Abstract
Background: Despite notable progress in the fight against iodine deficiency disorders in the Democratic Republic of Congo, a recent study has shown that pregnant women in Lubumbashi were still iodine deficient. Our objective was to assess thyroid function in this population.
Methods: In a cross-sectional study conducted in maternity units from three different socioeconomic areas in Lubumbashi, serum thyrotropin, free thyroxine, thyroglobulin, and thyroperoxidase antibodies were measured in 225 pregnant women attending antenatal visits, in 75 women who recently delivered, and in 75 nonpregnant controls. The outcome was the prevalence of thyroid dysfunction.
Results: Median values in pregnant women, women who recently delivered, and nonpregnant women were 1.80, 2.80, and 1.54 mIU/L for thyrotropin (p<0.001); 0.85, 1.11, and 1.16 ng/dL for free thyroxine (p<0.001); and 13.3, 9.5, and 10.4 ng/mL for thyroglobulin (p=0.01), respectively. The prevalence of thyroid dysfunction in pregnant women, in women who recently delivered, and in nonpregnant women was 31%, 8%, and 20% for isolated hypothyroxinemia (p<0.001); 12%, 24%, and 5% for subclinical hypothyroidism (p=0.002); 8%, 3%, and 3%, for overt hypothyroidism (p=0.09); and 5%, 13%, and 4%, for positive thyroperoxidase antibodies (p=0.03), respectively. In multiple logistic regression, women who were pregnant or who recently delivered, who lived in a poor socioeconomic area, and who had low urinary iodine concentration were more likely to have an increased serum thyrotropin: odds ratio (OR)=3.43 (95% confidence interval [CI] 1.23–9.53) for pregnancy, OR=4.49 [CI 1.66–15.01] for postpartum period, OR=3.68 [CI 1.85–7.35] for semiurban area, and OR=0.44 [CI 0.19–0.96] for urinary iodine concentration ≥250 μg/L.
Conclusions: Our results show that there is a high prevalence of thyroid dysfunction in pregnant women of Lubumbashi, and this high prevalence is associated with iodine deficiency. To prevent obstetrical adverse outcomes and neurological damage in children, iodine supplementation is needed before conception or in early pregnancy in Lubumbashi.
Introduction
Thyroid dysfunction is the most frequent endocrine disorder in pregnant women. Overt hypothyroidism and even subclinical hypothyroidism increases the risk of obstetric complications: miscarriage, fetal death, gestational hypertension, preterm birth, and low birth weight (1–5). When occurring early in pregnancy, hypothyroidism can cause cognitive and neurodevelopment retardation in children. Moreover, thyroid autoantibodies in pregnancy are also associated with recurrent miscarriage (6) and with maternal morbidity later in life (7). Maintaining a normal thyroid function during pregnancy is therefore of predominant importance for the mother and for the child.
Maintaining a pregnant woman in a euthyroid state is a challenge for the thyroid gland during gestation because of an increased thyroid hormone demand and decreased iodine availability due to iodine transfer to the fetus and intensified iodine urinary losses induced by the increased renal glomerular filtration (8,9). Physiological adaptations take place when iodine intake is sufficient and when there is no underlying thyroid pathology: the increase in estrogens induces an increase in thyroxine (T4)-binding globulin, which alters the ratio between free and bound thyroid hormones; the increase in human chorionic gonadotropin hormone, which has thyrotrophic activity, induces a slight increase in free thyroxine (FT4) with a peak at the end of the first trimester, and this causes a transient decrease in thyrotropin (TSH) through negative feedback on pituitary thyrotrophs (8,10–12); the placental deiodinase III alters metabolism, distribution, and availability of T4 for the mother and for the fetus in the second half of gestation (12,13).
When iodine intake is mildly to moderately deficient, there may not be enough T4 available to the fetus, and this may not be recognized because TSH does not necessarily increase because of stable or slightly increased triiodothyronine (T3) levels (12). Severe iodine deficiency can result in overt hypothyroxinemia, goiter, and the spectrum of iodine deficiency disorders (11).
The Democratic Republic of Congo has adopted a salt iodization strategy in 1993 to fight against iodine deficiency disorders, with a recommended iodine content of salt set between 30 and 100 ppm. The latest national surveys on iodine status reported a sufficient iodine intake at the national level, a prevalence of goiter below 1%, and availability of iodized table salt in more than 97% of households (14). However, a study by Kitwa et al. (15) showed that noniodized salt was still commercialized in Lubumbashi markets, despite the fact that salt iodization is mandatory, and the iodine content of table salt collected in households was under the threshold limit of 15 ppm for 36.3% of samples. In a recent study, we showed that iodine intake varied across socioeconomic levels in Lubumbashi, and that pregnant women in rural and semiurban areas of Lubumbashi were moderately and mildly iodine deficient, with a median urinary iodine of 97 and 145 μg/L, respectively, while pregnant women in an urban area had an adequate iodine intake, with a median urinary iodine of 168 μg/L (16). As thyroid dysfunction might be a consequence of iodine deficiency, the objective of the present study was to assess the prevalence of thyroid dysfunction and its potential link with iodine deficiency in these pregnant women in Lubumbashi.
Subjects and Methods
We conducted a cross-sectional study between March 2009 and February 2011 in three maternity units from Lubumbashi. As we did not know the size of the population in the different districts of Lubumbashi, and for the purpose of sample representativeness, we recruited women in three maternities serving populations from different socioeconomic strata. Lubumbashi University Clinics, located in an urban area, serves a population with the highest socioeconomic status; Bongonga Health Centre, located in a semiurban area, provides services to individuals with a middle status; and Katuba General Hospital, located in a rural area, serves a population with the lowest socioeconomic status. The study was designed to assess thyroid function of pregnant women and its relationship with their iodine intake.
The sample size was calculated to have a power of 80% to detect a difference of at least 50 μg/L in urinary iodine concentration (UIC) between pregnant women and nonpregnant controls, using a significance level of 5% and assuming a balance between the three trimesters of pregnancy and the three maternities. A total of 375 women were recruited, and 225 pregnant women were randomly selected among those attending antenatal visits: 25 in the 1st trimester of pregnancy, 25 in the 2nd, and 25 in the 3rd trimester, in each of the three maternity units. Seventy-five nonpregnant women of childbearing age were selected as controls among visitors, 25 by maternity; 75 women who recently delivered were also included in the study at their 3rd postpartum day (25 in each maternity unit).
Twin pregnancies were not included in the study (17), nor were women with known thyroid disease. All participants gave written informed consent. The study protocol was approved by the Ethics Committee of The University of Lubumbashi before implementation.
Each participant gave a blood sample and a spot urine sample for measurement of UIC. Women were questioned about sociodemographic, clinical, and epidemiologic data. Gestational age was calculated from the last menstrual date and verified by ultrasonography. All blood and urine samples were stored at −20°C until measurement in the Laboratory of Lubumbashi University Clinics.
Iodine was measured in spot urine samples using a modified Sandell–Kolthoff reaction between arsenic and cerium, after mineralization with ammonium persulfate digestion, by microplate reading (Multiskan EX Microplate Photometer, Primary EIA V.2.1–0, Labsystems; Thermo Scientific). UIC was used to categorize iodine intake as proposed by the World Health Organization (18): iodine intake was considered adequate during pregnancy when the median UIC was found to be between 150 and 249 μg/L, deficient when the median UIC was <150 μg/L, more than adequate when the median UIC was between 250 and 499 μg/L, and excessive when the median UIC was ≥500 μg/L. In nonpregnant women, iodine intake was considered adequate if the median UIC was between 100 and 199 μg/L. Results of the iodine status of women in Lubumbashi have been published previously and are summarized in Table 1 (16).
Table 1.
Age, Parity, and Urinary Iodine Concentration of 375 Childbearing-Aged Women in Lubumbashi, Democratic Republic of Congo
| All maternity units | Age, years, mean±SD (min–max) | Parity, p50 (p25–p75) | UIC, μg/L, p50 (p25–p75) |
|---|---|---|---|
| All pregnant (n=225) | 27±7 (15–43) | 3 (2–6) | 138 (57–321) |
| 1st trimester (n=76) | 28±6 (15–42) | 3 (2–6) | 238 (115–387) |
| 2nd trimester (n=71) | 26±7 (15–43) | 3 (2–5) | 129 (69–286) |
| 3rd trimester (n=78) | 27±7 (16–41) | 3 (1–6) | 77 (27–222) |
| p-valuea | 0.005 | 0.24 | <0.001 |
| Postpartum (n=75) | 27±7 (15–43) | 3 (2–6) | 144 (97–300) |
| Nonpregnant (n=75) | 32±8 (17–52) | 2 (0–7) | 204 (95–331) |
| p-valueb | <0.001 | <0.001 | 0.09 |
Adapted from Habimana et al. (16).
Comparison between trimesters of gestation.
Comparison between pregnant, postpartum, and nonpregnant women.
UIC, urinary iodine concentration.
We assessed thyroid function by measuring serum TSH and FT4. Blood samples were also analyzed for free triiodothyronine (FT3), thyroglobulin (Tg), and antithyroperoxidase antibody (TPOAb) concentrations. Measurements were made by an immunoenzymatic colorimetric method with the ETI-System fast reader ELX 800, and using commercial kits supplied by DiaSorin (DiaMetra; SA/NV DiaSorin Benelux). For TSH, we used the reference ranges established in 2011 by the American Thyroid Association Guidelines for pregnant women (19) and we used the Manufacturer's reference ranges for other biochemical analyses. The reference ranges for TSH were 0.1–2.5 mIU/L in the 1st trimester, 0.2–3.0 mIU/L in the 2nd trimester, 0.3–3.0 mIU/L in the 3rd trimester, 0.3–3.6 mIU/L for the postpartum period, and 0.3–4.0 mIU/L for nonpregnant women; for FT4, it was 0.8–2.0 ng/dL for nonpregnant women and 0.8–2.2 ng/dL for pregnant women; for FT3, it was 1.4–4.2 pg/mL for nonpregnant women and 1.8–4.2 pg/mL for pregnant women; for Tg, it was lower than 40 ng/mL in all cases; TPOAbs were considered positive if ≥20 AU/mL (AU=arbitrary units from DiaMetra kits).
Euthyroidism was defined as a normal TSH with normal FT4. Subclinical hypothyroidism was defined as an elevated TSH with normal FT4, overt hypothyroidism as high TSH with low FT4, isolated hypothyroxinemia as low FT4 with normal TSH, and subclinical hyperthyroidism was defined as low TSH with normal FT4 (20,21). TPOAb positives were considered apart.
Statistical analyses
Continuous data are reported as arithmetic mean and standard deviation when normally distributed, and as median with quartiles otherwise. Discrete data are reported as proportions. We used the Pearson's chi-square test to compare proportions, and Mann–Whitney U or ANOVA with the Kruskal–Wallis test to compare medians between groups. Correlation between continuous variables was assessed on a log scale, using the Pearson coefficient. Logistic regression was used to assess predictors of an increased TSH. Sociodemographic variables (age, parity, and socioeconomic area), group of women (pregnancy, early postpartum, or nonpregnant), UIC (<150 μg/L, 150–250 μg/L, or≥250 μg/L), and TPOAb status (13,22) were initially examined by univariate analyses; all variables with a p-value<0.20 were submitted to a multivariate regression analysis with a stepwise backward elimination procedure, using p>0.05 to remove from the model. All tests were two sided, and the significance level was set to 0.05. Statistical analyses were performed using SAS 9.3 statistical software.
Results
Characteristics of the participants and UICs are summarized in Table 1. Pregnant women and women who recently delivered were aged 27±7 years. Half of the pregnant women had 3 previous pregnancies and 25% had at least 6. Women with at most one previous pregnancy were younger than multiparous women (21±4 vs. 29±6 years, p<0.001). Most women were married and nearly all were housewives. The median UIC in pregnant women was 138 μg/L (interquartile range 57–321), lower than the median UIC in nonpregnant controls of 204 μg/L (interquartile range 95–331), p=0.04, with a gradient from the 1st to the 3rd trimester of gestation (median UIC=238 μg/L in the 1st trimester, 129 μg/L in the 2nd, and 77 μg/L in the 3rd trimester, p<0.001). About 52% of pregnant women had a UIC below 150 μg/L, 42% of pregnant women had a UIC below 100 μg/L, and 23% had a UIC below 50 μg/L. On a log scale, a lower UIC was associated with greater gestational age (r=−0.24, p<0.001); UIC did not correlate with age.
Tables 2, 3, and 4 report median values with quartiles for TSH, FT4, FT3, and Tg, and proportions of TPOAb-positive individuals, for pregnant and nonpregnant women in all maternity units (Table 2), according to socioeconomic area and trimesters of gestation (Table 3), as well as UIC levels and TPOAb status (Table 4). The median TSH in pregnant women was 1.80 mIU/L, higher than the 1.54 mIU/L found in nonpregnant women, but lower than the 2.80 mIU/L observed in postpartum women (p<0.001). During pregnancy, TSH increased with trimester in rural (p=0.19) and semiurban areas (p<0.001). TSH decreased with parity, but the association was borderline (r=−0.13, p=0.05), and maternal age did not modify this association. Pregnant women with a UIC <150 μg/L had higher TSH levels than women with a UIC ≥250 μg/L (p=0.002).
Table 2.
Biochemical Thyroid Indices in 375 Childbearing-Aged Women in Lubumbashi, Democratic Republic of Congo
| All maternity units | TSH, mIU/L | FT4, ng/dL | FT3, pg/mL | Tg, ng/mL | TPOAb positive |
|---|---|---|---|---|---|
| All pregnant (n=225) | 1.80 (1.13–2.63) | 0.85 (0.67–1.00) | 1.44 (1.01–2.23) | 13.3 (4.7–23.2) | 12 (5.3) |
| 1st trimester (n=76) | 1.54 (0.82–2.12) | 0.80 (0.55–0.99) | 1.56 (0.81–2.75) | 9.2 (3.0–16.4) | 7 (9.2) |
| 2nd trimester (n=71) | 1.99 (1.28–2.78) | 0.86 (0.71–0.98) | 1.41 (1.09–2.00) | 14.3 (5.3–23.7) | 5 (7.0) |
| 3rd trimester (n=78) | 2.06 (1.34–2.83) | 0.86 (0.68–1.04) | 1.52 (1.07–1.98) | 15.9 (7.4–25.5) | 0 (0.0) |
| p-valuea | 0.005 | 0.24 | 0.78 | 0.01 | 0.02 |
| Postpartum (n=75) | 2.80 (1.75–3.78) | 1.11 (0.94–1.45) | 0.91 (0.60–1.60) | 11.8 (8.0–15.1) | 10 (13.3) |
| Nonpregnant (n=75) | 1.54 (1.11–2.98) | 1.16 (0.79–1.38) | 1.71 (1.28–2.37) | 11.8 (8.5–15.6) | 3 (4.0) |
| p-valueb | <0.001 | <0.001 | <0.001 | 0.01 | 0.03 |
Data are medians with quartiles in parentheses, and number and percentages in parentheses for TPOAb positive.
Comparison between trimesters of gestation.
Comparison between pregnant, postpartum, and nonpregnant women.
FT3, free triiodothyronine; FT4, free thyroxine; Tg, thyroglobulin; TPOAb, antithyroperoxidase antibody; TSH, thyrotropin.
Table 3.
Biochemical Thyroid Indices of 375 Women in Lubumbashi, Democratic Republic of Congo, According to Socioeconomic Area
| TSH, mIU/L | FT4, ng/dL | FT3, pg/mL | Tg, ng/mL | TPOAb positive | |
|---|---|---|---|---|---|
| Rural area | |||||
| All pregnant (n=75) | 1.99 (1.30–2.81) | 0.81 (0.69–0.92) | 1.46 (1.09–2.19) | 15.4 (6.4–24.6) | 0 (0.0) |
| T1 (n=25) | 1.70 (0.89–2.50) | 0.99 (0.88–1.15) | 1.61 (0.94–2.19) | 11.3 (6.4–21.0) | 0 (0.0) |
| T2 (n=25) | 2.07 (1.26–2.79) | 0.78 (0.69–0.82) | 1.57 (1.15–2.28) | 14.3 (7.4–27.4) | 0 (0.0) |
| T3 (n=25) | 2.40 (1.73–2.96) | 0.73 (0.68–0.82) | 1.22 (1.04–1.67) | 18.7 (4.8–27.4) | 0 (0.0) |
| p-valuea | 0.19 | <0.001 | 0.27 | 0.57 | |
| Postpartum (n=25) | 2.51 (1.69–3.08) | 1.45 (1.04–1.58) | 1.61 (0.54–2.11) | 9.8 (7.2–12.7) | 0 (0.0) |
| Nonpregnant (n=25) | 1.71 (0.69–3.29) | 0.98 (0.66–1.22) | 1.64 (0.57–2.36) | 11.4 (8.5–16.5) | 1 (4.0) |
| p-valueb | 0.001 | <0.001 | 0.78 | 0.002 | |
| Semiurban area | |||||
| All pregnant (n=75) | 2.10 (1.15–3.38) | 0.95 (0.62–1.11) | 2.11 (1.42–3.22) | 12.0 (5.8–18.7) | 9 (12.0) |
| T1 (n=26) | 1.05 (0.33–1.75) | 0.58 (0.33–0.79) | 2.91 (1.06–3.79) | 6.2 (1.5–11.9) | 7 (26.9) |
| T2 (n=24) | 2.28 (1.41–3.34) | 0.98 (0.80–1.09) | 1.70 (1.33–2.29) | 14.3 (6.9–25.5) | 2 (8.3) |
| T3 (n=25) | 2.75 (2.14–4.26) | 1.10 (0.98–1.27) | 1.97 (1.61–2.78) | 14.2 (10.5–19.5) | 0 (0.0) |
| p-valuea | <0.001 | <0.001 | 0.10 | 0.002 | |
| Postpartum (n=25) | 3.73 (2.64–5.25) | 1.09 (0.95–1.49) | 0.67 (0.54–0.93) | 11.9 (7.9–17.0) | 1 (4.0) |
| Nonpregnant (n=25) | 2.26 (1.78–3.88) | 1.38 (1.32–1.48) | 2.25 (1.63–2.61) | 9.4 (6.9–15.1) | 1 (4.0) |
| p-valueb | <0.001 | <0.001 | <0.001 | 0.01 | |
| Urban area | |||||
| All pregnant (n=75) | 1.43 (0.97–2.14) | 0.84 (0.60–0.96) | 1.10 (0.71–1.43) | 12.3 (0.0–23.8) | 3 (4.0) |
| T1 (n=25) | 1.86 (1.15–2.59) | 0.76 (0.58–0.91) | 1.05 (0.67–1.55) | 10.1 (1.9–18.3) | 0 (0.0) |
| T2 (n=22) | 1.43 (1.16–2.03) | 0.89 (0.74–0.96) | 1.09 (0.75–1.33) | 14.5 (0.0–22.6) | 3 (13.6) |
| T3 (n=28) | 1.27 (0.81–2.02) | 0.80 (0.51–0.96) | 1.15 (0.83–1.69) | 16.4 (0.0–36.3) | 0 (0.0) |
| p-valuea | 0.17 | 0.34 | 0.68 | 0.35 | |
| Postpartum (n=25) | 2.08 (1.05–3.34) | 1.05 (0.78–1.12) | 1.06 (0.74–1.52) | 11.9 (8.0–15.4) | 9 (36.0) |
| Nonpregnant (n=25) | 1.72 (1.08–2.60) | 1.02 (0.65–1.23) | 1.60 (1.32–1.88) | 11.9 (9.5–15.6) | 1 (4.0) |
| p-valueb | 0.14 | 0.009 | <0.001 | 0.83 | |
Data are medians with quartiles in parentheses, and number and percentages in parentheses for TPOAb positive.
Comparison between trimesters of gestation.
Comparison between pregnant, postpartum, and nonpregnant women.
T1, 1st trimester; T2, 2nd trimester; T3, 3rd trimester.
Table 4.
Biochemical Thyroid Indices in 220 Pregnant Women in Lubumbashi, Democratic Republic of Congo, According to Urinary Iodine Concentration and Thyroperoxidase Antibody Status
| TSH, mIU/L | FT4, ng/dL | FT3, pg/mL | Tg, ng/mL | TPOAb positive | |
|---|---|---|---|---|---|
| UIC, μg/L | |||||
| <150 (n=115) | 2.06 (1.29–3.07) | 0.86 (0.67–1.03) | 1.57 (1.09–2.28) | 14.2 (5.6–24.3) | 1 (0.9) |
| 150–249 (n=35) | 2.00 (1.44–2.81) | 0.79 (0.67–0.97) | 1.30 (1.01–2.18) | 13.0 (5.6–20.9) | 2 (5.7) |
| ≥250 (n=70) | 1.42 (0.90–2.05) | 0.85 (0.67–0.98) | 1.38 (0.90–2.23) | 12.3 (4.5–20.4) | 8 (11.4) |
| p-valuea | 0.002 | 0.58 | 0.44 | 0.45 | <0.001 |
| TPOAb status | |||||
| Positive (n=12) | 1.06 (0.45–2.85) | 0.35 (0.02–0.84) | 1.86 (0.70–3.09) | 4.3 (0.7–9.8) | |
| Negative (n=213) | 1.86 (1.20–2.63) | 0.85 (0.69–1.00) | 1.44 (1.04–2.19) | 13.8 (5.4–23.4) | |
| p-valueb | 0.14 | 0.002 | 0.88 | 0.007 | |
Data are medians with quartiles in parentheses, and number and percentages in parentheses for TPOAb positive.
Comparison between UIC category.
Comparison between TPOAb status.
The median FT4 of pregnant women was 0.85 ng/dL, lower than the one found in nonpregnant and in postpartum women (1.16 and 1.11 ng/dL, respectively, p<0.001). The median FT3 was 1.44 pg/mL in pregnant women, higher than the 0.91 pg/mL found in women who had delivered and lower than the 1.71 pg/mL observed in nonpregnant women (p<0.001); FT3 and FT4 did not correlate with UIC.
Thyroid peroxidase autoantibodies were positive (TPOAb >20 AU/mL) in 25 women: 12/225 (5%) pregnant women (with a gradient in trimesters of gestation: 7/76 [9.2%] in the 1st trimester, 5/71 [7.0%] in the 2nd, and none [0%] in the 3rd trimester); 10/75 (13%) women who recently delivered; and 3/75 (4%) nonpregnant women (p=0.03). TPOAbs were more often positive in pregnant women with a UIC >250 μg/L (11.4%, compared with 5.7% in individuals with an adequate UIC and 0.9% with a UIC <150 μg/L, p<0.01). TPOAb-positive women had lower FT4 and lower Tg levels than TPOAb-negative pregnant women (p<0.01). TPOAbs were more frequently elevated in pregnant women in the semiurban area (12%) than in the urban environment (4%), and they were absent in the rural area (p=0.03). Multiparous women were more often TPOAb positive (8.7% vs. 1.0%, p=0.008).
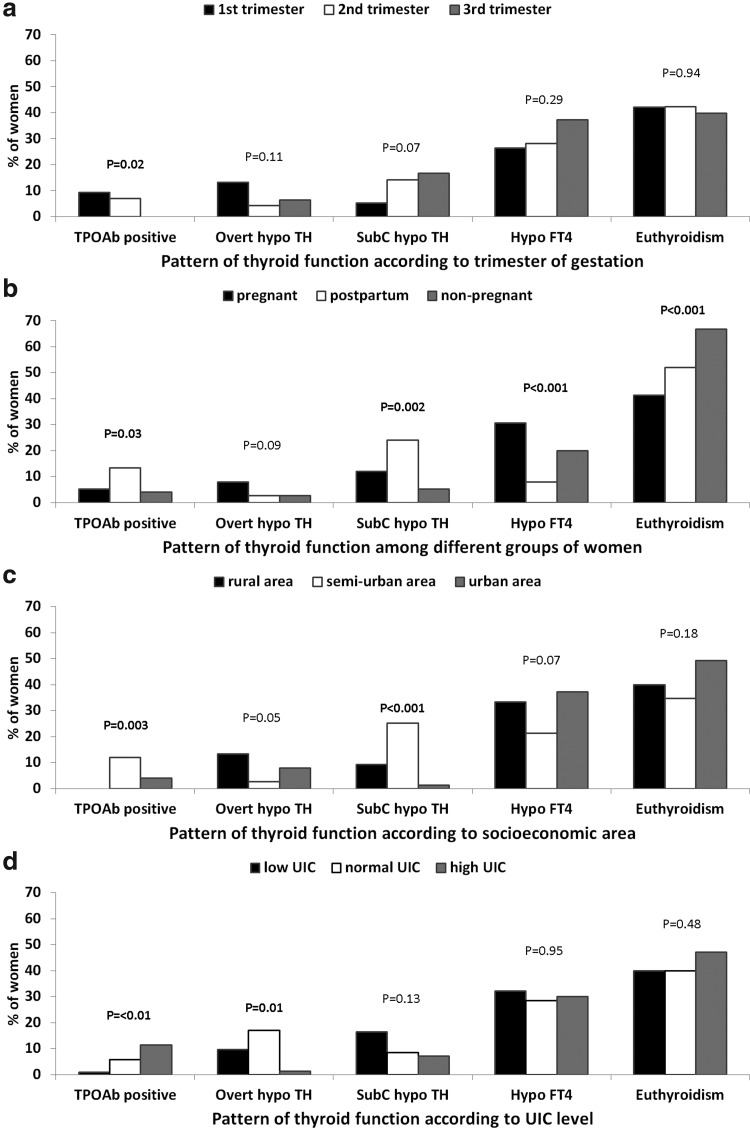
Figure 1 shows the patterns of thyroid function in participants. About 59% of pregnant women and 48% of postpartum women had alterations in thyroid function, as compared with 33% in nonpregnant women (p<0.001). Isolated hypothyroxinemia was more frequent in pregnant women than in other women (31% vs. 8% in the early postpartum period, and 20% in nonpregnant women, p<0.001), but the prevalence of isolated hypothyroxinemia was not statistically different according to the trimester of gestation, socioeconomic area, and UIC level. Overt hypothyroidism was also more frequent in pregnant women: 8% versus 3% in women in the early postpartum period and in nonpregnant women (p=0.09); it was less frequent when the UIC was high: 1.4% when the UIC was ≥250 μg/L compared with 10% when the UIC <150 μg/L, and 17% when the UIC was adequate (p=0.01). Subclinical hypothyroidism was present in 12% of pregnant women, in 24% of women in the early postpartum period, and in 5% of nonpregnant control women (p=0.002); subclinical hypothyroidism was more frequent in pregnant women in semiurban and rural areas than in the urban area (p<0.001) and when UIC was low: 17% when the UIC <150 μg/L, and 9% or 7% when the UIC was adequate or excessive, respectively (p=0.13).
FIG. 1.
Patterns of thyroid function in 225 pregnant women from three maternity units in Lubumbashi: (a) according to trimester of pregnancy; (b) as compared with 75 postpartum and 75 nonpregnant women; (c) according to socioeconomic area (rural, semiurban, and urban areas); (d) according to urinary iodine concentration (UIC; low, <150 μg/L; normal, 150–249 μg/L; high, ≥250 μg/L). Patterns of thyroid function are positive anti-thyroid peroxidase antibodies (TPOAb positive); overt hypothyroidism (overt hypo TH); subclinical hypothyroidism (SubC hypo TH); isolated hypothyroxinemia (Hypo FT4,); and euthyroidism.
Considering together overt and subclinical hypothyroidism in pregnant women, proportions of hypothyroidism decreased as UIC increased: 26.1% of pregnant women with a UIC <150 μg/L, 25.7% of pregnant women with a UIC of 150–249 μg/L, and 8.6% of pregnant women with a UIC >250 μg/L were hypothyroid (trend χ2, p=0.01).
Table 5 presents factors associated with a high TSH in women in Lubumbashi. In univariate analysis, we found a higher TSH among women who were pregnant or who recently delivered, in women who were living in a semiurban area, and in those who had a normal or low UIC. TPOAb status, age, and number of previous pregnancies were not associated with an increased TSH. In a multivariate logistic regression model performed with interaction terms, none of the interactions were significant, reflecting similar effects of one covariate across levels of another one. Moreover, multivariate ORs were close to univariate ORs, indicating independent effects of significant covariates. The effect of socioeconomic status on TSH can be therefore considered as independent of the effect of UIC on TSH.
Table 5.
Factors Associated with a High Serum Thyrotropin Level Using Logistic Regression
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| n (%) | Odds ratio | 95% confidence interval | p | Odds ratio | 95% confidence interval | p | |
| Socioeconomic area | <0.001 | <0.001 | |||||
| Rural | 22 (17.6) | 1.59 | [0.77–3.29] | 1.55 | [0.74–3.26] | ||
| Semiurban | 40 (32.0) | 3.58 | [1.82–7.03] | 3.68 | [1.85–7.35] | ||
| Urban | 14 (11.2) | 1 | 1 | ||||
| Group | 0.004 | 0.01 | |||||
| Pregnancy | 48 (21.3) | 3.03 | [1.24–7.43] | 3.43 | [1.23–9.53] | ||
| Postpartum | 21 (28.0) | 4.34 | [1.64–11.52] | 4.49 | [1.66–15.01] | ||
| Control | 7 (9.3) | 1 | 1 | ||||
| UIC, μg/L | 0.01 | 0.02 | |||||
| <150 | 37 (24.0) | 0.90 | [0.49–1.68] | 1.11 | [0.55–2.25] | ||
| 150–249 | 21 (25.9) | 1 | 1 | ||||
| ≥250 | 16 (12.0) | 0.39 | [0.19–0.80] | 0.44 | [0.19–0.96] | ||
| TPOAb status | 0.97 | ||||||
| Positive | 5 (20.0) | 0.98 | [0.36–2.71] | ||||
| Negative | 71 (20.3) | ||||||
| Parity | 0.27 | 0.05 | |||||
| 0–1 | 24 (24.2) | 1 | 1 | ||||
| 2 or plus | 52 (18.8) | 0.73 | [0.42–1.28] | 0.49 | [0.23–1.02] | ||
| Age, years | 0.19 | ||||||
| <25 | 35 (23.8) | 1 | |||||
| ≥25 | 41 (18.1) | 0.71 | [0.43–1.18] | ||||
A high serum TSH: >3 mIU/L in 2nd and 3rd trimester, >2.5 mIU/L in 1st trimester, >3.6 mIU/L in early postpartum, and >4.0 mIU/L in nonpregnant women. Bold values are statistically significant odds ratios.
Discussion
In the present study, we found that almost 60% of pregnant women had either isolated hypothyroxinemia or thyroid dysfunction, compared with less than 40% of nonpregnant women. The most frequent patterns of thyroid dysfunction were subclinical hypothyroidism, present in 12% of pregnant women, overt hypothyroidism in 8%, and thyroid peroxidase antibodies positive in 5% of pregnant women.
The overall prevalence of 20% of hypothyroidism found in this study is quite higher than what is commonly described: 0.3–0.5% for overt hypothyroidism and 2–3% for subclinical hypothyroidism in populations (19), or 4.6–5.0% in screened pregnant women (23). Nevertheless, our results are consistent with the observations on iodine deficiency in pregnant women from Lubumbashi: hypothyroidism was more frequent in pregnant women with low UIC. When iodine intake is restricted, TSH can rise up to twofold between the first trimester and the end of gestation (13). Irrespective of our cross-sectional design, pregnant women in the 3rd trimester had a TSH that was 1.5-fold higher than in women in the 1st trimester. About 18.5% of pregnant women in the 1st trimester had a TSH higher than the recommend upper limit of 2.5 mU/L. This finding is very important as an increased TSH in the first trimester of pregnancy has been associated with neurologic retardation and lower intellectual quotient in children in the study by Haddow et al. (24). In contrast, Lazarus et al. (23) could not confirm a lower intellectual quotient in children of mothers with subclinical hypothyroidism at age 3.5 years in the Controlled Antenatal Thyroid Screening study. Nevertheless, an association of maternal subclinical hypothyroidism with a neurocognitive delay remains biologically plausible (19).
We found two to three more pregnant women with hypothyroidism in rural and semiurban areas, characterized by moderate or mild iodine deficiency, than in the iodine-sufficient urban area. Semiurban areas of Lubumbashi are suburban agglomerations with displaced people looking for a job and for better life conditions; these individuals are between the unfavorable socioeconomic conditions of rural areas and the more favorable socioeconomic conditions of urban areas. They have more often poor infrastructures and are usually left behind during health promotion campaigns. The iodine status of pregnant women from the semiurban area showed a vast spectrum, with the highest median UIC level in the 1st trimester (306 μg/L) and the lowest in the 3rd trimester (68 μg/L) (16). This might explain the higher prevalence of hypothyroidism found in the semiurban area than in the rural area.
TSH decreased with parity in our study. This relation has been described also by Pearce et al. (25). In a previous study with Twite et al. (26), we showed that the thyroid volume in this population increased with parity, and that multiparous women had more often a goiter than nulliparous women. This finding is in agreement with previous observations by Rotondi et al. (27), who indicated that parity was one of the thyroid-size-determining factors at least in condition of moderate iodine deficiency. This corroborates also the notion that thyroid alterations occurring during pregnancy continue after pregnancy in a situation of iodine deficiency, and they tend to persist during later pregnancies, especially when pregnancies are very close (13). However, the previous iodine status of these multiparous has never been assessed before our study. Nonetheless, the problem remains unsolved for women who begin their motherhood in conditions of iodine deficiency and who develop hypothyroidism.
We found a quite high proportion of isolated hypothyroxinemia in pregnant women: 26% in the 1st trimester, 28% in the 2nd, and 37% in the 3rd trimester. Pop et al. (28,29) have shown that an uncorrected low concentration of FT4 at 12 weeks of gestation was associated with neurodevelopmental retardation in children, even when the TSH was normal. Hypothyroxinemia is frequently overestimated in pregnancy when measuring FT4 by immunometric assays (12,30). Nonetheless, the prevalence of hypothyroxinemia in our study was also high in nonpregnant women (20%), where immunoassays are not affected by alterations in binding proteins; this argues for an actual decrease in FT4 rather than an assay phenomenon.
We found a gradient in TPOAb positivity in pregnant women from low (UIC <150 μg/L) to more than adequate iodine intake (UIC ≥250 μg/L); this is consistent with studies showing an association between excessive iodine intake and an increased risk of thyroid autoimmunity (31,32). Nevertheless, the proportion of women with TPOAb was not high in our study. This underlines the important role of iodine deficiency, rather than thyroid autoimmunity, in the occurrence of hypothyroidism in pregnant women from Lubumbashi.
Our study has some limitations. The cross-sectional design does not allow following changes between trimesters of pregnancy for a given woman. The nonprobability sampling limits the external validity of pooled results. The iodine status at an individual level cannot be assessed with a single spot urine. A single evaluation performed in the early postpartum period does not adequately reflect thyroid function, but women of Lubumbashi are difficult to follow after discharge from the maternity unit. Another limitation is the sensitivity of FT4 immunologic assays to alterations in binding proteins (30). Actually, the immunoenzymatic assays have less good correlation with the gold standard, liquid chromatography in tandem with mass spectrometry, but this sophisticated analysis is too expensive, technically complex (12), and not available in the Lubumbashi laboratory. We partially overcome this last limitation by using, as recommended, reference ranges established by the manufacturer for pregnant women, but they were not trimester-specific or ethnicity-specific or adjusted for iodine deficiency.
Strengths of this work include the evaluation of both iodine intake and maternal thyroid function in pregnant women from the three trimesters of pregnancy, the comparison with nonpregnant women of childbearing age, and the assessment of these parameters in maternity units from different socioeconomic areas of Lubumbashi. This is the first study on thyroid function of pregnant women in the Democratic Republic of Congo.
Our findings suggest a high prevalence of thyroid dysfunction in pregnant women in Lubumbashi. Pregnant women who are iodine deficient are more likely to have hypothyroidism. Our results provide evidence for the need (i) to ensure higher availability of iodized salt in households of Lubumbashi, (ii) to include a key group of pregnant women in national surveys of iodine status, and (iii) to seriously consider implementing iodine supplementation during antenatal visits in early pregnancy or even before conception.
Acknowledgments
This study was supported by a grant from the Commission Universitaire pour le Développement (CUD—PIC 2008 RD Congo; www.cud.be) of the Belgian Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ.2009Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 160:985–991 [DOI] [PubMed] [Google Scholar]
- 2.Casey BM.2006Subclinical hypothyroidism and pregnancy. Obstet Gynecol Surv 61:415–420 [DOI] [PubMed] [Google Scholar]
- 3.Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, Locorotondo G, Caroli P, Pezzarossa A, Dazzi D, Hassan H.2005Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Hum Reprod 20:1529–1533 [DOI] [PubMed] [Google Scholar]
- 4.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H.2006Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 91:2587–2591 [DOI] [PubMed] [Google Scholar]
- 5.Leung AM.2012Thyroid function in pregnancy. J Trace Elem Med Biol 26:137–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazzarin N, Moretti C, De FG, Vaquero E, Manfellotto D.2012Further evidence on the role of thyroid autoimmunity in women with recurrent miscarriage. Int J Endocrinol 2012:717185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannisto T, Vaarasmaki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, Bloigu A, Jarvelin MR, Suvanto E.2010Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metab 95:1084–1094 [DOI] [PubMed] [Google Scholar]
- 8.Glinoer D.2004The regulation of thyroid function during normal pregnancy: importance of the iodine nutrition status. Best Pract Res Clin Endocrinol Metab 18:133–152 [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann MB.2009Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr 89:668S–672S [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick DL, Russell MA.2010Diagnosis and management of thyroid disease in pregnancy. Obstet Gynecol Clin North Am 37:173–193 [DOI] [PubMed] [Google Scholar]
- 11.Glinoer D.2007The importance of iodine nutrition during pregnancy. Public Health Nutr 10:1542–1546 [DOI] [PubMed] [Google Scholar]
- 12.Klubo-Gwiezdzinska J, Burman KD, Van ND, Wartofsky L.2011Levothyroxine treatment in pregnancy: indications, efficacy, and therapeutic regimen. J Thyroid Res 2011:843591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glinoer D.1997The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 18:404–433 [DOI] [PubMed] [Google Scholar]
- 14.Ntambue K 2007 From Severe Endemic Cretinism to Iodine Sufficiency: An IDD Success Story in the Democratic Republic of the Congo Volume 26 Fourth edition. IDD Newsletter 26, 1–4. Zürich, Switzerland: pp 1–4 [Google Scholar]
- 15.Kitwa KE, Habimana L, Lumbu SJ, Donnen P, Twite KE, Mpoyo KE, De NP, Kalenga MK, Robert A.2012Evaluation of iodine content in table salt consumed in Democratic Republic of Congo. Food Nutr Bull 33:217–223 [DOI] [PubMed] [Google Scholar]
- 16.Habimana L, Twite KE, Wallemacq P, De NP, Daumerie C, Donnen P, Kalenga MK, Robert A.2013Iodine and iron status of pregnant women in Lubumbashi, Democratic Republic of Congo. Public Health Nutr 16:1362–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dashe JS, Casey BM, Wells CE, McIntire DD, Byrd EW, Leveno KJ, Cunningham FG.2005Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol 106:753–757 [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization UNICEF, ICCIDD 2007 Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination A Guide for Programme Managers. Third edition. World Health Organization, Geneva [Google Scholar]
- 19.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W.2011Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21:1081–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dayan CM.2001Interpretation of thyroid function tests. Lancet 357:619–624 [DOI] [PubMed] [Google Scholar]
- 21.Gurnell M, Halsall DJ, Chatterjee VK.2011What should be done when thyroid function tests do not make sense? Clin Endocrinol (Oxf) 74:673–678 [DOI] [PubMed] [Google Scholar]
- 22.Prummel MF, Wiersinga WM.2004Thyroid autoimmunity and miscarriage. Eur J Endocrinol 150:751–755 [DOI] [PubMed] [Google Scholar]
- 23.Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, Chiusano E, John R, Guaraldo V, George LM, Perona M, Dall'amico D, Parkes AB, Joomun M, Wald NJ.2012Antenatal thyroid screening and childhood cognitive function. N Engl J Med 366:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ.1999Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341:549–555 [DOI] [PubMed] [Google Scholar]
- 25.Pearce EN, Oken E, Gillman MW, Lee SL, Magnani B, Platek D, Braverman LE.2008Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr Pract 14:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twite KE, Habimana L, Bernard P, Donnen P, Makenga JC, Kat KF, Kitwa KE, Mpoyo KE, Twite BE, Kalwaba KS, Gruson D, Mutamba LG, Kalenga MK, Robert A.2010Aspects échographiques de la glande thyroïde chez la femme enceinte à Lubumbashi. Ann Afr Méd 4:647–655 [Google Scholar]
- 27.Rotondi M, Amato G, Biondi B, Mazziotti G, Del BA, Rotonda NM, Balzano S, Bellastella A, Glinoer D, Carella C.2000Parity as a thyroid size-determining factor in areas with moderate iodine deficiency. J Clin Endocrinol Metab 85:4534–4537 [DOI] [PubMed] [Google Scholar]
- 28.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL.1999Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 50:149–155 [DOI] [PubMed] [Google Scholar]
- 29.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ.2003Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 59:282–288 [DOI] [PubMed] [Google Scholar]
- 30.Lee RH, Spencer CA, Mestman JH, Miller EA, Petrovic I, Braverman LE, Goodwin TM.2009Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol 200:260–266 [DOI] [PubMed] [Google Scholar]
- 31.Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C.2006Effect of iodine intake on thyroid diseases in China. N Engl J Med 354:2783–2793 [DOI] [PubMed] [Google Scholar]
- 32.Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, Bai X, Li Y, Li N, Li Z, Wang S, Xing Q, Xue H, Zhu L, Hou X, Fan C, Teng W.2011More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 164:943–950 [DOI] [PubMed] [Google Scholar]