Abstract
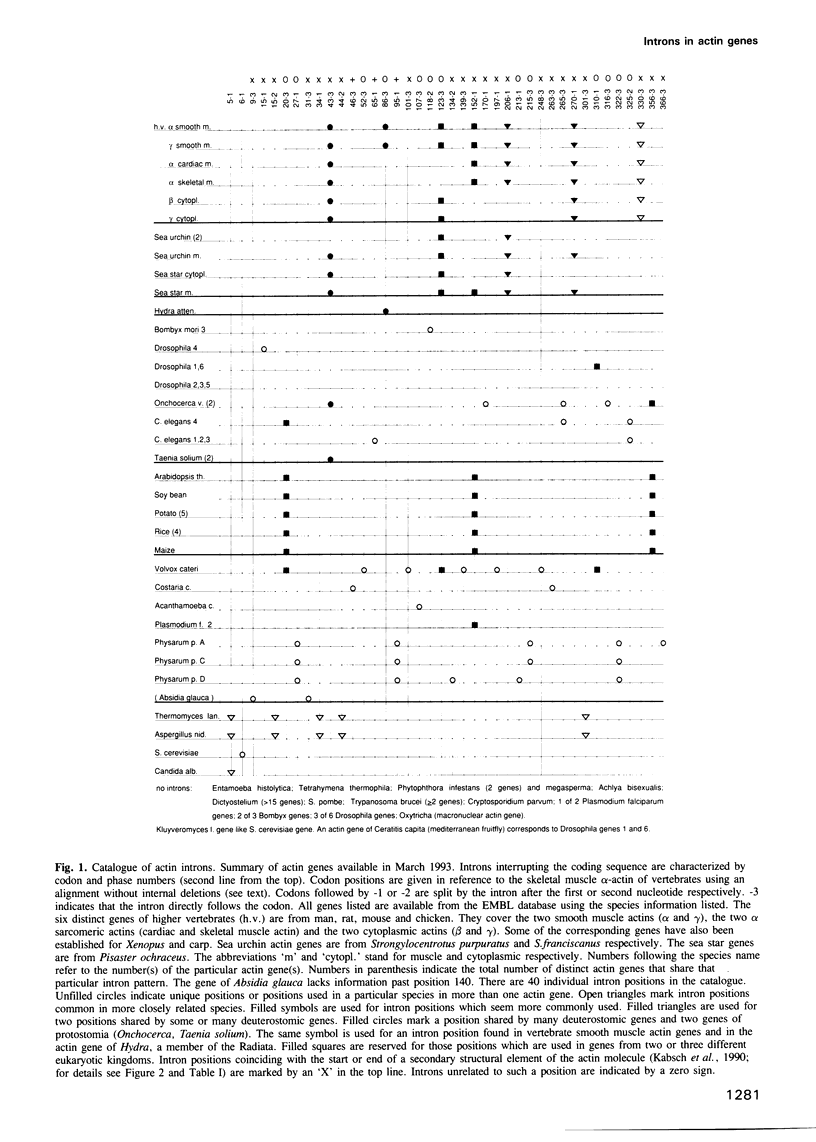
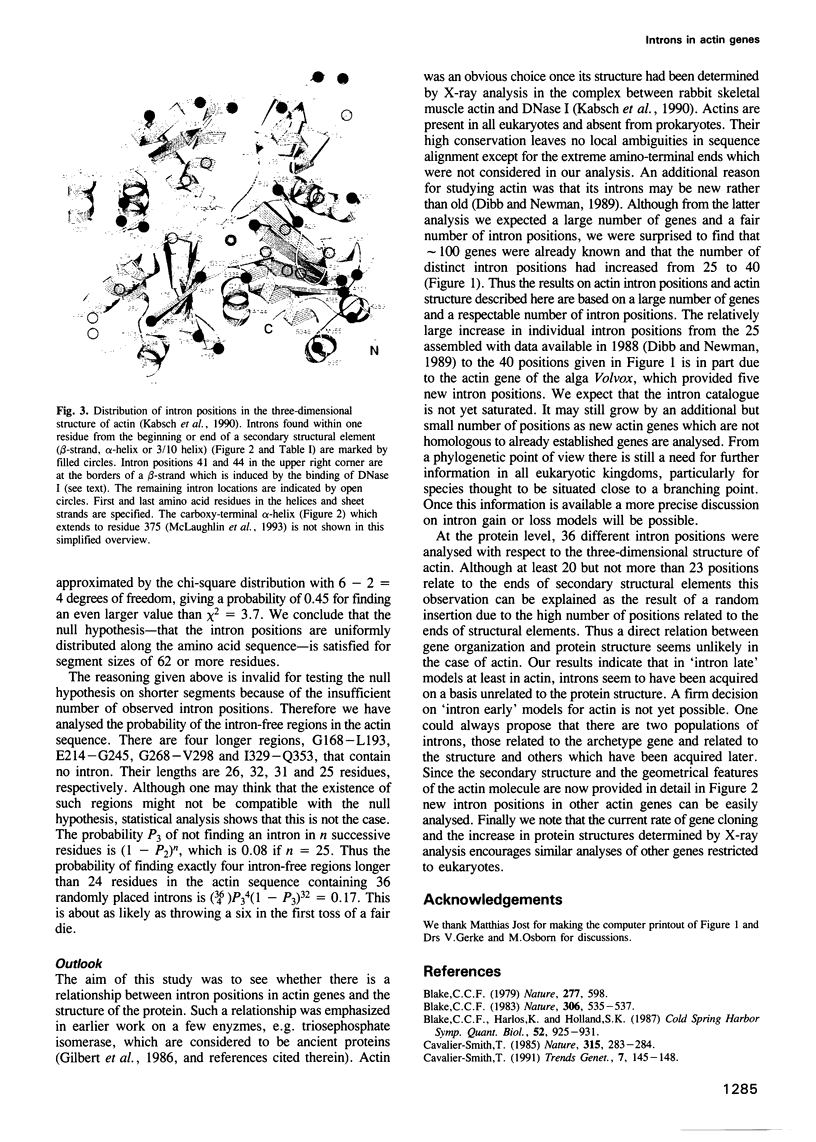
A catalogue of intron positions along the coding sequence was assembled from the large number of actin genes known for different eukaryotes. 36 positions in the amino acid sequence were compared with the known three-dimensional structure of actin. At least 20 but not more than 23 intron positions are at the start or end of a secondary structural element (beta-strand, alpha-helix or 3/10 helix) while eight positions interrupt such an element. Statistical analysis shows that due to the large number of end positions the boundaries of secondary structural elements are not correlated with the intron positions. In addition, the observed intron pattern seems compatible with the null hypothesis, i.e. intron positions are randomly distributed along the actin sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake C. C. Exons encode protein functional units. Nature. 1979 Feb 22;277(5698):598–598. doi: 10.1038/277598a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Harlos K., Holland S. K. Exon and domain evolution in the proenzymes of blood coagulation and fibrinolysis. Cold Spring Harb Symp Quant Biol. 1987;52:925–931. doi: 10.1101/sqb.1987.052.01.101. [DOI] [PubMed] [Google Scholar]
- Blake C. Exons--present from the beginning? Nature. 1983 Dec 8;306(5943):535–537. doi: 10.1038/306535a0. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Intron phylogeny: a new hypothesis. Trends Genet. 1991 May;7(5):145–148. [PubMed] [Google Scholar]
- Cavalier-Smith T. Selfish DNA and the origin of introns. Nature. 1985 May 23;315(6017):283–284. doi: 10.1038/315283b0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science. 1978 Dec 22;202(4374):1257–1260. doi: 10.1126/science.364651. [DOI] [PubMed] [Google Scholar]
- Dibb N. J., Newman A. J. Evidence that introns arose at proto-splice sites. EMBO J. 1989 Jul;8(7):2015–2021. doi: 10.1002/j.1460-2075.1989.tb03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb N. J. Proto-splice site model of intron origin. J Theor Biol. 1991 Aug 7;151(3):405–416. doi: 10.1016/s0022-5193(05)80388-1. [DOI] [PubMed] [Google Scholar]
- Dibb N. J. Why do genes have introns? FEBS Lett. 1993 Jun 28;325(1-2):135–139. doi: 10.1016/0014-5793(93)81429-4. [DOI] [PubMed] [Google Scholar]
- Fink G. R. Pseudogenes in yeast? Cell. 1987 Apr 10;49(1):5–6. doi: 10.1016/0092-8674(87)90746-x. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Marchionni M., McKnight G. On the antiquity of introns. Cell. 1986 Jul 18;46(2):151–153. doi: 10.1016/0092-8674(86)90730-0. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Hickey D. A., Benkel B. F., Abukashawa S. M. A general model for the evolution of nuclear pre-mRNA introns. J Theor Biol. 1989 Mar 7;137(1):41–53. doi: 10.1016/s0022-5193(89)80148-1. [DOI] [PubMed] [Google Scholar]
- Hunkapiller T., Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–63. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- McLaughlin P. J., Gooch J. T., Mannherz H. G., Weeds A. G. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993 Aug 19;364(6439):685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- Rogers J. Exon shuffling and intron insertion in serine protease genes. Nature. 1985 Jun 6;315(6019):458–459. doi: 10.1038/315458a0. [DOI] [PubMed] [Google Scholar]
- Solomon L. R., Rubenstein P. A. Correct NH2-terminal processing of cardiac muscle alpha-isoactin (class II) in a nonmuscle mouse cell. J Biol Chem. 1985 Jun 25;260(12):7659–7664. [PubMed] [Google Scholar]
- Tittiger C., Whyard S., Walker V. K. A novel intron site in the triosephosphate isomerase gene from the mosquito Culex tarsalis. Nature. 1993 Feb 4;361(6411):470–472. doi: 10.1038/361470a0. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]