CM, Amiard V, van Zadelhoff G, Veldink GA, Muller O, Adams WW., III 2013. Emerging trade-offs—impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins and biotic defense. New Phytol. 197, 720–729. ( 10.1111/nph.12100) [DOI] [PubMed] [Google Scholar]
- 57.Maeda H, Song W, Sage TL, DellaPenna D. 2006. Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 18, 2710–2732. ( 10.1105/tpc.105.039404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amiard V, Demmig-Adams B, Mueh KE, Turgeon R, Combs AF, Adams WW., III 2007. Role of light and jasmonic signaling in regulating foliar phloem cell wall ingrowth development. New Phytol. 173, 722–731. ( 10.1111/j.1469-8137.2006.01954.x) [DOI] [PubMed] [Google Scholar]
- 59.Frenkel M, et al. 2009. Improper excess light energy dissipation in Arabidopsis results in a metabolic reprogramming. BMC Plant Biol. 9, 12 ( 10.1186/1471-2229-9-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munné-Bosch S, Weiler EW, Alegre L, Muller M, Duchting P, Falk J. 2007. α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 225, 681–691. ( 10.1007/s00425-006-0375-0) [DOI] [PubMed] [Google Scholar]
- 61.Semchuk NM, Lushchak OV, Falk J, Krupinska K, Lushchak VI. 2009. Inactivation of genes, encoding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana. Plant Physiol. Biochem. 47, 384–390. ( 10.1016/j.plaphy.2009.01.009) [DOI] [PubMed] [Google Scholar]
- 62.Havaux M, Eymery F, Profirova S, Rey P, Dorman P. 2005. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 17, 3451–3469. ( 10.1105/tpc.105.037036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sattler SE, Mene-Saffrane L, Farmer EE, Krischke M, Mueller MJ, DellaPenna D. 2006. Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 18, 3706–3720. ( 10.1105/tpc.106.044065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jänkänpää HJ, et al. 2013. Non-photochemical quenching capacity in Arabidopsis thaliana affects herbivore behaviour. PLoS ONE 8, e53232 ( 10.1371/journal.pone.0053232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rohwer CL, Erwin JE. 2008. Horticultural application of jasmonates: a review. J. Hortic. Sci. 83, 283–304. [Google Scholar]
- 66.Gilbertson RL, Lucas WJ. 1996. How do viruses traffic on the ‘Vascular highway’? Trends Plant Sci. 1, 260–268. ( 10.1016/1360-1385(96)10029-7) [DOI] [Google Scholar]
- 67.Waigman E, Ueki S, Trutnyeva K, Citovsky V. 2004. The ins and outs of nondestructive cell-to-cell systemic movement of plant viruses. Crit. Rev. Plant Sci. 23, 195–250. ( 10.1080/07352680490452807) [DOI] [Google Scholar]
- 68.Scholthof HB. 2005. Plant virus transport: motions of functional equivalence. Trends Plant Sci. 10, 376–382. ( 10.1016/j.tplants.2005.07.002) [DOI] [PubMed] [Google Scholar]
- 69.Sattler SE, Cajon EB, Coughlin SJ, DellaPenna D. 2003. Characterization of tocopherol cyclases from higher plants and cyanobacteria: evolutionary implications for tocopherol synthesis and function. Plant Physiol. 132, 2184–2195. ( 10.1104/pp.103.024257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sattler SE, Gilliland LU, Magallanes-Lundback M, Pollard M, DellaPenna D. 2004. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16, 1419–1432. ( 10.1105/tpc.021360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hofius D, Hajirezael MR, Geiger M, Tschiersch H, Melzer M, Sonnewald U. 2004. RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol. 135, 1256–1268. ( 10.1104/pp.104.043927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maeda H, Sage TL, Isaac G, Welti R, DellaPenna D. 2008. Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in Arabidopsis at low temperature. Plant Cell 20, 452–470. ( 10.1105/tpc.107.054718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasidharan R, Voesenek LACJ, Pierik R. 2011. Cell wall modifying proteins mediate plant acclimatization to biotic and abiotic stresses. Crit. Rev. Plant Sci. 30, 548–562. ( 10.1080/07352689.2011.615706) [DOI] [Google Scholar]
- 74.Atkinson NJ, Urwin PE. 2012. The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 63, 3523–3543. ( 10.1093/jxb/ers100) [DOI] [PubMed] [Google Scholar]
- 75.Bari R, Jones JDG. 2008. Role of plant hormones in plant defence responses. Plant Mol. Biol. 69, 473–488. ( 10.1007/s11103-008-9435-0) [DOI] [PubMed] [Google Scholar]
- 76.Kim TH. 2012. Plant stress surveillance monitored by ABA and disease signaling interactions. Mol. Cells 33, 1–7. ( 10.1007/s10059-012-2299-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Derksen H, Rampitsch C, Daayf F. 2013. Signaling cross-talk in plant disease resistance. Plant Sci. 207, 79–87. ( 10.1016/j.plantsci.2013.03.004) [DOI] [PubMed] [Google Scholar]
- 78.Maharijaya A, Vosman B, Verstappen F, Steenhuis-Broers G, Mumm R, Purwito A, Visser RGF, Voorrips RE. 2012. Resistance factors in pepper inhibit larval development of thrips (Frankliniella occidentalis). Entomol. Exp. Appl. 145, 62–71. ( 10.1111/j.1570-7458.2012.01304.x) [DOI] [Google Scholar]
- 79.Adams WW, III, Cohu CM, Muller O, Demmig-Adams B. 2013. Foliar phloem infrastructure in support of photosynthesis. Front. Plant Sci. 4, 194 ( 10.3389/fpls.2013.00194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohu CM, Muller O, Demmig-Adams B, Adams WW., III 2013. Minor loading vein acclimation for three Arabidopsis thaliana ecotypes in response to growth under different temperature and light regimes. Front. Plant Sci. 4, 240 ( 10.3389/fpls.2013.00240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohu CM, Muller O, Stewart JJ, Demmig-Adams B, Adams WW., III 2013. Association between minor loading vein architecture and light- and CO2-saturated rates of photosynthetic oxygen evolution among Arabidopsis thaliana ecotypes from different latitudes. Front. Plant Sci. 4, 264 ( 10.3389/fpls.2013.00264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dumlao MR, Darehshouri A, Cohu CM, Muller O, Mathias J, Adams WW, III, Demmig-Adams B. 2012. Low temperature acclimation of photosynthetic capacity and leaf morphology in the context of phloem loading type. Photosynth. Res. 113, 181–189. ( 10.1007/s11120-012-9762-5) [DOI] [PubMed] [Google Scholar]
- 83.Thomashow MF. 2010. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Phys. 154, 571–577. ( 10.1104/pp.110.161794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akhtar M, Jaiswal A, Taj G, Jaiswal JP, Qureshi MI, Singh NK. 2012. DREB1/CBF transcription factors: their structure, function and role in abiotic stress tolerance in plants. J. Genet. 91, 385–395. ( 10.1007/s12041-012-0201-3) [DOI] [PubMed] [Google Scholar]
- 85.Muller O, Cohu CM, Stewart JJ, Protheroe JA, Demmig-Adams B, Adams WW., III In press Association between photosynthesis and contrasting features of minor veins in leaves of summer annuals loading phloem via symplastic versus apoplastic routes. Physiologia Plantarum. ( 10.1111/ppl.12155). [DOI] [PubMed] [Google Scholar]