Abstract
Rationale and Objectives
The objectives of this study were to measure the parallel changes of transverse relaxation times (T2), spin-lattice relaxation time in the rotating frame (T1ρ), and the delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC)-T1 mapping of human knee cartilage in detecting cartilage degeneration at 3.0T.
Materials and Methods
Healthy volunteers (n = 10, mean age 35.6 years) and patients (n = 10, mean age 65 years) with early knee osteoarthritis (OA) were scanned at 3.0T MR using an 8-channel phased array knee coil (transmit–receive). Quantitative assessment of T2, T1ρ, and dGEMRIC-T1 values (global and regional) were correlated between asymptomatic subjects and patients with OA.
Results
The average T2 (39 ± 2 milliseconds [mean ± standard deviation] vs. 47 ± 6 milliseconds, P < .0007) and T1ρ (48 ± 3 vs. 62 ± 8 milliseconds, P < .0002) values were all markedly increased in all patients with OA when compared to healthy volunteers. The average dGEMRIC-T1 (1244 ± 134 vs. 643 ± 227 milliseconds, P < .000002) value was sharply decreased after intravenous administration of gadolinium contrast agent in all patients with OA.
Conclusions
The research results showed that all the T2, T1ρ, and dGEMRIC-T1 relaxation times varied with the cartilage degeneration. The dGEMRIC-T1 and T1ρ relaxation times seem to be more sensitive than T2 in detecting early cartilage degeneration. The preliminary study demonstrated that the early biochemical changes in knee osteoarthritic patients could be detected noninvasively in in vivo using T1ρ and dGEMRIC-T1 mapping.
Keywords: dGEMRIC, osteoarthritis, T2, T1ρ, cartilage imaging
The earliest biochemical changes in osteoarthritis (OA) are the modifications at the molecular level of cartilage matrix that occur without obvious morphologic changes (1,2). The loss of glycosaminoglycan (GAG) and collagen breakdown are the typical characteristics of early OA.
Several attempts have been made to quantify the changes concerning the loss of GAG in knee cartilage using various quantitative magnetic resonance methods (3–22). In recent years, three relaxation methods, based on the measurement of the delayed gadolinium-enhanced magnetic resonance imaging (MRI) of cartilage (dGEMRIC)-T1, transverse relaxation times (T2), and spin-lattice relaxation time in the rotating frame (T1rho or T1ρ), have been reported and shown to be promising (2,11,22,23). The dGEMRIC technique has been validated in both basic scientific and clinical studies by the intravenous injection of negatively charged gadolinium-based contrast agent as a marker of proteoglycan (PG) depletion (4,23).
T2 has been investigated extensively in cartilage in vivo (1,11–14). T2 mapping is one of the most promising approaches for assessing the underlying collagen microstructure in the extracellular matrix of articular cartilage. Damages to the extracellular matrix of articular cartilage and the increase of water content in degenerated cartilage may increase the T2 relaxation times (12).
T1ρ-weighted MRI has recently been proposed as an attractive alternative to the existing MRI methods (2,11,15–17). The T1ρ-weighted MRI method was first described by Redfield (18), and the related techniques have been used to investigate the slow-motion interactions between the macromolecule protons and motion-restricted water molecules (11,16). T1ρ mapping has been shown to be sensitive to changes in PG loss of cartilage (17). Previous studies have demonstrated that the T1ρ values are elevated in patients with OA when compared to corresponding healthy subjects (2,11,22). T2 and T1ρ are different MR parameters and may provide complementary information about cartilage degeneration. Although previous work (24) has indicated that T1ρ has a greater dynamic range (>100%) for detecting early pathology than T2, which can be used to measure even smaller macromolecular changes with greater accuracy in comparison with T2, to the best of our knowledge, there has been no quantitative comparison concerning the previously mentioned three methods. In present study, we measured and compared the global and regional changes in T2, T1ρ, and dGEMRIC-T1 relaxation times in healthy subjects and in patients with OA using quantitative relaxation methods at 3.0Twith parallel imaging.
MATERIALS AND METHODS
Study Population
Ten healthy volunteers (n = 6 men and n = 4 women, ranging in age from 24 to 45 years, with an average age of 35.6 years) and 10 patients (n = 8 men and n = 2 women, ranging in age from 53 to 82 years, with an average age of 65 years) with clinically documented early knee OA by radiography (Kellgren–Lawrence [K-L] grading scale 1, 2) (19) were recruited. All healthy volunteers and patients with OA were scanned for both T2 and T1ρ mapping. Then, to validate the T1 mapping of the subjects with OA before the contrast agent gadolinium diethylenetriamine pentaacetic acid [Gd(DTPA)2−] injection, the 10 clinically diagnosed patients with OA were first imaged using the T1 mapping method. Later, all patients with OA were injected intravenously with 0.2 mmol/kg of gadopentetate dimeglumine (Magnevist, Berlex, Wayne, NJ); and ~90 minutes after the contrast agent injection, the patients with OA were imaged again using the same imaging protocol (8). To limit patient motion between acquisitions, the knee was fixed with foam padding. The volunteers’ body height and weight were obtained to calculate the body mass index (BMI). Volunteers with a BMI >24.9 kg/m2 were classified as overweight, and volunteers with a BMI >29.9 kg/m2 were classified as obese (25). All the human subjects provided informed consent to participate in the research after the nature of the procedure had been fully explained, which was approved by our institutional review board.
Imaging Hardware
All MRI experiments were performed on a 3.0T clinical MR scanner (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen, Germany). An 18-cm diameter, 8-channel transmit–receive phased array knee coil was used for all the imaging measurements.
Imaging Protocol
The protocol included the following sequences: two-dimensional (2D)-sagittal T2-weighted multiecho spin echo (MESE) with fat saturation. For three-dimensional (3D)-T1rho imaging, 3D T1rho-weighted images with parallel imaging (acceleration factor [AF] = 2) were acquired using the GRE sequence based on the spin-lock technique (2). Three-dimensional–sagittal T1-weighted FLASH (fast low angle shot) imaging with variable flip angle method (VFA) and parallel imaging (AF = 2) were acquired before and after contrast agent injection, respectively.
All sagittal images covered the regions of femoral, tibial, and patellar cartilages. The acquisition parameters of all MRI sequence were listed in Table 1.
TABLE 1.
Magnetic Resonance Imaging Sequence Parameters
| Imaging Parameters | 2D MESE | 3D GRE | 3D FLASH |
|---|---|---|---|
| Weighting | T2 | T1rho | T1 |
| Plane | Sagittal | Sagittal | Sagittal |
| Fat sat | Yes | Yes | Yes |
| Matrix | 256 × 128 | 256 × 128 | 320 × 320 |
| Number of slices | 31 | 30 | 80 |
| FOV (mm) | 150 | 150 | 130 |
| Slice thickness (mm) | 1.5 | 3 | 1.5 |
| Flip angle (°) | 180 | 25 | 3.9/23 (Before); 5.5/32.5 (After) |
| TE (ms) | 16.5/33/49.5/66/82.5 | 2.04 | 7.24 |
| TR (ms) | 4000 | 175 | 17 |
| BW (Hz/pixel) | 130 | 260 | 130 |
| Interpolated in-plane spatial resolution (mm) | 0.59 × 0.59 | 0.59 × 0.59 | 0.7 × 0.7 |
| Echo train length | 5 | 1 | 1 |
| NEX | 1 | 1 | 2 |
| Duration of each 90° pulse (μs) | 1536 | 200 | 200 |
| Acceleration factor | — | 2 | 2 |
| Spin-lock frequency (Hz) | — | 300 | — |
| Time of spin-lock (TSL) [ms] | — | 2/10/20/30 | — |
| Acquisition time | 8 minutes 30 seconds | 5 minutes 31 seconds/TSL | <15 minutes |
BW, band width; FLASH, fast low angle shot; FOV, field of view; GRE, gradient recalled echo; MESE, multiecho spin echo (MESE); NEX, number of excitations; TE, echo time; TR, relaxation time.
MR Images Analysis and Processing
All the MR images were analyzed based on global and regional compartments. Three regions were defined in each subject: femoral, tibial, and patellar cartilages. The in-house developed routines in MATLAB version 7.1(Mathworks, Natick, MA) and C++ were used for offline processing of the acquired MR images.
T2-weighted images with the shortest echo time (TE = 16.5 milliseconds) and T1ρ-weighted images with the shortest spin-lock length (TSL = 2 milliseconds) were used for the segmentation of femoral, tibial, and patellar cartilages. Regions of interest (ROIs) were segmented manually for each slice for all the subjects. These segmentations were used to draw ROIs for each MR image with different TE and TSL values. T2, T1ρ, and dGEMRIC maps were computed with custom-built MATLAB routines using respective signal expressions (2,5,9–11,22).
The intersubject variability of the T2, T1ρ, and dGEMRIC-T1 maps was quantified using root mean square coefficients of variation percentage (RMS-CV %) and a nonparametric rank test (Wilcoxon signed rank test) to determine whether there were any statistically significant differences among these values for asymptomatic and osteoarthritic subjects.
RESULTS
The mean BMI of the volunteers included in this study was 25.6 ± 4.2 kg/m2. The BMI was within the normal range in 15 volunteers (75%); two volunteers were overweight (10%) and three were obese (15%). There was no statistically significant difference (P > .05) in BMI between the two groups of healthy controls and patients with OA. For patients with OA, the standard deviations for the two parameters of T2 and T1ρ were statistically different (P < .05). The RMS-CV % was 11.95 for T2 measurement and 9.66 for T1ρ measurement.
Table 2 displays the average T2 and T1ρ relaxation times in each cartilage region and the average across the three cartilage compartments for all controls and patients with OA. The T2 and T1ρ values were elevated for patients with OA in each cartilage region when compared to healthy volunteers. However, the T1ρ values of patients with OA have a greater dynamic range than the T2 values. On the other hand, there was no statistically significant difference in T2 and T1ρ relaxation times across the different cartilage regions (patella, femur, and tibia) of controls and patients with OA, respectively.
TABLE 2.
Average T2, T1ρ (in Milliseconds) Relaxation Times (Mean ± Standard Deviation, n = 10 for Each Group) for Each Cartilage Region
| Femur | Tibia | Patella | Average | |
|---|---|---|---|---|
| Controls (T2) | 40 ± 3 | 37 ± 4 | 41 ± 2 | 39 ± 2 |
| Patients with OA (T2) | 46 ± 4 | 48 ± 5 | 46 ± 12 | 47 ± 6 |
| Controls (T1ρ) | 52 ± 5 | 47 ± 5 | 46 ± 6 | 48 ± 3 |
| Patients with OA (T1ρ) | 67 ± 8 | 57 ± 11 | 64 ± 13 | 62 ± 8 |
OA, osteoarthritis.
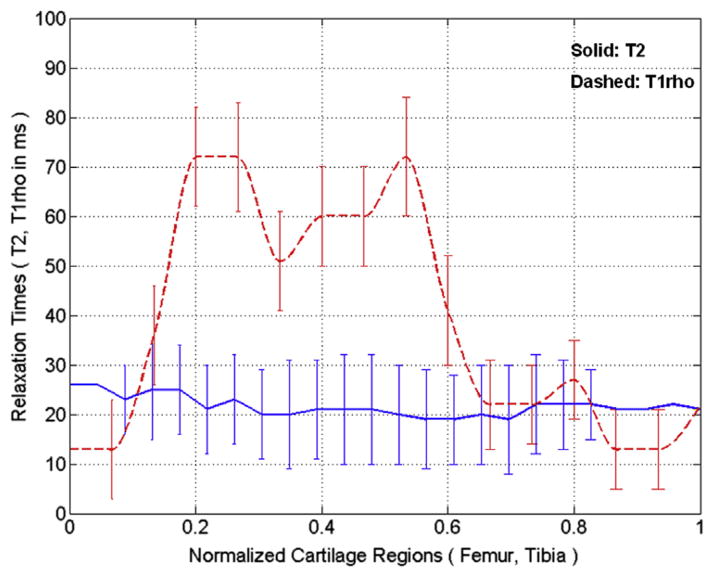
Two representative T2 (top row) and T1ρ (bottom row) slices obtained from a patient with OA overlaid onto the shortest TE (16.5 milliseconds) and the shortest TSL (2 milliseconds), respectively are displayed in Figure 1. As shown in Figure 1, the T1ρ maps have greater dynamic range when compared to corresponding T2 maps. Figure 2 shows the representative profile plots of the relaxation times across cartilage regions along the vertical lines shown in Figure 1a and c, respectively. The error bars show the standard deviations of values of 5 × 5 pixels centered on each point along the vertical line within the cartilage regions.
Figure 1.
Two representative sagittal T2 (a,b) and T1ρ (c,d) weighted images obtained from patient with OA along with overlaid maps, respectively. The relaxation times for the T2 and T1ρ mapping are distinct in different range of values with the obvious greater dynamic range in T1ρ for the patient with OA.
Figure 2.
The profile plots of the T2 and T1ρ relaxation times measured in the same subject as shown in Figure 1a and c. The vertical rectangular regions of interest were used for profile plotting (solid line for T2 and dashed line for T1ρ, respectively). Each point on the profile is an average of 5 × 5 pixels and error bars show the respective standard deviation.
The average T2 values were greater in subjects with OA than those with healthy controls (39 ± 2 milliseconds [mean ± standard deviation] vs. 47 ± 6 milliseconds, P < .0007). As shown in Figure 3, the average T2 values were 46 ± 4, 48 ± 5, 46 ± 12, and 47 ± 6 milliseconds for the femoral, tibial, patellar, and the average across the three regions in subjects with OA, respectively, whereas the average T2 values were 40 ± 3, 37 ± 4, 41 ± 2, and 39 ± 2 milliseconds for the femoral, tibial, patellar, and the average across the three regions in healthy subjects, respectively. There was no significant difference in T2 values among the three regions in subjects with OA with the range between 46 ± 4 and 48 ± 5 milliseconds and in healthy subjects with the range between 37 ± 4 and 41 ± 2 milliseconds, respectively. However, there is a statistically significant difference between the two groups of OA and healthy subjects. The P values between OA and healthy subjects were .0006, .0001, .0107, and .0007 for the femoral, tibial, patellar, and the average across the three regions, respectively. The RMS-CV % were 7.7, 9.5, 5.4, and 4.4 for the femoral, tibial, patellar, and the average across the three regions in healthy subjects, respectively. The RMS-CV % were 8.4, 9.8, 17.1, and 11.0 for the femoral, tibial, patellar, and the average across the three regions in subjects with OA, respectively.
Figure 3.

The comparison of average T2 and T1ρ values between healthy volunteers and patients with OA. In patients with OA, the average T2 values varied from 39 ± 2 to 47 ± 6 milliseconds when compared to healthy volunteers (n = 10, P < .0007). The P values between OA and healthy subjects were .0006, .0001, .0107, and .0007 for the femoral, tibial, patellar, and the average across the three regions, respectively. Similarly, the average T1ρ values varied from 48 ± 3 to 62 ± 8 milliseconds in subjects with OA compared to healthy controls (n = 10, P < .0002). The P values between OA and healthy subjects were .0001, .0293, .0001, and .0002 for the femoral, tibial, patellar, and the average across the three regions, respectively.
The average T1ρ values were markedly increased in subjects with OA compared to healthy controls (48 ± 3 vs. 62 ± 8 milliseconds, P < .0002). As shown in Figure 3, the average T1ρ values were 67 ± 8, 57 ± 11, 64 ± 13, and 62 ± 8 milliseconds for the femoral, tibial, patellar, and the average across the three regions in subjects with OA, respectively. On the other hand, the average T1ρ values were 52 ± 5, 47 ± 5, 46 ± 6, and 48 ± 3 milliseconds for the femoral, tibial, patellar, and the average across the three regions in healthy subjects, respectively. Similar to T2, there was no significant difference in T1ρ values among the three regions in subjects with OA with the range between 57 ± 11 and 67 ± 8 milliseconds and in healthy subjects with the range between 46 ± 6 and 52 ± 5 milliseconds, respectively; but, there is a statistically significant difference between the two groups of OA and healthy subjects. The P values between OA and healthy subjects were .0001, .0293, .001, and .0002 for the femoral, tibial, patellar, and the average across the three regions, respectively. The RMS-CV % were 10.0, 11.7, 12.2, and 6.8 for the femoral, tibial, patellar, and the average across the three regions in healthy subjects, respectively. The RMS-CV % were 11.9, 19.8, 19.6, and 12.6 for the femoral, tibial, patellar, and the average across the three regions in subjects with OA, respectively.
Table 3 displays the average T1 relaxation times in each cartilage region and the average across the three cartilage regions before and after contrast agent injection for all patients with OA. As expected, there is a large difference in T1 relaxation times between before and after contrast agent injection. The T1 values before contrast agent injection, however, have a greater range than those after contrast agent injection.
TABLE 3.
Average T1 (in Milliseconds) Relaxation Times (Mean ± Standard Deviation, n = 10) before and after Contrast Agent Injection for Each Cartilage Region
| Femur | Tibia | Patella | Average | |
|---|---|---|---|---|
| Before (T1) | 1239 ± 145 | 1235 ± 148 | 1304 ± 114 | 1244 ± 134 |
| After (T1) | 639 ± 235 | 621 ± 193 | 658 ± 252 | 643 ± 227 |
Two representative slices obtained from a subject with OA, with the T1 mapping before and after contrast agent injection, are shown in Figure 4. The top and bottom rows of Figure 4 show the T1 mapping before and after contrast agent injection, respectively.
Figure 4.
Two representative T1 maps obtained from the same patient with OA before (a,b) and after (c,d) contrast agent injection respectively. The color bar scale at the right indicates the T1 values in milliseconds.
Figure 5 shows the profile plots of the T1 relaxation times variation of cartilage regions along the vertical lines in Figure 4a and c with T1 mapping before and after contrast agent injection, respectively. The T1 relaxation times varied in different range before and after contrast agent injection with the obvious much greater range before contrast agent injection. The error bars show the standard deviations of values of 5 × 5 pixels centered on each point along the vertical line within the cartilage regions.
Figure 5.
The profile plots of the T1 relaxation times before and after contrast agent injection measured in the same subject as shown in Figure 4a and c. The vertical rectangular regions of interest were used for profile plotting (solid line for after and dashed line for before contrast agent injection, respectively). Each point on the profile is an average of 5 × 5 pixels and error bars show the respective standard deviation.
The average dGEMRIC-T1 value was sharply decreased after intravenous administration of gadolinium contrast agent in patients with OA (1244 ± 134 vs. 643 ± 227 milliseconds, P < .000002), depending upon the degree of cartilage degeneration. As shown in Figure 6, the average T1 values before contrast agent were 1239 ± 145, 1235 ± 148, 1304 ± 114, and 1244 ± 134 milliseconds for the femoral, tibial, patellar, and the average across the three regions in subjects with OA, respectively, whereas the average T1 values after contrast agent were 639 ± 235, 621 ± 193, 658 ± 252, and 643 ± 227 milliseconds for the femoral, tibial, patellar, and the average across the three regions in subjects with OA, respectively. There was no significant difference in T1 values among the three regions in subjects with OA before contrast agent injection with the range between 1235 ± 148 and 1304 ± 114 milliseconds and after contrast agent injection with the range between 621 ± 193 and 658 ± 252 milliseconds, respectively. However, there is a statistically significant difference between the before and after contrast agent injection images of each patient with OA. The P values between before and after contrast agent injection in subjects with OA were .000001, .000003, .000006, and .000003 for the femoral, tibial, patellar, and the average across the three regions, respectively. The RMS-CV % before contrast agent were 10.3, 10.1, 8.5, and 9.0 for the femoral, tibial, patellar, and the average across the three regions in subjects with OA, respectively. The RMS-CV % after contrast agent were 26.9, 22.0, 30.1, and 23.0 for the femoral, tibial, patellar, and the average across the three regions in subjects with OA, respectively.
Figure 6.

The average dGEMRIC-T1 value was sharply decreased after intravenous administration of gadolinium contrast agent in patients with OA (1244 ± 134 vs. 643 ± 227 milliseconds, P < .000002). The P values between before and after contrast agent injection in patients with OA were .000001, .000003, .000006, and .000003 for the femoral, tibial, patellar, and the average across the three regions, respectively.
DISCUSSION
In this study, we measured the parallel changes of T2, T1ρ, and dGEMRIC-T1 relaxation times of human knee cartilage in detecting early cartilage degeneration.
The T2 and T1ρ values of human cartilage were all strongly increased in patients with OA when compared to healthy controls. The T2 relaxation time is sensitive to slow molecular motions of water protons and anisotropy of the human cartilage and has been shown to demonstrate modifications in anatomically intact tissue in vivo (20,21). The inherent T2 relaxation time of cartilage is derived from the interactions of cartilage water with the cartilage macromolecules (predominantly from collagen integrity), which are modulated by their chemical and structural states. Our T2 values corresponded well with those already reported in the literature (11,22,26), with a range from 37 to 41 milliseconds for healthy controls and from 46 to 48 milliseconds for patients with OA. Previous in vitro studies (11,26–35) have reported that T2 relaxation time showed poor correlation with PG content and have shown that PG depletion did not affect T2 values significantly. This lack of ability in quantifying PG loss may make T2 mapping techniques less attractive in detecting early cartilage degeneration.
Although it was unlikely that the magic angle effect for T2 map accounted for regional differences in cartilage signal intensity in the case of the small orientation effect (11,40), the angular dependence on the external B0 magnetic field for T2 values has made it difficult in acquiring a correct appearance of T2 maps. On the other hand, previous in vivo (36–42) T2 values showed a strong increase in values in patients with OA when compared to healthy subjects, but the delta from controls was relatively limited, quite similar to our results. Therefore, it may be difficult to use T2 mapping to quantify cartilage degeneration longitudinally.
T1ρ-weighted MR imaging methods have been recently proposed as an attractive alternative to evaluate the biochemical changes in cartilage matrix noninvasively. This technique has been used to investigate the slow-motion interactions between the macromolecule protons and bulk water protons, as well as chemical exchange between different proton surroundings. This method has several advantages compared to the other existing methods. It can be easily translated to a clinical setting without any hardware modifications and requires no exogenous contrast agent. It was reported that T1ρ relaxation time is sensitive to early biochemical changes in cartilage, especially the PG content (2). Preliminary results in our in vivo studies have shown that the T1ρ values increased markedly for human cartilage in patients with OA compared to healthy controls. As displayed in Table 2, there is a larger delta in T1ρ values than in T2 values between healthy controls and patients with OA. Our work may indicate that T1ρ quantitation method is more sensitive than T2 in detecting the early cartilage degradation. T1ρ has been shown a close correlation with the PG content in in vitro studies (1,3,11,17,43–45), and can be provided with a more-sensitive indicator of PG degeneration than T2 in trypsin-depleted cartilage. Our T1ρ in vivo studies correspond well with those reported in the literature (2,11,22,46,47) with a more obvious increase in patients with OA. T1ρ relaxation times are comparably isolated from the orientation of collagen that can affect T2 mapping techniques (11).
The dGEMRIC-T1 mapping technique has been validated in many studies to allow the evaluation of the PG degeneration of human cartilage (4–10). Two separate imaging sessions before and after contrast agent administration with an intervention of 90-minute waiting (8) make this quantitation method time-consuming. Previous work has, however, shown it to be perhaps the most sensitive MR quantitation method in detecting early cartilage degradation. In vitro studies (22,48–52) have found that regions of trypsin-depleted cartilage showed histologic differences and marked changes in the postcontrast Gd(DTPA)2− signal intensity and T1 relaxation times. Our dGEMRIC-T1 results were similar (22,53–57). The dGEMRIC-T1 technique is reliable in evaluating early OA because it can provide the valid information on the distribution and content of GAG about cartilage (22). There are still some disadvantages about this technique, such as the issues of the concentration of contrast agent and the delay time for imaging after contrast agent injection.
T2 and T1ρ methods do not require any hardware modifications or contrast agent administration and may provide independent or complementary information about the disease process. Similarly, dGEMRIC and T1ρ relaxation times seem to provide similar information regarding the cartilage degeneration and are more robust than T2 in detecting early cartilage degeneration. However, further studies are required for exact slice-by-slice spatial correlation. Generally, greater T1ρ values can show the degeneration of PG in cartilage regions. Likewise, the dGEMRIC-T1 also shows the same lesions with the degeneration of PG in cartilage. Loss of PG in cartilage will give rise to more contrast agent penetration in cartilage and then lowered dGEMRIC-T1 values with the cartilage regions.
Our present study has some limitations in terms of small sample size, and we did not compare the relaxation times within the OA population. Our preliminary results suggested that the T2 and T1ρ were increased with cartilage degeneration. However, in the case of dGEMRIC, we have to compare the range of T1 values with that of controls. Currently, we do not have control dGEMRIC-T1 values in the presence of contrast agent for some technical reasons, such as involuntary administration of contrast agent with healthy controls. Likewise, the age mismatch between healthy controls and patients with OA is a deficiency of the present study, which possibly affects the significance of the comparison results. We cannot recruit enough appropriate healthy controls with the same age range as the OA group (53–82 years); further work is warranted concerning this drawback.
CONCLUSION
The preliminary results indicate that the in vivo T2, T1ρ, and dGEMRIC-T1 mapping techniques have demonstrated their parallel changes in detecting the cartilage degeneration. All the T2, T1ρ, and dGEMRIC-T1 relaxation times varied obviously with the cartilage degeneration. The dGEMRIC-T1 and T1ρ relaxation times seem to be more sensitive than T2 in detecting early cartilage degeneration.
Acknowledgments
The authors acknowledge the support by research grants RO1 AR053133, RO1 AR056260, and RO1 AR060238 A2 from the National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health, USA. This work was also supported in part by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China. Dr. Jeff L. Zhang, Department of Radiology, University of Utah, is thanked for technical support.
References
- 1.Menezes NM, Gray ML, Hartke JR, et al. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51:503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 2.Pakin SK, Xu J, Schweitzer ME, et al. Rapid 3D-T1rho mapping of the knee joint at 3. 0T with parallel imaging. Magn Reson Med. 2006;56:563–571. doi: 10.1002/mrm.20982. [DOI] [PubMed] [Google Scholar]
- 3.Mlynarik V, Trattnig S, Huber M, et al. The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. J Magn Reson Imaging. 1999;10:497–502. doi: 10.1002/(sici)1522-2586(199910)10:4<497::aid-jmri1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Williams A, Gillis A, McKenzie C, et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. AJR Am J Roentgenol. 2004;182:167–172. doi: 10.2214/ajr.182.1.1820167. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie CA, Williams A, Prasad PV, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5T and 3. 0T. J Magn Reson Imaging. 2006;24:928–933. doi: 10.1002/jmri.20689. [DOI] [PubMed] [Google Scholar]
- 6.Masi JN, Newitt D, Sell CA, et al. Optimization of gadodiamide concentration for MR arthrography at 3 T. AJR Am J Roentgenol. 2005;184:1754–1761. doi: 10.2214/ajr.184.6.01841754. [DOI] [PubMed] [Google Scholar]
- 7.Tiderius CJ, Olsson LE, Leander P, et al. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49:488–492. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]
- 8.Burstein D, Velyvis J, Scott KT, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Bashir A, Gray ML, Hartke J, et al. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857–865. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Kimelman T, Vu A, Storey P, et al. Three-dimensional T1 mapping for dGEMRIC at 3. 0 T using the Look Locker method. Invest Radiol. 2006;41:198–203. doi: 10.1097/01.rli.0000195842.49255.ea. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Osteoarthr Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier CF, Tan SG, Hariharan H, et al. T2 quantitation of articular cartilage at 1. 5 T. J Magn Reson Imaging. 2003;17:358–364. doi: 10.1002/jmri.10263. [DOI] [PubMed] [Google Scholar]
- 13.Deichmann R, Adolf H, Noth U, et al. Fast T2-mapping with snapshot flash imaging. Magn Reson Imaging. 1995;13:633–639. doi: 10.1016/0730-725x(95)00004-z. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie CA, Chen Z, Drost DJ, et al. Fast acquisition of quantitative T2 maps. Magn Reson Med. 1999;41:208–212. doi: 10.1002/(sici)1522-2594(199901)41:1<208::aid-mrm30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Regatte RR, Akella SV, Borthakur A, et al. In vivo proton MR three-dimensional T1rho mapping of human articular cartilage: initial experience. Radiology. 2003;229:269–274. doi: 10.1148/radiol.2291021041. [DOI] [PubMed] [Google Scholar]
- 16.Regatte RR, Akella SV, Borthakur A, et al. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging. 2003;17:114–121. doi: 10.1002/jmri.10228. [DOI] [PubMed] [Google Scholar]
- 17.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 18.Redfield AG. Nuclear magnetic resonance saturation and rotary saturation in solids. Phys Rev. 1955;98:1787–1809. [Google Scholar]
- 19.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieminen MT, Menezes NM, Williams A, et al. T2 of articular cartilage in the presence of Gd-DTPA2. Magn Reson Med. 2004;51:1147–1152. doi: 10.1002/mrm.20083. [DOI] [PubMed] [Google Scholar]
- 21.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 22.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 23.Klocke NF, Amendola A, Thedens DR. Comparison of T1ρ, dGEMRIC, and quantitative T2 MRI in preoperative ACL rupture patients. Acad Radiol. 2013;20:99–107. doi: 10.1016/j.acra.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regatte RR, Akella SV, Lonner JH, et al. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich KM, Shepard T, de Oliveira VS, et al. T2 measurements of cartilage in osteoarthritis patients with meniscal tears. AJR Am J Roentgenol. 2009;193:W411–W415. doi: 10.2214/AJR.08.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David-Vaudey E, Ghosh S, Ries M, et al. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin DW, Wadghiri YZ, Dunn JF. Micro-imaging of articular cartilage: T2, proton density, and the magic angle effect. Acad Radiol. 1998;5:790–798. doi: 10.1016/s1076-6332(98)80264-x. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin DW, Dunn JF. High-resolution magnetic resonance imaging of articular cartilage: correlation with histology and pathology. Top Magn Reson Imaging. 1998;9:337–347. [PubMed] [Google Scholar]
- 29.Goodwin DW, Wadghiri YZ, Zhu H, et al. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004;182:311–318. doi: 10.2214/ajr.182.2.1820311. [DOI] [PubMed] [Google Scholar]
- 30.Lammentausta E, Kiviranta P, Nissi MJ, et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9. 4 T: relationships with tissue mechanical properties. J Orthop Res. 2006;24:366–374. doi: 10.1002/jor.20041. [DOI] [PubMed] [Google Scholar]
- 31.Watrin-Pinzano A, Ruaud JP, Cheli Y, et al. Evaluation of cartilage repair tissue after biomaterial implantation in rat patella by using T2 mapping. Magma. 2004;17:219–228. doi: 10.1007/s10334-004-0071-7. [DOI] [PubMed] [Google Scholar]
- 32.Watrin-Pinzano A, Ruaud JP, Cheli Y, et al. T2 mapping: an efficient MR quantitative technique to evaluate spontaneous cartilage repair in rat patella. Osteoarthr Cartilage. 2004;12:191–200. doi: 10.1016/j.joca.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Watrin-Pinzano A, Ruaud JP, Olivier P, et al. Effect of proteoglycan depletion on T2 mapping in rat patellar cartilage. Radiology. 2005;234:162–170. doi: 10.1148/radiol.2341030394. [DOI] [PubMed] [Google Scholar]
- 34.Xia Y, Farquhar T, Burton-Wurster N, et al. Diffusion and relaxation mapping of cartilage-bone plugs and excised disks using microscopic magnetic resonance imaging. Magn Reson Med. 1994;31:273–282. doi: 10.1002/mrm.1910310306. [DOI] [PubMed] [Google Scholar]
- 35.Xia Y. Relaxation anisotropy in cartilage by NMR microscopy (muMRI) at 14-microm resolution. Magn Reson Med. 1998;39:941–949. doi: 10.1002/mrm.1910390612. [DOI] [PubMed] [Google Scholar]
- 36.Dardzinski BJ, Mosher TJ, Li S, et al. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205:546–550. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 37.Dardzinski BJ, Laor T, Schmithorst VJ, et al. Mapping T2 relaxation time in the pediatric knee: feasibility with a clinical 1. 5-T MR imaging system. Radiology. 2002;225:233–239. doi: 10.1148/radiol.2251011461. [DOI] [PubMed] [Google Scholar]
- 38.Dunn TC, Lu Y, Jin H, et al. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2-preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 40.Mosher TJ, Smith H, Dardzinski BJ, et al. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol. 2001;177:665–669. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]
- 41.Mosher TJ, Smith HE, Collins C, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234:245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 42.Smith HE, Mosher TJ, Dardzinski BJ, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 43.Duvvuri U, Reddy R, Patel SD, et al. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 44.Mlynarik V, Szomolanyi P, Toffanin R, et al. Transverse relaxation mechanisms in articular cartilage. J Magn Reson. 2004;169:300–307. doi: 10.1016/j.jmr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Wheaton AJ, Dodge GR, Elliott DM, et al. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54:1087–1093. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 46.Wheaton AJ, Dodge GR, Borthakur A, et al. Detection of changes in articular cartilage proteoglycan by T(1rho) magnetic resonance imaging. J Or-thop Res. 2005;23:102–108. doi: 10.1016/j.orthres.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheaton AJ, Borthakur A, Kneeland JB, et al. In vivo quantification of T1rho using a multislice spin-lock pulse sequence. Magn Reson Med. 2004;52:1453–1458. doi: 10.1002/mrm.20268. [DOI] [PubMed] [Google Scholar]
- 48.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 49.Kurkijarvi JE, Nissi MJ, Kiviranta I, et al. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: topographical variation and relationships to mechanical properties. Magn Reson Med. 2004;52:41–46. doi: 10.1002/mrm.20104. [DOI] [PubMed] [Google Scholar]
- 50.Nieminen MT, Toyras J, Laasanen MS, et al. Prediction of biomechanical properties of articular cartilage with quantitative magnetic resonance imaging. J Biomech. 2004;37:321–328. doi: 10.1016/s0021-9290(03)00291-4. [DOI] [PubMed] [Google Scholar]
- 51.Nissi MJ, Toyras J, Laasanen MS, et al. Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res. 2004;22:557–564. doi: 10.1016/j.orthres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Samosky JT, Burstein D, Eric Grimson W, et al. Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. J Orthop Res. 2005;23:93–101. doi: 10.1016/j.orthres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham T, Jessel R, Zurakowski D, et al. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage to predict early failure of Bernese periacetabular osteotomy for hip dysplasia. J Bone Joint Surg Am. 2006;88:1540–1548. doi: 10.2106/JBJS.E.00572. [DOI] [PubMed] [Google Scholar]
- 54.Gillis A, Bashir A, McKeon B, et al. Magnetic resonance imaging of relative glycosaminoglycan distribution in patients with autologous chondrocyte transplants. Invest Radiol. 2001;36:743–748. doi: 10.1097/00004424-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosami-noglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–3514. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 56.Tiderius C, Hori M, Williams A, et al. dGEMRIC as a function of BMI. Osteoarthr Cartilage. 2006;14:1091–1097. doi: 10.1016/j.joca.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Williams A, Sharma L, McKenzie CA, et al. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage in knee osteoarthritis: findings at different radiographic stages of disease and relationship to malalignment. Arthritis Rheum. 2005;52:3528–3535. doi: 10.1002/art.21388. [DOI] [PubMed] [Google Scholar]