Abstract
Serum thyrotropin (TSH) is considered the single most sensitive and specific measure of thyroid function in the general population owing to its negative logarithmic association with free triiodothyronine and free thyroxine concentrations. It is therefore often the test of choice for screening, diagnosis, and monitoring of primary hypothyroidism. Serum TSH concentrations can be analyzed quantitatively using third-generation immunoassays, whereas its bioactivity can be measured by TSH activity assays in cell culture. Theoretically, if serum TSH concentrations are directly related to TSH activity, the two tests should yield comparable results. However, on occasion, the results are discordant, with serum concentrations being higher than TSH biological activity. This review focuses on the dissociation between the clinical state and serum TSH concentrations and addresses clinically important aspects of TSH analysis.
Thyrotropin Synthesis
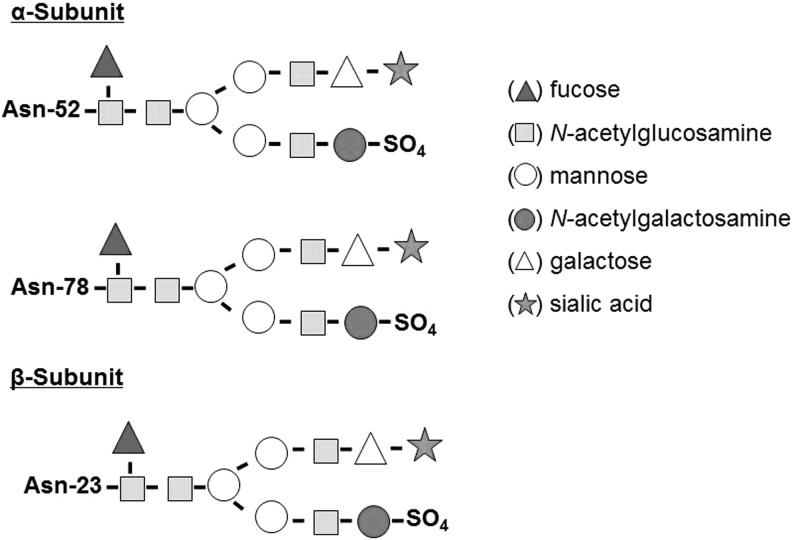
Thyrotropin (TSH) is a heterodimeric 28-kDa-glycoprotein hormone released from the anteromedial pituitary and is a regulator of thyroid function. Its synthesis is controlled by the hypothalamic neuropeptide TSH-releasing hormone (TRH). The two peptide subunits of TSH are noncovalently linked and cotranslationally glycosylated with mannose-rich oligosaccharides (1). Posttranslationally, the two subunits are combined and the attached oligosaccharides are further processed. Synthesis of a mature TSH molecule requires the excision of signal peptides from both TSH α- and β-subunits, followed by trimming of mannose and further addition of fucose, galactose, and sialic acids (2). Thus, mature TSH molecules are asparagine-(N)-linked [Asp(N)-linked] complex carbohydrate structures capped with sulfate and/or sialic acid molecules (3,4) (Fig. 1). TSH oligosaccharide structures vary according to the source of TSH: human pituitary–derived TSH comprises fucosylated biantennary glycans with terminal N-acetylgalactosamine sulfate and low sialic acid content (5–7), while recombinant human TSH (rhTSH), synthesized in Chinese hamster ovary cells, specifically terminates in α2,3-linked sialic acid (8–11) and rhTSH formed in yeast lacks sialic acid residues altogether (12).
FIG. 1.
TSH α- and β-subunits. A normal human TSH molecule contains an α-subunit with two oligosaccharide chains (located at Asn-52 and Asn-78) and a β-subunit with one oligosaccharide chain (located at Asn-23). Each subunit contains a terminal sialic acid and a sulfate residue that confer TSH binding and biological activity. Asn, asparagine; TSH, thyrotropin.
Glycosylation Patterns and TSH Bioactivity
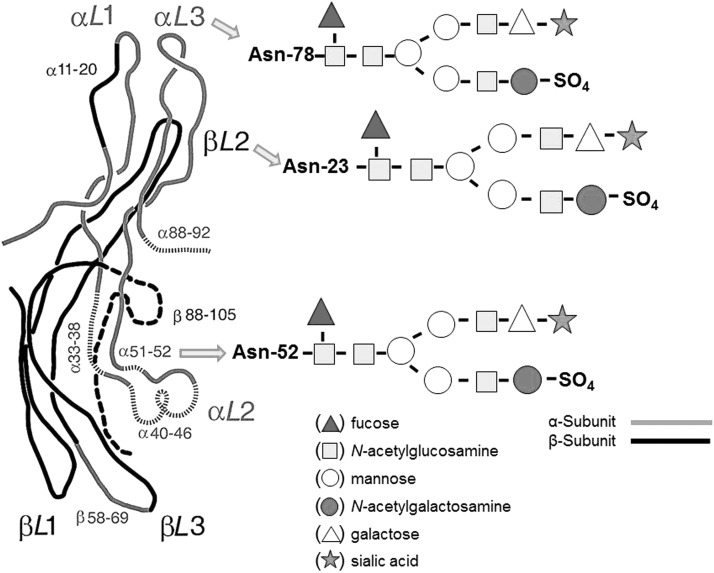
Appropriate glycosylation is necessary to maintain normal TSH bioactivity (13). Indeed, both α- and β-subunits of TSH have functionally important domains associated with TSH receptor (TSHR) binding and activation. The α-subunit has two Asp(N)-linked oligosaccharide chains with a typical biantennary structure, while TSH β-subunit has only one chain (Fig. 2). The transcriptional and posttranscriptional mechanisms involved in TSH glycosylation result in folding of these subunits, leading to their heterodimerization. These mechanisms also qualitatively regulate TSH secretion, prevent intracellular degradation, and influence TSH clearance rate from the circulation (14,15). A three-dimensional TSH structure has been proposed in the 1990s based on crystallographic studies and comparisons with other glycoprotein hormones (16), but the schemes have not been fully confirmed by more recent studies, including structures of the follicle stimulating hormone extracellular domain and the TSH extracellular domain (17–19).
FIG. 2.
Suggested structure of the human TSH molecule with two major subunits that confer biological and immunological activity. Each subunit contains glycan branches (designated by the arrows) whose terminal ends can alter TSH binding to its receptors and prevent proper renal clearance. [Structure is modified from (88).]
Human pituitary–derived TSH carbohydrate chains are subject variations in glycan structures, effecting TSH bioactivity (6,7). Such variations in glycosylation can be normal and have been observed in healthy subjects during the nocturnal TSH surge, normal fetuses during the last trimester of pregnancy, primary hypothyroidism, nonthyroidal illnesses, and in TSH-secreting pituitary adenomas (6,7).
TSH can be modified by changes in its terminal mannose, N-acetylgalactosamine sulfate, GlcNAc galactose, and/or core fucose. Specific hepatic receptors rapidly capture these modified TSH forms (20–23), resulting in blood enriched with highly branched and sialylated TSH glycoforms. Thus, patients with primary hypothyroidism exhibit TSH molecules with increased sialylation (6,7,24,25) and decreased inner fucosylation (26). Despite the loss of N-linked oligosaccharides, TSH isoforms maintain effective binding to the cognate receptor. However, loss of glycosylation leads to the loss of TSH biological activity (27). Moreover, deglycosylated isoforms may disrupt the biological activity of normal TSH and human thyroid-stimulating immunoglobulins (28), possibly by preventing the binding of other isoforms (29,30).
On the other hand, a higher glycosylation rate lowers the TSH clearance rate from the circulation; therefore, modulations of the TSH molecule result in changes in hepatic and renal clearance. Because of the nonhepatocyte cellular location of various liver lectin receptors, even in cases of hepatic insufficiency, such as chronic liver disease, fatty liver and cirrhosis serum TSH concentrations, as assessed by immunoassay, remain normal (31–33). In cases of chronic kidney disease patients, impaired glycosylation is associated with a change in TSH clearance rate, resulting in increased TSH biological half-life, decreased pulsatility, and a response to hypothalamic TRH feedback. Yet, in these patients as well, normal TSH levels are maintained (34–36).
TSH Measurements
As a single hormone determination, serum TSH immunoassays provide the most sensitive index to reliably detect thyroid function abnormalities (37–39). The current standards of care call for the use of “third-generation” TSH assays with a functional sensitivity of <0.02 mIU/L (37,40–42) in order to detect differing degrees of TSH suppression. Of note, not all immunoassays perform consistently in measuring very low levels of TSH, and the sensitivity of TSH assays used in clinical laboratories (functional sensitivity) may differ significantly from that stated by the manufacturer (analytical sensitivity) (38).
The usefulness of population-based TSH reference intervals to detect thyroid dysfunction in individuals may be somewhat limited (43,44). This is because it has been demonstrated that for TSH the between-person variability is more variable than within-person variability (45–47). Several epidemiological studies indicate that within-person (intraindividual) TSH variability is relatively narrow, and varies by only about 0.5 mIU/L when tested every month over a span of 1 year. Theoretically, it may be important to evaluate individuals with marginally (yet confirmed) low (e.g., 0.3–0.4 mIU/L) or high (3.0–4.5 mIU/L) TSH levels relative to patient-specific risk factors for cardiovascular disease, rather than relative to the normal TSH reference interval (48). However, it has not been demonstrated that serum TSH levels outside a person's individual range (but still within the population reference interval) can result in increased morbidity and mortality.
TSH is used in some U.S. states and regions of the world to screen newborns for underactive thyroid. It is also used for monitoring thyroid replacement therapy, in the workup of female infertility, and to screen adults for thyroid disorders. An abnormal serum TSH concentration indicates an excess or deficiency in thyroid hormone levels but does not provide information about the underlying etiology.
Baseline serum TSH concentrations are important when thyroid abnormalities are first suspected, and then again when equilibrium has been reached or the clinical status has changed (Table 1). Close monitoring of TSH and free thyroxine (FT4) is recommended for pregnant women with thyroid disease, for severely ill patients with nonthyroidal illness (49), and for severely ill hypothyroid patients with malabsorption of oral levothyroxine (LT4).
Table 1.
Clinical Situations in Which Measurements of Serum Thyrotropin Alone May Yield Misleading Results
| Condition | Serum TSH | Consequences of clinical action based on serum TSH value alone | Serum FT4 |
|---|---|---|---|
| Heterophile antibodies | Normal | Failure to diagnose thyrotoxicosis | High |
| Central hypothyroidism | Low, normal, or elevated | Failure to diagnose hypothyroidism and investigate hypothalamic–pituitary structure function | Low |
| TSH-secreting pituitary adenoma | Normal or elevated | Failure to diagnose thyrotoxicosis and investigate pituitary structure and function | High |
| Thyroid hormone resistance | Normal or elevated | Failure to recognize the condition | High |
| Poor compliance with T4 therapy | High | Inappropriate increase in dose of T4 | High |
| Delayed recovery of TSH secretion | Normal or low | Failure to diagnose impending hypothyroidism | Low |
FT4, free thyroxine; TSH, thyrotropin.
Nonthyroidal illness can frequently alter thyroid hormone peripheral metabolism and hypothalamic–pituitary–thyroidal function, resulting in thyroid test abnormalities, including decreased or increased serum TSH levels (50). It is important to distinguish the generally mild, transient TSH alterations typical of nonthyroidal illness from the more profound and persistent TSH changes associated with hyper- or hypothyroidism (51).
Primary hypothyroidism is the most common cause of elevated serum TSH. In such patients, serum FT4 is low-normal or reduced, and serum free triiodothyronine (FT3) concentrations usually remain normal until thyroid function level has markedly declined (52). In an iodine-sufficient patient, a transient elevation in TSH occurs during the recovery phase after a severe illness (15), when the hypothalamic–pituitary–thyroid axis is temporarily overactive. In general, such patients do not have an underlying thyroid dysfunction.
Hypothalamic–pituitary dysfunction may be associated with normal or modest increases in TSH, resulting from abnormal TSH glycosylation in the TRH-deficient patient. TSH resistance is associated with molecular defects that hinder adequate transmission of TSH stimulatory signals into thyrocytes. The defect may affect steps along the cascade after TSH binding to its receptor. Resistance to TSH because of either TSHR or Gs alpha mutations (53) and the resulting TSH elevations does not necessarily indicate hypothyroidism (54). Compound heterozygotes or homozygotes for partially inactivating mutations of the TSHR may have an elevated TSH, but with normal peripheral hormones, and be euthyroid, while monoallelic mutations in the Gs alpha subunit are associated with pseudohypoparathyroidism. If the mutation is on the maternal allele, then this can be associated with mild TSH elevations. Because of silencing of the paternal allele caused by imprinting, there is no thyroid phenotype if the mutation is on the paternal allele (55).
Isolated TSH elevations (with normal serum FT4) may be observed in a variety of conditions, including subclinical (mild) hypothyroidism, recovery from hypothyroxinemia of nonthyroidal illnesses, partial TSH resistance, hypothalamic hypothyroidism, and the presence of medications (such as amiodarone), which can inhibit thyroid hormone synthesis and metabolism and may cause transient reversible elevation of serum TSH (56).
In older adults, serum TSH may be higher than the normal reference interval for healthy adults (57–60). Epidemiological studies indicate elevated TSH levels with aging (61,62). Theoretically, this may be because of the presence of TSH isoforms with low bioactivity, especially when there are no accompanying clinical symptoms of hypothyroidism. The treatment of subclinical hypothyroidism in the elderly (48) may be resolved once the issue of declining TSH bioactivity in aging is resolved, by creating appropriate reference intervals for TSH for older adults, or by new technologies for TSH testing that can differentiate and quantify only TSH isoforms with normal bioactivity (63).
Fine-tuning the degree of TSH suppression plays a critical role in the management of thyroid cancer (64,65). It is well recognized that in patients with advanced-stage differentiated thyroid cancer, maintaining serum TSH below the normal range ensures greater progression-free disease and longevity (64). Suppression may be persistent for a prolonged period. In such cases, it is important to note that TSH concentrations ranging between 0.01 and 0.1 mIU/L can indicate a significant risk for atrial fibrillation and bone loss in older patients (66,67).
In the case of hyperthyroidism, including cases of Graves' disease, toxic adenoma and nodular goiter, subacute and lymphocytic (silent, postpartum) thyroiditis, iodine-induced hyperthyroidism, and exogenous thyroid hormone excess, serum FT4 and FT3 measurements are indicated when TSH levels are less than 0.1 mIU/L and also when TSH is in the range of 0.1–0.4 mIU/L. Further, in cases of Graves' disease, TSH should not be used to monitor therapy, as it may not respond to alterations in circulating thyroid hormones in an appropriate and rapid fashion. Patients with overt hyperthyroidism are expected to have serum TSH concentrations of less than 0.01 mIU/L except in TSH-induced thyrotoxicosis and T4/T3 resistance. In the case of resistance to thyroid hormones, the patients may have a mixed phenotype, with signs of hypothyroidism in some tissues and thyrotoxicosis in others. Because of the impaired feedback at the level of the thyrotrophs, the TSH levels are normal or mildly elevated serum. Isolated TSH suppression may be seen in hyperthyroidism resulting from causes such as subclinical hyperthyroidism, recovery from overt hyperthyroidism, nonthyroidal illnesses, during the first trimester of pregnancy, and in patients using medications such as dopamine and high-dose glucocorticoids.
Methods for Serum TSH Analysis
TSH immunoassays
The immunometric assay methodology (“sandwich” or “noncompetitive” methodology) is based on the excess antibody approach, and provides functional sensitivity of 0.1 mIU/L to reliably detect the low serum TSH values characteristic of hyperthyroidism (68,69). TSH assay sensitivity and specificity have been further enhanced by the adoption of nonisotopic (chemiluminescent and fluorescent) signals, resulting in narrower TSH reference intervals because of reduced cross-reactivity and improved precision, and offer the additional advantage of being easier to automate (70,71). Automated third-generation TSH assays have become the current standard of care. Fourth-generation assays, which have a sensitivity of 0.001–0.002 mIU/L, have been developed for research purposes (72).
TSH testing provides better sensitivity for detecting thyroid dysfunction than does FT4 testing (37,42,73,74). Because serum T4 has a half-life of approximately 7 days, it does not change sufficiently in 1 day to raise TSH secretion, and therefore there is no need to withhold LT4 therapy on the day of blood testing for TSH (37). TSH normally exhibits a diurnal variation peaking between midnight and 04:00 am, with the lowest levels between 10:00 and 16:00 hours (75). This variation should not usually influence the diagnostic interpretation of test results since most clinical TSH measurements are performed on ambulatory patients during similar hours. Similarly, a diurnal variation exists in T3 levels (76).
Laboratory-Related Variability in TSH Measurements
Although assay antibodies recognize peptide determinants of TSH, studies indicate that the presence or absence of specific glycans modify the immunoreactivity of the hormone, particularly when these changes occur in the β-subunit—the subunit specific to TSH (77–79). Furthermore, although pituitary and recombinant TSH usually share high cross-reactivity, clearly, they do not behave in an identical manner (10). Differences between the glycosylation patterns of a pituitary TSH calibrator and serum samples have been shown to be responsible for differences in epitope conformation and for introducing discrepancies in TSH measurements (10).
TSH is the primary test for thyroid function and one of the most common tests performed in the clinical chemistry laboratory (80). Inter- and intralaboratory variations in TSH measurements have been observed depending on the different methods used for analysis (41,81–85). Assays typically measure a nondetermined, highly heterogeneous mixture of glycoforms in addition to degradation products of TSH against a distinct internal standard or reference material (86). Although both preparations may share common glycoforms, they should not be assumed to behave identically (87). There are indications that circulating forms of TSH differ between euthyroid and hypothyroid persons (6,7,24–26). Under pathophysiologic conditions, TSH, similar to many other circulating glycoproteins, exhibits large structural variations (88). For instance, TSH glycosylations related to hypothyroidism may alter epitope expression between serum samples and the calibrator resulting in discordances in TSH measurements (79,89–91).
Monoclonal anti-TSH antibodies can be divided into two groups depending on their capacity to bind different forms of TSH (92). Some antibodies are unable to bind sialylated forms of TSH, whereas others fail to detect nonfucosylated forms of TSH (92). Both forms are seen in patients developing common thyroid disorders, such as hypothyroidism.
TSH sialylation and sulfation
Sialylation of the terminal end of the N-linked oligosaccharides is subject to variation that affects biological activity (93). Highly sialylated terminal ends lead to reduced intrinsic biological activity in vitro since the negatively charged sialic acid residues tend to repel the negatively charged TSHRs, while the addition of sialic acids increases the circulation half-life of TSH in vivo by the reduction of TSH binding to hepatic asialoglycoprotein receptors. The net effect of reduced intrinsic in vitro activity, yet longer in vivo half-life, is the increased bioactivity of sialylated TSH in vivo (14). Sulfation effectively increases TSH biological activity and reverses the effects of highly sialylated carbohydrate chains (94). Sialylation and sulfation of termini are two separate processes; sulfation does not necessarily follow desialylation, and immunoassay analysis does not differentiate between the sialylated and sulfated forms.
Alterations in TSH bioactivity relative to immunoactivity ratio (B/I) have physiological implications that are important in explaining unexpected laboratory results when clinical findings seem discordant. The sulfated TSH isoforms have higher affinity for TSHRs and increased in vitro bioactivity, and therefore a higher B/I ratio when measured by in vitro cyclic adenosine monophosphate (cAMP) production (although the in vivo bioactivity might be reduced by desialylation because of a faster clearance of TSH molecules by the liver). For example, even the normal circadian rhythm influences sialylation, reflected in higher levels of sialic acid–rich TSH isoforms at night compared with daytime (14). The sialic acid–rich TSH forms have lower biological activity in vitro than the normal sulfated forms. Examples are adults and fetuses with resistance to thyroid hormone who have an inappropriately normal or elevated TSH because of the resistance at the pituitary level and demonstrate upregulated α-2,6 sialyltransferase activity, which greatly increases the sialic acid content of secreted TSH molecules, resulting in lower bioactivity in vitro (14).
Fucosylation
TSH contains a fucose group attached to the GlcNAc residue, leading to increased activation of the IP3 pathway, stimulating cAMP (88,95). In addition, fucosylation is known to increase TSH antibody recognition and immunoreactivity (92). In primary hypothyroidism, fucose residues are decreased, forming a TSH isoform with reduced immunological and biological activity (92).
Terminal truncation
Human TSH has been shown to be heterogeneous at the amino-terminus of each subunit because of terminal truncation of both unit polypeptide chains. Shortened isoforms may affect antibody-binding interactions at the carboxy terminus while conducting immunoassay analysis, resulting in lower immunoreactivity.
Macro-TSH
Case reports suggest an alternative form of TSH, namely, macro-TSH, a form that leads to falsely elevated levels of TSH (96,97). Macro-TSH is a rare macromolecule composed of a bond between TSH and anti-TSH IgG molecules (96). These rare macromolecules have reduced biological activity and similar binding efficiency to immunoassay antibodies. In such patients, FT4 levels appear to be normal and the clinical presentation suggests the absence of thyroid dysfunction despite elevated serum TSH because of macro-TSH presence.
Antibody Recognition of TSH
Most existing antibodies target three antigenic regions in the TSH molecule. Each cluster comprises at least two close epitopes (92), with a main immunogenic region that has been characterized as dependent on changes in glycosylation (92). Some antibodies display a preference for specific glycoforms and thereby induce discordances among assays, particularly when these assays have been calibrated against the normal pituitary TSH standard.
Both the presence and the nature of specific linkages of the sialic acid residues are of high importance for optimizing antibody recognition. Moreover, fucosylation adds a further level of complexity as evidenced by the preference of some antibodies for nonfucosylated forms of TSH. This indicates that discordance in measurements can arise when an internal calibrator lacks fucose, such as in rhTSH. Thus, the identity of the TSH calibrator is of eminent importance to ensure that serum TSH can be measured with higher accuracy and possibly on a molar basis. Most of the formats tested were quite divergent when the calibrator was missing sialic acid, as in pituitary TSH, or a core fucose, as in nonfucosylated rhTSH fractions (98). The use of a pituitary international reference preparation is likely to mask part, if not all, of the influence of sialic acid and may explain the variable measurements encountered with rhTSH in 1999 and noted in most third-generation assays (41,99).
The use of monoclonal antibodies in TSH immunoassays has virtually eliminated cross-reactivity with other glycoprotein hormones (such as luteinizing hormone or human chorionic gonadotropin [hCG]). However, because monoclonal antibodies differ in their specificity for recognizing various circulating TSH isoforms, these antibody differences can result in the reporting of TSH values that may differ by as much as 1.0 mIU/L between different laboratories (who use different TSH assays) for the same serum specimen (100).
Endogenous antibodies
Some patients may develop autoantibodies against the TSH molecule. Endogenous antibody interferences are most frequently characterized by falsely high TSH values, depending on the type and composition of the antibody assay employed.
Heterophile antibodies
Heterophile antibodies are a group of relatively weak, multispecific, polyreactive antibodies with specificity for poorly defined antigens that react with immunoassays derived from two or more species (101,102). Most frequently, heterophile antibody interferences result from IgM rheumatoid factor or human antimouse antibodies (HAMA).
Human antimouse antibodies
Immunometric assays based on monoclonal antibodies of murine origin are more prone to HAMA interference than competitive immunoassays because HAMA are able to form a bridge between the capture and signal antibody reagents and create a signal that is reported as a falsely high value (15,103). Of concern are the instances when such HAMA interferences produce inappropriately normal values in patients who eventually prove to have clinical disease (104). Conducting serial dilutions of the suspect serum, and finding nonlinear results upon comparison with that of patients with a suppressed TSH can usually identify such assay-related artifacts. Despite the measures used by manufacturers to neutralize interferences, both the clinician and the laboratory must be aware of this possibility when an apparently inappropriate test result is encountered.
TSH Bioactivity Assays
Serum TSH concentration measurement alone may fail to detect the presence of pituitary and/or hypothalamic disease such as central hypothyroidism or TSH-secreting pituitary tumors, because TSH levels are often within the normal reference interval in these circumstances (105,106). Moreover, current TSH immunoassays cannot distinguish between normal and biologically altered TSH isoforms. TSH isoforms with impaired biologic activity are typically secreted in central hypothyroidism, whereas TSH isoforms with enhanced biologic activity are often secreted by TSH-secreting pituitary tumors (105,106). These abnormal TSH isoforms can result in paradoxically low, normal, or high reported TSH in the face of clinical and biochemical hypo- or hyperthyroidism (106). Therefore, in cases of suspected pituitary or hypothalamic disease, it is important to obtain serum FT4 and TSH concentrations concomitantly.
TSH bioactivity assays indicate the total amount of the biologic potencies of the various circulating TSH isoforms (107). Two methodologies are used to detect TSH antibodies, namely, bioassays and TSH-binding or receptor assays. Bioassays assess the functionality of the antibodies, and as such are able to distinguish between thyroid-stimulating immunoglobulins and thyroid-blocking antibodies (TBAb) (108). On the other hand, TSH-binding inhibiting immunoglobulin (TBII) assays are able to detect antibodies at the TSHR on membranes of the thyroid follicular cells, but they do not measure their functionality, and are thus unable to distinguish between stimulating and blocking antibodies (109).
TSH bioactivity measured in vitro provides a different parameter than the bioactivity of TSH measured in vivo. For example, in vitro, sialic acid (a negatively charged residue) tends to lower the affinity of TSH for the negatively charged TSHR. In comparison, in vivo, sialic acid binds and covers a galactose residue in the oligosaccharides, which then “hides” that galactose from hepatic galactose receptors, prolonging the half-life of circulating TSH. Thus, adding sialic acid to TSH tends to decrease intrinsic in vitro bioactivity slightly, but tends to increase in vivo bioactivity dramatically. This may be the reason why organisms evolved a mechanism to increase the sialylation of TSH when hypothyroid, a biochemical effect demonstrated in hypothyroid patients (25).
Bioassay methods (thyroid-stimulating antibody/TBAb)
Bioactivity assays detect cAMP released to indicate serum antibody action (110). Increased cAMP production indicates stimulating antibody activity at the receptor level, while inhibition of cAMP production indicates a blocking action (TBAb). As such, the bioassay methods are generally able to differentiate between the presence of thyroid-stimulating antibody (TSAb) and TBAb. However, the interpretation of these tests can prove to be difficult, because both TSAb and TBAb can be present in the same patient, and because TBAb may demonstrate stimulating activity in some cases.
TSAb bioassays measure biological activity by using a cAMP-inducible luciferase gene stably transfected in cell lines to measure cAMP levels through light production (111). Alternatively, TSH bioactivity and TSAb activity can be measured by determining cAMP in the extracellular fluid of Chinese hamster ovary cells transfected with recombinant human TSHR (CHO-R cells-JP26) (111–114). CHO-R testing has proven more reliable in determining autoantibody action and possesses better sensitivity, specificity, and reproducibility than its immediate precursor, the FRTL-5 bioassay (112). Currently, assays for TBAb are not available for routine clinical use.
Receptor methods (TBII)
TSHR binding assays, as noted above, are unable to distinguish between TSAb and TBAb. However, in a clinical setting, a physical examination together with biochemical testing can be used to interpret the binding assay results and determine the difference between these effects for a patient. TBII assays have evolved over time (115–118). The current third-generation assays do not use labeled TSH, but rather human monoclonal thyroid-stimulating immunoglobulins binding to recombinant TSHR. The third-generation assay has 100% specificity (119) and a detection limit of about 0.01 mIU/L, and can therefore reliably distinguish between normal and hyperthyroid patients. However, these assays have restrictive clinical application and are not generally widely utilized because the distinction between normal and hyperthyroid patients is usually not a clinical problem. These assays are more frequently used when the distinction between Graves' disease and other causes of hyperthyroidism is difficult, and in pregnant women with Graves' disease, when appropriate, to assess the likelihood of the fetus developing thyrotoxicosis in utero or after delivery. The assay can also be employed to help determine if a patient with Graves' disease will develop or has developed a remission.
Bioassays Versus Immunometric Assays of TSH and B/I Ratio
TSH bioassays reflect the sum of the biopotencies of the various circulating TSH isoforms, whereas immunometric assays quantify serum TSH concentrations. Analyzed simultaneously by immunometric and biological assays, the ratio between the bioactivity and immunoactivity serves as an index of the overall potency of circulating TSH molecules (120). Thus, variations in the B/I ratio of immunopurified TSH samples result from changes in the amount of biological activity per unit of immunological activity. This serves as an estimate of the circulating TSH molecules' overall biological potency, or intrinsic bioactivity. Multiplying the B/I ratio by the concentration of TSH in serum produces the bioactive TSH concentration or intrinsic TSH bioactivity (107).
Alterations to the TSH structure affect its binding capacity and clearance. Since TSH undergoes modifications before its release from the anterior pituitary, it is subject to a variety of glycosylations that would affect its binding action. In a case of subclinical hypothyroidism in a young adult with Down's syndrome, antibody recognition of TSH significantly increased with sialylation and fucosylation (121). The modified TSH demonstrated a much stronger and more efficient binding to the assay antibodies compared with the normal pituitary TSH. This competitive binding preference for the modified TSH suggests that the normal active TSH was not as accurately detected in serum, at least in this one patient. This sialylation modification is known to prevent liver uptake and metabolic clearance, and therefore prolongs the half-life of serum TSH (121).
TSH isoform bioactivity is similarly subject to modifications that affect bioassay measurements. TSH glycosylation affects antibody binding activity and its ability to induce cAMP release. Altered glycan groups on TSH affect its bioactivity; deglycosylation may increase receptor binding and decrease signal transduction. Sialylation increases receptor binding action on TSH proteins (88). Thus, to reach accurate diagnoses, both bioactivity and biochemical analysis should be taken into account, especially in cases such as TSH-secreting pituitary tumors and hypothalamic hypothyroidism. This potential discordance should also be considered when routine TSH immunoassay values are not congruent to FT4 and T3 and/or the clinical circumstance (14,122).
Of note, in some clinical conditions (Table 1), the measurements of serum TSH alone as a first-line test may yield misleading information; it is difficult for the laboratory to proactively detect interference from a single measurement such as an isolated TSH test. A more common occurrence is for the physician to suspect assay interference when a reported value is inconsistent with the clinical status of a patient. The most practical way to investigate possible interference is to test the specimen by a different manufacturer's method and check for discordance between the test results. This approach is effective because methods vary in their susceptibility to interfering substances. Occasionally, a biological check can be made using TRH stimulation (which is not presently clinically available in the United States) or thyroid hormone suppression to validate a suspected inappropriate serum TSH level. Interferences producing a falsely elevated TSH value will usually be associated with a blunted (<2-fold increase) response to stimulation.
Disease states associated with apparently higher serum TSH
Extremely rare conditions associated with elevated TSH include inherited autosomal recessive forms of partial (euthyroid hyperthyrotropinemia) or complete (congenital hypothyroidism) TSH resistance that are associated with biallelic inactivating point mutations of the TSHR gene (15). Inherited dominant forms of partial TSH resistance have also been described in the absence of TSHR gene mutations (15). More frequently, in patients who have elevated serum FT4, normal or elevated TSH requires further investigation for resistance to thyroid hormone or a thyrotroph tumor (123,124).
Psychiatric illness may be associated with either elevated or suppressed TSH, but abnormal levels are not usually in the range typically associated with symptomatic thyroid dysfunction. In patients with Addison's disease (with slightly elevated TSH in the absence of primary thyroid disease) corticosteroid results in the normalization of TSH levels.
Central hypothyroidism
Idiopathic central hypothyroidism (seen in various hypothalamic–pituitary conditions) may result from the secretion of biologically inactive TSH. In these cases, serum TSH levels are normal or slightly elevated with decreased bioactivity (112,125–127). TRH regulates not only the secretion of TSH but also its specific molecular and conformational characteristics required for TSH hormone action (126). In such cases, chronic TRH administration has been shown to increase TSH bioactivity and restore thyroid function (126). In patients with primary hypothyroidism and TSH-secreting pituitary adenomas, normal, reduced, and increased TSH bioactivities have all been reported (112,122,128–131).
Resistance to thyroid hormone
As previously mentioned, TSH bioactivity is increased in patients with thyroid hormone resistance (122,132). These patients demonstrate greatly increased sialic acid content of secreted TSH molecules (14).
Sheehan's syndrome (hypothyroidism caused by postpartum panhypopituitarism)
Sheehan's syndrome is a peripartum condition that follows massive necrosis of the anterior pituitary gland (133,134). Many patients with Sheehan's syndrome have low TSH levels (135). The majority of cases are classified as chronic since patients can remain asymptomatic for several months to years after massive postpartum uterine bleeding, which leads to amenorrhea and loss of lactation. An acute state is diagnosed when symptoms of headaches and hormonal deficiencies appear within days after a traumatic delivery (136). Despite the differences in time and diagnosis, these patients present with similar TSH characteristics and serum levels because of a necrotic anterior pituitary gland. While TSH secretion is elevated because of increased tonic (not pulsatile) TSH secretion, the TSH circadian rhythm is severely blunted (135,137). Patients with Sheehan's syndrome demonstrated mannose content of TSH isoforms similar to that of normal controls, but the degree of TSH sialylation is higher (135,137). Moreover, although intrinsic TSH bioactivity is decreased, serum TSH concentrations were higher than those in controls, so the resultant bioactive serum TSH concentration (the product B/I×I) in patients with Sheehan's syndrome did not differ from that of healthy controls. The observation that serum FT4 levels correlated significantly with bioactive TSH concentrations, but not with immunoreactive TSH levels or intrinsic TSH bioactivity in Sheehan's patients, reflects the relevant role of bioactive TSH in residual T4 secretion in Sheehan's patients. Thus, the paradox of the presence of hypothyroidism with increased serum immunoreactive TSH levels in patients with Sheehan's syndrome cannot be solved simply by demonstrating that serum TSH has decreased intrinsic bioactivity. Another paradox is posed by the observation that in Sheehan's patients bioactive serum TSH, although normal, fails to sustain normal T4 levels.
TSH and Aging
TSH immunoactivity and reference intervals in older adults
Several epidemiological studies indicate that the normal reference interval for serum TSH increases with age (61,62). The NHANES III survey of the U.S. population demonstrates that the 2.5th, 50th, and 97.5th percentiles of serum TSH rise with age, and the most significant effects are seen at the 97.5th percentile, which increases by 0.3 mIU/L with each 10-year increase in age (138). It was demonstrated that in individuals who are TPO antibody or thyroglobulin antibody negative, serum TSH concentrations >3.0 mIU/L were observed with increasing frequency with aging. Moreover, in elderly subjects (>80 years), 23.9% had serum TSH concentrations between 2.5 and 4.5 mIU/L, and 12% had concentrations greater than 4.5 mIU/L (60). The mild rise may indicate that slightly elevated TSH in the elderly may not necessary reflect subclinical thyroid dysfunction, but instead be a normal manifestation of aging. This observation has called into the question the applicability of adult TSH reference intervals to the elderly population, which may need to be adjusted with increasing age. Surks et al. acknowledge that serum TSH levels between 3.0 and 4.5 may indicate early signs for subclinical hypothyroidism (138,139). Nonetheless, if the upper limit is set for 2.5–3.0 mIU/L, in the United States alone, there would be a 300–400% increase in individuals diagnosed with hypothyroidism (22–28 million additional individuals). Therefore, Surks et al. suggest the use of age-specific reference intervals in order to avoid the misclassification of healthy patients as hypothyroid (60). Consequently, the upper limit will be set higher for older individuals than for younger individuals, averting unwarranted hypothyroidism diagnoses and, in turn, unnecessary thyroid hormone therapies for healthy, aging individuals (63).
TSH biological activity and aging
Although immunoassay discrepancy may be the underlying reason for the elevated serum TSH, the increase in serum TSH has also been associated with relatively higher concentrations of biologically inactive isoforms of TSH, as described above. When higher TSH is not accompanied by the normal thyroidal response of a rise in FT4/FT3, it suggests the presence of bioinactive TSH. Sera with bioinactive TSH would show elevated TSH concentrations (immunoactivity) although bioactivity may be normal.
The current standard of care calls for the use of third-generation TSH assays that have a functional sensitivity of <0.02 mIU/L (37,40–42). This level of sensitivity is important for determining the degree of TSH suppression. In older patients, serum TSH concentrations between 0.01–0.1 mIU/L (implying an increase in FT4/T3 for this individual patient) represents a significant risk for atrial fibrillation and are often an iatrogenic consequence of LT4 suppression or an unanticipated side effect of replacement therapy (66,67). Further, since T4 (and LT4) has a narrow therapeutic index, monitoring TSH levels provides an improved sensitivity than does FT4 testing.
TSH Assessment in Pregnancy
Thyroid disorders are relatively frequent in women of childbearing age (140,141); consequently, TSH measurements are useful in detecting subtle thyroid dysfunction associated with reproductive issues and poor pregnancy outcome. During pregnancy, the placenta secretes high levels of hCG, a glycoprotein hormone sharing a common α-subunit with TSH (142). As discussed above, currently used immunometric TSH assays have overcome the problems posed by hCG cross reactivity (141). Moreover, enhanced sensitivity of third-generation assays has established the lower TSH limit for nonpregnant women as ∼0.3 mIU/L and affords the accurate detection of the low serum TSH concentrations often detected in early pregnancy (143). During the first trimester, elevated hCG secretion results in higher T4 and T3 and suppressed serum TSH concentrations. Since hCG concentrations are higher in multiple pregnancies than in singleton pregnancies, a greater downward shift is observed in twin pregnancies (144). However, in the case of maternal hypothyroxinemia (low FT4 accompanied by normal TSH), which is increasingly recognized for its association with neurodevelopmental deficits, TSH is within the normal range and is therefore not a useful assay for detecting this problem (145). FT4 immunoassays are notoriously unreliable during pregnancy (146), but relying on TSH is not sufficient in this case.
Trimester-specific reference intervals of TSH
During pregnancy, increased hCG can lead to transient hCG-induced thyrotoxicosis. This can be reflected in lower limits of TSH during the first trimester. The lower and upper reference limits for serum TSH are decreased by about 0.1–0.2 mIU/L and 1.0 mIU/L, respectively, compared with the TSH reference interval of 0.4–4.0 mIU/L of nonpregnant women (142,147). Accordingly, the Endocrine Society and the recent American Thyroid Association guidelines recommend using a TSH upper limit of 2.5 mIU/L for preconception and first trimester, and 3.0 mIU/L for the second and third trimesters (74,148). Of note, in 1.7% of pregnancies, TSH can be greatly suppressed (<0.01 mIU/L) and yet still represent a euthyroid pregnant patient (149). The differences in normal TSH intervals in the various ethnic groups persist during pregnancy (150); therefore, providing specific normal ranges for the local population is of particular advantage. Taken together, and in accordance with the new American Thyroid Association guidelines for women in pregnancy, trimester-specific reference intervals for TSH, as defined in populations with optimal iodine intake, should be applied, although they are not generally provided (74). If trimester-specific reference intervals for TSH are not available in the laboratory, the following reference intervals are recommended by the American Thyroid Association: 1st trimester 0.1–2.5 mIU/L; 2nd trimester 0.2–3.0 mIU/L; 3rd trimester 0.3–3.0 mIU/L (74,151).
Finally, immunoassay methods for TSH analysis using nonisotopic, primarily chemiluminescent signals have become available on a variety of high-throughput immunoassay analyzer platforms that employ bar-coding, multiple-analyte random-access, primary tube sampling, auto dilution, STAT testing, and computerized data output (152). Since most thyroid disease is treated on an outpatient basis, point-of-care thyroid tests are unlikely to replace centralized automated thyroid testing. Liquid chromatography–tandem mass spectrometry testing of thyroid hormones requires equilibrium dialysis or ultrafiltration before analysis. Currently, there is no available methodology to measure TSH, since it is a larger molecule with variable secondary modifications. However, TSH analysis may change when liquid chromatography–tandem mass spectrometry for TSH is made available, by finding smaller fragments of the molecule that can adequately represent TSH activity.
Acknowledgments
Dr. O.P. Soldin was supported in part by NIH R01AG033867 and by the Flight Attendants Medical Research Institute. The project was also supported in part by award number P30CA051008 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank Chen Fei for help with constructing the figures.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Grossmann M, Weintraub BD, Szkudlinski MW.1997Novel insights into the molecular mechanisms of human thyrotropin action: structural, physiological, and therapeutic implications for the glycoprotein hormone family. Endocr Rev 18:476–501 [DOI] [PubMed] [Google Scholar]
- 2.Weintraub BD, Stannard BS, Magner JA, Ronin C, Taylor T, Joshi L, Constant RB, Menezes-Ferreira MM, Petrick P, Gesundheit N.1985Glycosylation and posttranslational processing of thyroid-stimulating hormone: clinical implications. Recent Prog Horm Res 41:577–606 [DOI] [PubMed] [Google Scholar]
- 3.Magner JA.1990Thyroid-stimulating hormone: biosynthesis, cell biology, and bioactivity. Endocr Rev 11:354–385 [DOI] [PubMed] [Google Scholar]
- 4.Shupnik MA, Ridgway EC, Chin WW.1989Molecular biology of thyrotropin. Endocr Rev 10:459–475 [DOI] [PubMed] [Google Scholar]
- 5.Pierce JG, Parsons TF.1981Glycoprotein hormones: structure and function. Annu Rev Biochem 50:465–495 [DOI] [PubMed] [Google Scholar]
- 6.Papandreou MJ, Persani L, Asteria C, Ronin C, Beck-Peccoz P.1993Variable carbohydrate structures of circulating thyrotropin as studied by lectin affinity chromatography in different clinical conditions. J Clin Endocrinol Metab 77:393–398 [DOI] [PubMed] [Google Scholar]
- 7.Trojan J, Theodoropoulou M, Usadel KH, Stalla GK, Schaaf L.1998Modulation of human thyrotropin oligosaccharide structures—enhanced proportion of sialylated and terminally galactosylated serum thyrotropin isoforms in subclinical and overt primary hypothyroidism. J Endocrinol 158:359–365 [DOI] [PubMed] [Google Scholar]
- 8.Cole ES, Lee K, Lauziere K, Kelton C, Chappel S, Weintraub B, Ferrara D, Peterson P, Bernasconi R, Edmunds T, Richards S, Dickrell L, Kleeman JM, McPherson JM, Pratt BM.1993Recombinant human thyroid stimulating hormone: development of a biotechnology product for detection of metastatic lesions of thyroid carcinoma. Nat Biotechnol 11:1014–1024 [DOI] [PubMed] [Google Scholar]
- 9.Grabenhorst E, Schlenke P, Pohl S, Nimtz M, Conradt HS.1999Genetic engineering of recombinant glycoproteins and the glycosylation pathway in mammalian host cells. Glycoconj J 16:81–97 [DOI] [PubMed] [Google Scholar]
- 10.Canonne C, Papandreou MJ, Medri G, Verrier B, Ronin C.1995Biological and immunochemical characterization of recombinant human thyrotrophin. Glycobiology 5:473–481 [DOI] [PubMed] [Google Scholar]
- 11.Szkudlinski MW, Thotakura NR, Weintraub BD.1995Subunit-specific functions of N-linked oligosaccharides in human thyrotropin: role of terminal residues of alpha- and beta-subunit oligosaccharides in metabolic clearance and bioactivity. Proc Natl Acad Sci USA 92:9062–9066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerngross TU.2004Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nat Biotechnol 22:1409–1414 [DOI] [PubMed] [Google Scholar]
- 13.Amir SM, Kubota K, Tramontano D, Ingbar SH, Keutmann HT.1987The carbohydrate moiety of bovine thyrotropin is essential for full bioactivity but not for receptor recognition. Endocrinology 120:345–352 [DOI] [PubMed] [Google Scholar]
- 14.Persani L.1998Hypothalamic thyrotropin-releasing hormone and thyrotropin biological activity. Thyroid 8:941–946 [DOI] [PubMed] [Google Scholar]
- 15.Baquedano MS, Ciaccio M, Dujovne N, Herzovich V, Longueira Y, Warman DM, Rivarola MA, Belgorosky A.2010Two novel mutations of the TSH-beta subunit gene underlying congenital central hypothyroidism undetectable in neonatal TSH screening. J Clin Endocrinol Metab 95:E98–E103 [DOI] [PubMed] [Google Scholar]
- 16.Szkudlinski MW, Teh NG, Grossmann M, Tropea JE, Weintraub BD.1996Engineering human glycoprotein hormone superactive analogues. Nat Biotechnol 14:1257–1263 [DOI] [PubMed] [Google Scholar]
- 17.Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM.2003Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol 24:528–533 [DOI] [PubMed] [Google Scholar]
- 18.Graves PN, Vlase H, Davies TF.1995Folding of the recombinant human thyrotropin (TSH) receptor extracellular domain: identification of folded monomeric and tetrameric complexes that bind TSH receptor autoantibodies. Endocrinology 136:521–527 [DOI] [PubMed] [Google Scholar]
- 19.Vlase H, Graves PN, Magnusson RP, Davies TF.1995Human autoantibodies to the thyrotropin receptor: recognition of linear, folded, and glycosylated recombinant extracellular domain. J Clin Endocrinol Metab 80:46–53 [DOI] [PubMed] [Google Scholar]
- 20.Fiete D, Srivastava V, Hindsgaul O, Baenziger JU.1991A hepatic reticuloendothelial cell receptor specific for SO4-4GalNAc beta 1,4GlcNAc beta 1,2Man alpha that mediates rapid clearance of lutropin. Cell 67:1103–1110 [DOI] [PubMed] [Google Scholar]
- 21.Joziasse DH, Lee RT, Lee YC, Biessen EA, Schiphorst WE, Koeleman CA, van den Eijnden DH.2000Alpha3-galactosylated glycoproteins can bind to the hepatic asialoglycoprotein receptor. Eur J Biochem 267:6501–6508 [DOI] [PubMed] [Google Scholar]
- 22.Smedsrod B, Einarsson M.1990Clearance of tissue plasminogen activator by mannose and galactose receptors in the liver. Thromb Haemost 63:60–66 [PubMed] [Google Scholar]
- 23.Lehrman MA, Haltiwanger RS, Hill RL.1986The binding of fucose-containing glycoproteins by hepatic lectins. The binding specificity of the rat liver fucose lectin. J Biol Chem 261:7426–7432 [PubMed] [Google Scholar]
- 24.Miura Y, Perkel VS, Papenberg KA, Johnson MJ, Magner JA.1989Concanavalin-A, lentil, and ricin lectin affinity binding characteristics of human thyrotropin: differences in the sialylation of thyrotropin in sera of euthyroid, primary, and central hypothyroid patients. J Clin Endocrinol Metab 69:985–995 [DOI] [PubMed] [Google Scholar]
- 25.Persani L, Borgato S, Romoli R, Asteria C, Pizzocaro A, Beck-Peccoz P.1998Changes in the degree of sialylation of carbohydrate chains modify the biological properties of circulating thyrotropin isoforms in various physiological and pathological states. J Clin Endocrinol Metab 83:2486–2492 [DOI] [PubMed] [Google Scholar]
- 26.Schaaf L, Trojan J, Helton TE, Usadel KH, Magner JA.1995Serum thyrotropin (TSH) heterogeneity in euthyroid subjects and patients with subclinical hypothyroidism: the core fucose content of TSH-releasing hormone-released TSH is altered, but not the net charge of TSH. J Endocrinol 144:561–571 [DOI] [PubMed] [Google Scholar]
- 27.Fares F.2006The role of O-linked and N-linked oligosaccharides on the structure-function of glycoprotein hormones: development of agonists and antagonists. Biochim Biophys Acta 1760:560–567 [DOI] [PubMed] [Google Scholar]
- 28.Fares FA, Levi F, Reznick AZ, Kraiem Z.2001Engineering a potential antagonist of human thyrotropin and thyroid-stimulating antibody. J Biol Chem 276:4543–4548 [DOI] [PubMed] [Google Scholar]
- 29.Azzam N, Bar-Shalom R, Fares F.2012Conversion of TSH heterodimer to a single polypeptide chain increases bioactivity and longevity. Endocrinology 153:954–960 [DOI] [PubMed] [Google Scholar]
- 30.Azzam N, Bar-Shalom R, Kraiem Z, Fares F.2005Human thyrotropin (TSH) variants designed by site-directed mutagenesis block TSH activity in vitro and in vivo. Endocrinology 146:2845–2850 [DOI] [PubMed] [Google Scholar]
- 31.Shimada T, Higashi K, Umeda T, Sato T.1988Thyroid functions in patients with various chronic liver diseases. Endocrinol Jpn 35:357–369 [DOI] [PubMed] [Google Scholar]
- 32.L'Age M, Meinhold H, Wenzel KW, Schleusener H.1980Relations between serum levels of TSH, TBG, T4, T3, rT3 and various histologically classified chronic liver diseases. J Endocrinol Invest 3:379–383 [DOI] [PubMed] [Google Scholar]
- 33.Agha F, Qureshi H, Khan RA.1989Serum thyroid hormone levels in liver cirrhosis. J Pak Med Assoc 39:179–183 [PubMed] [Google Scholar]
- 34.Kaptein EM.1996Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev 17:45–63 [DOI] [PubMed] [Google Scholar]
- 35.Carrero JJ, Stenvinkel P, Lindholm B.2012Endocrine aspects of chronic kidney disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM. (eds) Brenner and Rector's The Kidney. Ninth edition. Elsevier Saunders, Philadelphia, PA, pp 2122–2137 [Google Scholar]
- 36.Spector DA, Davis PJ, Helderman JH, Bell B, Utiger RD.1976Thyroid function and metabolic state in chronic renal failure. Ann Intern Med 85:724–730 [DOI] [PubMed] [Google Scholar]
- 37.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR.2003Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13:3–126 [DOI] [PubMed] [Google Scholar]
- 38.Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL.2002American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract 8:457–469 [PubMed] [Google Scholar]
- 39.Haugen BR.2009Drugs that suppress TSH or cause central hypothyroidism. Best Practice Res 23:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckett GJ, Toft AD.2003First-line thyroid function tests—TSH alone is not enough. Clin Endocrinol (Oxf) 58:20–21 [DOI] [PubMed] [Google Scholar]
- 41.Rawlins ML, Roberts WL.2004Performance characteristics of six third-generation assays for thyroid-stimulating hormone. Clin Chem 50:2338–2344 [DOI] [PubMed] [Google Scholar]
- 42.Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, Smith SA, Daniels GH, Cohen HD.2000American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 160:1573–1575 [DOI] [PubMed] [Google Scholar]
- 43.Boas M, Forman JL, Juul A, Feldt-Rasmussen U, Skakkebaek NE, Hilsted L, Chellakooty M, Larsen T, Larsen JF, Petersen JH, Main KM.2009Narrow intra-individual variation of maternal thyroid function in pregnancy based on a longitudinal study on 132 women. Eur J Endocrinol 161:903–910 [DOI] [PubMed] [Google Scholar]
- 44.Benhadi N, Fliers E, Visser TJ, Reitsma JB, Wiersinga WM.2010Pilot study on the assessment of the setpoint of the hypothalamus-pituitary-thyroid axis in healthy volunteers. Eur J Endocrinol 162:323–329 [DOI] [PubMed] [Google Scholar]
- 45.Andersen S, Pedersen KM, Bruun NH, Laurberg P.2002Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 87:1068–1072 [DOI] [PubMed] [Google Scholar]
- 46.Andersen S, Bruun NH, Pedersen KM, Laurberg P.2003Biologic variation is important for interpretation of thyroid function tests. Thyroid 13:1069–1078 [DOI] [PubMed] [Google Scholar]
- 47.Ankrah-Tetteh T, Wijeratne S, Swaminathan R.2008Intraindividual variation in serum thyroid hormones, parathyroid hormone and insulin-like growth factor-1. Ann Clin Biochem 45:167–169 [DOI] [PubMed] [Google Scholar]
- 48.Biondi B, Cooper DS.2008The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
- 49.Beckett G, MacKenzie F.2007Thyroid guidelines—are thyroid-stimulating hormone assays fit for purpose? Ann Clin Biochem 44:203–208 [DOI] [PubMed] [Google Scholar]
- 50.Mebis L, van den Berghe G.2009The hypothalamus-pituitary-thyroid axis in critical illness. Neth J Med 67:332–340 [PubMed] [Google Scholar]
- 51.Stockigt JR.1996Guidelines for diagnosis and monitoring of thyroid disease: nonthyroidal illness. Clin Chem 42:188–192 [PubMed] [Google Scholar]
- 52.Bigos ST, Ridgway EC, Kourides IA, Maloof F.1978Spectrum of pituitary alterations with mild and severe thyroid impairment. J Clin Endocrinol Metab 46:317–325 [DOI] [PubMed] [Google Scholar]
- 53.Calebiro D, Gelmini G, Cordella D, Bonomi M, Winkler F, Biebermann H, de Marco A, Marelli F, Libri DV, Antonica F, Vigone MC, Cappa M, Mian C, Sartorio A, Beck-Peccoz P, Radetti G, Weber G, Persani L.2012Frequent TSH receptor genetic alterations with variable signaling impairment in a large series of children with nonautoimmune isolated hyperthyrotropinemia. J Clin Endocrinol Metab 97:E156–E160 [DOI] [PubMed] [Google Scholar]
- 54.Persani L, Calebiro D, Cordella D, Weber G, Gelmini G, Libri D, de Filippis T, Bonomi M.2010Genetics and phenomics of hypothyroidism due to TSH resistance. Mol Cell Endocrinol 322:72–82 [DOI] [PubMed] [Google Scholar]
- 55.Weinstein LS, Yu S, Warner DR, Liu J.2001Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev 22:675–705 [DOI] [PubMed] [Google Scholar]
- 56.Melmed S, Nademanee K, Reed AW, Hendrickson JA, Singh BN, Hershman JM.1981Hyperthyroxinemia with bradycardia and normal thyrotropin secretion after chronic amiodarone administration. J Clin Endocrinol Metab 53:997–1001 [DOI] [PubMed] [Google Scholar]
- 57.Atzmon G, Barzilai N, Hollowell JG, Surks MI, Gabriely I.2009Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab 94:1251–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atzmon G, Barzilai N, Surks MI, Gabriely I.2009Genetic predisposition to elevated serum thyrotropin is associated with exceptional longevity. J Clin Endocrinol Metab 94:4768–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boucai L, Surks MI.2009Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin Endocrinol (Oxf) 70:788–793 [DOI] [PubMed] [Google Scholar]
- 60.Surks MI, Boucai L.2010Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab 95:496–502 [DOI] [PubMed] [Google Scholar]
- 61.Brochmann H, Bjoro T, Gaarder PI, Hanson F, Frey HM.1988Prevalence of thyroid dysfunction in elderly subjects. A randomized study in a Norwegian rural community (Naeroy). Acta Endocrinol 117:7–12 [DOI] [PubMed] [Google Scholar]
- 62.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE.2002Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 63.Deary M, Buckey TM, Soldin OP.2012TSH—clinical aspects of its use in determining thyroid disease in the elderly. How does it impact the practice of medicine in aging? Adv Pharmacoepidemiol Drug Saf 1: DOI: 10.4172/2167-1052.1000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM.2009Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 65.Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI.2006Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16:1229–1242 [DOI] [PubMed] [Google Scholar]
- 66.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC.2000The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- 67.Sawin CT.1995Subclinical hypothyroidism in older persons. Clin Geriatr Med 11:231–238 [PubMed] [Google Scholar]
- 68.Hales CN, Woodhead JS.1980Labeled antibodies and their use in the immunoradiometric assay. Methods Enzymol 70:334–355 [DOI] [PubMed] [Google Scholar]
- 69.Kohler G, Milstein C.1975Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497 [DOI] [PubMed] [Google Scholar]
- 70.Nicoloff JT, Spencer CA.1990Clinical review 12: the use and misuse of the sensitive thyrotropin assays. J Clin Endocrinol Metab 71:553–558 [DOI] [PubMed] [Google Scholar]
- 71.Spencer CA, Takeuchi M, Kazarosyan M.1996Current status and performance goals for serum thyrotropin (TSH) assays. Clin Chem 42:140–145 [PubMed] [Google Scholar]
- 72.Spencer CA, Schwarzbein D, Guttler RB, LoPresti JS, Nicoloff JT.1993Thyrotropin (TSH)-releasing hormone stimulation test responses employing third and fourth generation TSH assays. J Clin Endocrinol Metab 76:494–498 [DOI] [PubMed] [Google Scholar]
- 73.Soldin OP.2012Thyroid function tests Measuring serum thyrotropin and thyroid hormone and assessing thyroid hormone transport and antibodies. In: Braverman L, Cooper D. (eds) Werner and Ingbar's The Thyroid. Lippincott Williams & Wilkins, Philadelphia, PA, pp 279–296 [Google Scholar]
- 74.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W.2011Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21:1081–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brabant G, Prank K, Hoang-Vu C, Hesch RD, von zur Muhlen A.1991Hypothalamic regulation of pulsatile thyrotopin secretion. J Clin Endocrinol Metab 72:145–150 [DOI] [PubMed] [Google Scholar]
- 76.Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, Ross RJ.2008Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab 93:2300–2306 [DOI] [PubMed] [Google Scholar]
- 77.Papandreou MJ, Sergi I, Benkirane M, Ronin C.1990Carbohydrate-dependent epitope mapping of human thyrotropin. Mol Cell Endocrinol 73:15–26 [DOI] [PubMed] [Google Scholar]
- 78.Sergi I, Papandreou MJ, Medri G, Canonne C, Verrier B, Ronin C.1991Immunoreactive and bioactive isoforms of human thyrotropin. Endocrinology 128:3259–3268 [DOI] [PubMed] [Google Scholar]
- 79.Zerfaoui M, Ronin C.1996Glycosylation is the structural basis for changes in polymorphism and immunoreactivity of pituitary glycoprotein hormones. Eur J Clin Chem Clin Biochem 34:749–753 [PubMed] [Google Scholar]
- 80.Dayan CM.2001Interpretation of thyroid function tests. Lancet 357:619–624 [DOI] [PubMed] [Google Scholar]
- 81.Thienpont LM, Van Uytfanghe K, Beastall G, Faix JD, Ieiri T, Miller WG, Nelson JC, Ronin C, Ross HA, Thijssen JH, Toussaint B.2010Report of the IFCC Working Group for Standardization of Thyroid Function Tests; part 2: free thyroxine and free triiodothyronine. Clinical Chem 56:912–920 [DOI] [PubMed] [Google Scholar]
- 82.Wood WG, Waller D, Hantke U.1985An evaluation of six solid-phase thyrotropin (TSH) kits. J Clin Chem Clin Biochem 23:461–471 [DOI] [PubMed] [Google Scholar]
- 83.Chan BY, Swaminathan R.1990Analytical and clinical performance of six sensitive thyrotrophin kits. Pathology 22:11–15 [DOI] [PubMed] [Google Scholar]
- 84.Laurberg P.1993Persistent problems with the specificity of immunometric TSH assays. Thyroid 3:279–283 [DOI] [PubMed] [Google Scholar]
- 85.Spencer CA, Takeuchi M, Kazarosyan M, MacKenzie F, Beckett GJ, Wilkinson E.1995Interlaboratory/intermethod differences in functional sensitivity of immunometric assays of thyrotropin (TSH) and impact on reliability of measurement of subnormal concentrations of TSH. Clin Chem 41:367–374 [PubMed] [Google Scholar]
- 86.Muller MM.2000Implementation of reference systems in laboratory medicine. Clin Chem 46:1907–1909 [PubMed] [Google Scholar]
- 87.Lequin RM.2002Standardization: comparability and traceability of laboratory results. Clin Chem 48:391–393 [PubMed] [Google Scholar]
- 88.Szkudlinski MW, Fremont V, Ronin C, Weintraub BD.2002Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiol Rev 82:473–502 [DOI] [PubMed] [Google Scholar]
- 89.Kashiwai T, Ichihara K, Endo Y, Tamaki H, Amino N, Miyai K.1991Immunological and biological characteristics of recombinant human thyrotropin. J Immunol Methods 143:25–30 [DOI] [PubMed] [Google Scholar]
- 90.Thotakura NR, Desai RK, Bates LG, Cole ES, Pratt BM, Weintraub BD.1991Biological activity and metabolic clearance of a recombinant human thyrotropin produced in Chinese hamster ovary cells. Endocrinology 128:341–348 [DOI] [PubMed] [Google Scholar]
- 91.Ribela MT, Bianco AC, Bartolini P.1996The use of recombinant human thyrotropin produced by Chinese hamster ovary cells for the preparation of immunoassay reagents. J Clin Endocrinol Metab 81:249–256 [DOI] [PubMed] [Google Scholar]
- 92.Donadio S, Morelle W, Pascual A, Romi-Lebrun R, Michalski JC, Ronin C.2005Both core and terminal glycosylation alter epitope expression in thyrotropin and introduce discordances in hormone measurements. Clin Chem Lab Med 43:519–530 [DOI] [PubMed] [Google Scholar]
- 93.Green ED, Adelt G, Baenziger JU, Wilson S, Van Halbeek H.1988The asparagine-linked oligosaccharides on bovine fetuin. Structural analysis of N-glycanase-released oligosaccharides by 500-megahertz 1H NMR spectroscopy. J Biol Chem 263:18253–18268 [PubMed] [Google Scholar]
- 94.Thotakura NR, Desai RK, Szkudlinski MW, Weintraub BD.1992The role of the oligosaccharide chains of thyrotropin alpha- and beta-subunits in hormone action. Endocrinology 131:82–88 [DOI] [PubMed] [Google Scholar]
- 95.Medri G, Sergi I, Papandreou MJ, Beck-Peccoz P, Verrier B, Ronin C.1994Dual activity of human pituitary thyrotrophin isoforms on thyroid cell growth. J Mol Endocrinol 13:187–198 [DOI] [PubMed] [Google Scholar]
- 96.Loh TP, Kao SL, Halsall DJ, Toh SA, Chan E, Ho SC, Tai ES, Khoo CM.2012Macro-thyrotropin: a case report and review of literature. J Clin Endocrinol Metab 97:1823–1828 [DOI] [PubMed] [Google Scholar]
- 97.Sakai H, Fukuda G, Suzuki N, Watanabe C, Odawara M.2009Falsely elevated thyroid-stimulating hormone (TSH) level due to macro-TSH. Endocr J 56:435–440 [DOI] [PubMed] [Google Scholar]
- 98.Donadio S, Pascual A, Thijssen JH, Ronin C.2006Feasibility study of new calibrators for thyroid-stimulating hormone (TSH) immunoprocedures based on remodeling of recombinant TSH to mimic glycoforms circulating in patients with thyroid disorders. Clin Chem 52:286–297 [DOI] [PubMed] [Google Scholar]
- 99.Rafferty B, Gaines Das R.1999Comparison of pituitary and recombinant human thyroid-stimulating hormone (rhTSH) in a multicenter collaborative study: establishment of the first World Health Organization reference reagent for rhTSH. Clin Chem 45:2207–2215 [PubMed] [Google Scholar]
- 100.Silvio R, Swapp KJ, La'ulu SL, Hansen-Suchy K, Roberts WL.2009Method specific second-trimester reference intervals for thyroid-stimulating hormone and free thyroxine. Clin Biochem 42:750–753 [DOI] [PubMed] [Google Scholar]
- 101.Van Houcke SK, Van Uytfanghe K, Shimizu E, Tani W, Umemoto M, Thienpont LM, International Federation of Clinical C, Laboratory Medicine Working Group for Standardization of Thyroid Function T 2011IFCC international conventional reference procedure for the measurement of free thyroxine in serum: International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Working Group for Standardization of Thyroid Function Tests (WG-STFT)(1). Clin Chem Lab Med 49:1275–1281 [DOI] [PubMed] [Google Scholar]
- 102.Kaplan IV, Levinson SS.1999When is a heterophile antibody not a heterophile antibody? When it is an antibody against a specific immunogen. Clin Chem 45:616–618 [PubMed] [Google Scholar]
- 103.Preissner CM, O'Kane DJ, Singh RJ, Morris JC, Grebe SK.2003Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab 88:3069–3074 [DOI] [PubMed] [Google Scholar]
- 104.Frost SJ, Hine KR, Firth GB, Wheatley T.1998Falsely lowered FT4 and raised TSH concentrations in a patient with hyperthyroidism and human anti-mouse monoclonal antibodies. Ann Clin Biochem 35():317–320 [DOI] [PubMed] [Google Scholar]
- 105.Lania A, Persani L, Beck-Peccoz P.2008Central hypothyroidism. Pituitary 11:181–186 [DOI] [PubMed] [Google Scholar]
- 106.Roelfsema F, Pereira AM, Veldhuis JD, Adriaanse R, Endert E, Fliers E, Romijn JA.2009Thyrotropin secretion profiles are not different in men and women. J Clin Endocrinol Metab 94:3964–3967 [DOI] [PubMed] [Google Scholar]
- 107.Oliveira JH, Persani L, Beck-Peccoz P, Abucham J.2001Investigating the paradox of hypothyroidism and increased serum thyrotropin (TSH) levels in Sheehan's syndrome: characterization of TSH carbohydrate content and bioactivity. J Clin Endocrinol Metab 86:1694–1699 [DOI] [PubMed] [Google Scholar]
- 108.Kasagi K, Konishi J, Arai K, Misaki T, Iida Y, Endo K, Torizuka K.1986A sensitive and practical assay for thyroid-stimulating antibodies using crude immunoglobulin fractions precipitated with polyethylene glycol. J Clin Endocrinol Metab 62:855–862 [DOI] [PubMed] [Google Scholar]
- 109.Ajjan RA, Weetman AP.2008Techniques to quantify TSH receptor antibodies. Nat Clin Pract Endocrinol Metab 4:461–468 [DOI] [PubMed] [Google Scholar]
- 110.Menezes-Ferreira MM, Petrick PA, Weintraub BD.1986Regulation of thyrotropin (TSH) bioactivity by TSH-releasing hormone and thyroid hormone. Endocrinology 118:2125–2130 [DOI] [PubMed] [Google Scholar]
- 111.Murakami M, Miyashita K, Kakizaki S, Saito S, Yamada M, Iriuchijima T, Takeuchi T, Mori M.1995Clinical usefulness of thyroid-stimulating antibody measurement using Chinese hamster ovary cells expressing human thyrotropin receptors. Eur J Endocrinol 133:80–86 [DOI] [PubMed] [Google Scholar]
- 112.Persani L, Tonacchera M, Beck-Peccoz P, Vitti P, Mammoli C, Chiovato L, Elisei R, Faglia G, Ludgate M, Vassart G, et al. . 1993Measurement of cAMP accumulation in Chinese hamster ovary cells transfected with the recombinant human TSH receptor (CHO-R): a new bioassay for human thyrotropin. J Endocrinol Invest 16:511–519 [DOI] [PubMed] [Google Scholar]
- 113.Perret J, Ludgate M, Libert F, Gerard C, Dumont JE, Vassart G, Parmentier M.1990Stable expression of the human TSH receptor in CHO cells and characterization of differentially expressing clones. Biochem Biophys Res Commun 171:1044–1050 [DOI] [PubMed] [Google Scholar]
- 114.Takano T, Sumizaki H, Amino N.1997Detection of thyroid-stimulating antibody using frozen stocks of Chinese hamster ovary cells transfected with cloned human thyrotropin receptor. Endocr J 44:431–435 [DOI] [PubMed] [Google Scholar]
- 115.Paunkovic J, Paunkovic N.2006Does autoantibody-negative Graves' disease exist? A second evaluation of the clinical diagnosis. Horm Metab Res 38:53–56 [DOI] [PubMed] [Google Scholar]
- 116.Preissner CM, Wolhuter PJ, Sistrunk JW, Homburger HA, Morris JC., 3rd2003Comparison of thyrotropin-receptor antibodies measured by four commercially available methods with a bioassay that uses Fisher rat thyroid cells. Clin Chem 49:1402–1404 [DOI] [PubMed] [Google Scholar]
- 117.Smith BR, Hall R.1974Thyroid-stimulating immunoglobulins in Graves' disease. Lancet 2:427–431 [DOI] [PubMed] [Google Scholar]
- 118.Villalta D, Orunesu E, Tozzoli R, Montagna P, Pesce G, Bizzaro N, Bagnasco M.2004Analytical and diagnostic accuracy of “second generation” assays for thyrotrophin receptor antibodies with radioactive and chemiluminescent tracers. J Clin Pathol 57:378–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kamijo K.2007TSH-receptor antibodies determined by the first, second and third generation assays and thyroid-stimulating antibody in pregnant patients with Graves' disease. Endocr J 54:619–624 [DOI] [PubMed] [Google Scholar]
- 120.Chappel S.1990Biological to immunological ratios: reevaluation of a concept. J Clin Endocrinol Metab 70:1494–1495 [DOI] [PubMed] [Google Scholar]
- 121.Gauchez AS, Pizzo M, Alcaraz-Galvain D, Chikh K, Orgiazzi J, Brabant G, Ronin C, Charrie A.2010TSH Isoforms: about a case of hypothyroidism in a Down's syndrome young adult. J Thyroid Res 2010:703978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beck-Peccoz P, Persani L.1994Variable biological activity of thyroid-stimulating hormone. Eur J Endocrinol 131:331–340 [DOI] [PubMed] [Google Scholar]
- 123.Bonomi M, Busnelli M, Beck-Peccoz P, Costanzo D, Antonica F, Dolci C, Pilotta A, Buzi F, Persani L.2009A family with complete resistance to thyrotropin-releasing hormone. N Engl J Med 360:731–734 [DOI] [PubMed] [Google Scholar]
- 124.Collu R, Tang J, Castagne J, Lagace G, Masson N, Huot C, Deal C, Delvin E, Faccenda E, Eidne KA, Van Vliet G.1997A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropin-releasing hormone receptor gene. J Clin Endocrinol Metab 82:1561–1565 [DOI] [PubMed] [Google Scholar]
- 125.Faglia G, Bitensky L, Pinchera A, Ferrari C, Paracchi A, Beck-Peccoz P, Ambrosi B, Spada A.1979Thyrotropin secretion in patients with central hypothyroidism: evidence for reduced biological activity of immunoreactive thyrotropin. J Clin Endocrinol Metab 48:989–998 [DOI] [PubMed] [Google Scholar]
- 126.Beck-Peccoz P, Amr S, Menezes-Ferreira MM, Faglia G, Weintraub BD.1985Decreased receptor binding of biologically inactive thyrotropin in central hypothyroidism. Effect of treatment with thyrotropin-releasing hormone. N Engl J Med 312:1085–1090 [DOI] [PubMed] [Google Scholar]
- 127.Lee KO, Persani L, Tan M, Sundram FX, Beck-Peccoz P.1995Thyrotropin with decreased biological activity, a delayed consequence of cranial irradiation for nasopharyngeal carcinoma. J Endocrinol Invest 18:800–805 [DOI] [PubMed] [Google Scholar]
- 128.Horimoto M, Nishikawa M, Yoshikawa N, Yoshimura M, Inada M.1990A sensitive and practical bioassay for thyrotropin (TSH): comparison of the bioactivity of TSH in normal subjects and in patients with primary hypothyroidism. Endocrinol Jpn 37:577–581 [DOI] [PubMed] [Google Scholar]
- 129.Dahlberg PA, Petrick PA, Nissim M, Menezes-Ferreira MM, Weintraub BD.1987Intrinsic bioactivity of thyrotropin in human serum is inversely correlated with thyroid hormone concentrations. Application of a new bioassay using the FRTL-5 rat thyroid cell strain. J Clin Invest 79:1388–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Persani L, Terzolo M, Asteria C, Orlandi F, Angeli A, Beck-Peccoz P.1995Circadian variations of thyrotropin bioactivity in normal subjects and patients with primary hypothyroidism. J Clin Endocrinol Metab 80:2722–2728 [DOI] [PubMed] [Google Scholar]
- 131.Beck-Peccoz P, Piscitelli G, Amr S, Ballabio M, Bassetti M, Giannattasio G, Spada A, Nissim M, Weintraub BD, Faglia G.1986Endocrine, biochemical, and morphological studies of a pituitary adenoma secreting growth hormone, thyrotropin (TSH), and alpha-subunit: evidence for secretion of TSH with increased bioactivity. J Clin Endocrinol Metab 62:704–711 [DOI] [PubMed] [Google Scholar]
- 132.Persani L, Asteria C, Tonacchera M, Vitti P, Krishna V, Chatterjee K, Beck-Peccoz P.1994Evidence for the secretion of thyrotropin with enhanced bioactivity in syndromes of thyroid hormone resistance. J Clin Endocrinol Metab 78:1034–1039 [DOI] [PubMed] [Google Scholar]
- 133.Sheehan HL.1948Post-partum necrosis of the anterior pituitary. Ir J Med Sci 270:241–255 [DOI] [PubMed] [Google Scholar]
- 134.Whitehead R.1963The hypothalamus in post-partum hypopituitarism. J Pathol Bacteriol 86:55–67 [DOI] [PubMed] [Google Scholar]
- 135.Abucham J, Castro V, Maccagnan P, Vieira JG.1997Increased thyrotrophin levels and loss of the nocturnal thyrotrophin surge in Sheehan's syndrome. Clin Endocrinol (Oxf) 47:515–522 [DOI] [PubMed] [Google Scholar]
- 136.Dejager S, Gerber S, Foubert L, Turpin G.1998Sheehan's syndrome: differential diagnosis in the acute phase. J Intern Med 244:261–266 [DOI] [PubMed] [Google Scholar]
- 137.MacCagnan P, Oliveira JH, Castro V, Abucham J.1999Abnormal circadian rhythm and increased non-pulsatile secretion of thyrotrophin in Sheehan's syndrome. Clin Endocrinol (Oxf) 51:439–447 [DOI] [PubMed] [Google Scholar]
- 138.Boucai L, Hollowell JG, Surks MI.2011An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid 21:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Surks MI.2008Should the upper limit of the normal reference range for TSH be lowered? Nat Clin Pract Endocrinol Metab 4:370–371 [DOI] [PubMed] [Google Scholar]
- 140.Glinoer D, Soto MF, Bourdoux P, Lejeune B, Delange F, Lemone M, Kinthaert J, Robijn C, Grun JP, de Nayer P.1991Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab 73:421–427 [DOI] [PubMed] [Google Scholar]
- 141.Glinoer D, Spencer CA.2010Serum TSH determinations in pregnancy: how, when and why? Nat Rev 6:526–529 [DOI] [PubMed] [Google Scholar]
- 142.Lockwood CM, Grenache DG, Gronowski AM.2009Serum human chorionic gonadotropin concentrations greater than 400,000 IU/L are invariably associated with suppressed serum thyrotropin concentrations. Thyroid 19:863–868 [DOI] [PubMed] [Google Scholar]
- 143.Feldt-Rasmussen U, Bliddal Mortensen AS, Rasmussen AK, Boas M, Hilsted L, Main K.2011Challenges in interpretation of thyroid function tests in pregnant women with autoimmune thyroid disease. J Thyroid Res 2011:598712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dashe JS, Casey BM, Wells CE, McIntire DD, Byrd EW, Leveno KJ, Cunningham FG.2005Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol 106:753–757 [DOI] [PubMed] [Google Scholar]
- 145.Negro R, Soldin OP, Obregon MJ, Stagnaro-Green A.2011Hypothyroxinemia and pregnancy. Endocr Pract 17:422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Soldin SJ, Cheng LL, Lam LY, Werner A, Le AD, Soldin OP.2010Comparison of FT4 with log TSH on the Abbott Architect ci8200: pediatric reference intervals for free thyroxine and thyroid-stimulating hormone. Clin Chim Acta 411:250–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stricker R, Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, Stricker R.2007Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. Eur J Endocrinol 157:509–514 [DOI] [PubMed] [Google Scholar]
- 148.Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A.2007Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 92:S1–S47 [DOI] [PubMed] [Google Scholar]
- 149.Casey BM, Dashe JS, Wells CE, McIntire DD, Leveno KJ, Cunningham FG.2006Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol 107:337–341 [DOI] [PubMed] [Google Scholar]
- 150.Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, van der Wal MF, Bonsel GJ.2007Ethnic differences in TSH but not in free T4 concentrations or TPO antibodies during pregnancy. Clin Endocrinol (Oxf) 66:765–770 [DOI] [PubMed] [Google Scholar]
- 151.Casey BM, Leveno KJ.2006Thyroid disease in pregnancy. Obstet Gynecol 108:1283–1292 [DOI] [PubMed] [Google Scholar]
- 152.Martel J, Despres N, Ahnadi CE, Lachance JF, Monticello JE, Fink G, Ardemagni A, Banfi G, Tovey J, Dykes P, John R, Jeffery J, Grant AM.2000Comparative multicentre study of a panel of thyroid tests using different automated immunoassay platforms and specimens at high risk of antibody interference. Clin Chem Lab Med 38:785–793 [DOI] [PubMed] [Google Scholar]