Abstract
Background: Thyroid cancer incidence is increasing, potentially due to enhanced diagnostic practices. However, access to healthcare may be dependent on socioeconomic status (SES) and race/ethnicity. Consequently, certain segments of the population may experience thyroid cancer overdiagnosis as a result of greater access to and use of enhanced diagnostic technology. The current study examined trends by SES in thyroid cancer incidence at the census tract level from 1995 to 2008 for the population of Texas, as well as by racial/ethnic subgroup.
Methods: Joinpoint regressions were used to examine incidence trends over time by SES for the study population, and for the non-Hispanic white, non-Hispanic black, and Hispanic subgroups separately. Other race/ethnicities were not adequately represented for subgroup analyses.
Results: There were 22,390 incident thyroid cancer cases (65.0% white, 6.7% black, 24.3% Hispanic, 4.1% Asian/other races; 85.9% papillary histology). The low SES group experienced a steady increase in incidence since 1995 (6.7% per year, p<0.05), whereas incidence among the high SES group has increased at a rate of 8.6% per year since 1999 (p<0.05). The joinpoint projected incidence trends for the low and high SES groups were significantly different (p=0.047). Whites experienced a steady increase in incidence over time among both high and low SES groups (7.6% per year p<0.05), whereas blacks and Hispanics of higher SES had a much more pronounced increase in incidence over time relative to their lower SES counterparts (blacks=12.8% vs. 4.1%; Hispanics=11.2% vs. 8.3%, p<0.05). For blacks and Hispanics, joinpoint projected incidence trends for the low and high SES groups were significantly different from one another (p<0.001–0.004).
Conclusions: These results identify groups experiencing the greatest problem of increasing thyroid cancer incidence, and raise concern that greater access to healthcare may be accompanied by thyroid cancer overdiagnosis. A dual focus on delineating and preventing disease-related causal factors and focusing clinical attention on avoiding overdiagnosis among certain populations (e.g., high SES) may be advisable to address thyroid cancer in Texas. Clinicians are encouraged to adhere to ATA/NCCN guidelines when choosing patients for thyroid ultrasound, selecting which nodules to examine, and deciding which patients should proceed to biopsy.
Introduction
Although thyroid cancer is a relatively rare carcinoma, several studies support rising incidence over the last few decades in the United States (1–6). A number of factors have been cited for these increases (7–9), most prominently, improvements in diagnostic practices (e.g., the use of ultrasound with fine needle aspiration biopsies) resulting in an increased ability to detect smaller tumors (1,10,11). Several studies have suggested that diagnostic improvements cannot wholly account for observed incidence trends, indicating a potential role for other factors such as increased environmental or hormonal exposures (8,12–16). However, a lack of concomitant increases in thyroid cancer mortality and evidence of a relatively high subclinical reservoir of occult papillary thyroid cancers suggests that the bulk of rising incidence rates are most likely attributable to the enhanced detection of small, asymptomatic thyroid cancers (1,17–19). If so, it is likely that increases in thyroid cancer incidence (particularly of small tumors) would be pronounced among segments of the population with greater access to improved diagnostic technology, such as among patients of higher socioeconomic status (SES). Recent research conducted with the National Cancer Institute (NCI)'s Survival, Epidemiology and End Results (SEER) 9 data supports this possibility (12). However, additional research with other data sources is needed to substantiate these findings, and to understand better whether these associations are universal or are restricted to non-Hispanic white patients. This research is important inasmuch as rising incidence trends may at least partially reflect the overdiagnosis of thyroid carcinomas that would otherwise not elicit symptoms or require clinical intervention.
Thyroid cancer incidence in the United States is known to vary by race (14,20). Unlike many other types of cancer, however, thyroid cancer incidence is higher among whites than among blacks (14,20), although both racial groups have experienced increasing incidence trends in recent years (14,21). Although incidence differences may reflect true racial/ethnic dissimilarities in disease rates, SEER data indicate that thyroid cancer incidence among whites (including Hispanics) is rising more sharply than the incidence among blacks (including Hispanics), particularly from the late 1990s onward (1975–2009 data) (22). This pattern suggests the possibility of overdiagnosis of thyroid cancers among whites due to potentially better access to new diagnostic technologies or healthcare (23). Higher SES and accompanying health insurance coverage may contribute to a higher likelihood of referral for diagnostic procedures in response to the potentially ambiguous symptoms of thyroid cancer (10), and these factors may be more relevant to whites than blacks and patients of other racial/ethnic minority groups. Although several recent studies have focused on racial/ethnic or socioeconomic differences in thyroid incidence, few examine both race/ethnicity and SES in conjunction with one another [notable exceptions include Morris et al. (23) and Brown et al. (24)], and additional research is needed to tease apart their relations better with incidence trends.
Cancer registries in the United States do not collect individual-level SES information of cases. Therefore, registry-based studies examining SES trends in cancer incidence and cancer survival generally use county-level (10,12) or zip-code-level (23) economic data to estimate SES information for cases. However, counties and even zip codes tend to be rather large areas with a great deal of economic diversity that is masked in a combined average estimate of residents' SES. On the other hand, census tracts are smaller statistical subdivisions averaging about 4000 residents each that were originally designed to be homogeneous in terms of population characteristics including residents' SES (25). Census tracts have been supported as suitable proxies for neighborhoods in previous research (26,27), and neighborhood-level SES has been used as a proxy for individual-level SES in previous studies (28). Therefore, compared to county- and zip-code-level estimates, SES data generated at the census tract level may be more representative of the individual-level SES of the inhabitants, and may be a more relevant estimate of patients' SES. However, we are aware of no previous studies on socioeconomic trends in thyroid cancer incidence conducted in the United States that have used this potentially more representative conceptualization of SES.
In addition, many previous studies focused on trends in thyroid cancer incidence have used national data from the SEER database (12,23), which focuses on certain states and geographical regions not including Texas or its metropolitan areas. Consequently, to the best of our knowledge, trends over time in thyroid cancer incidence by SES have not been previously examined using Texas Cancer Registry data. Significantly, Texas has the highest uninsurance rates, with 31% of adult Texans living without health insurance coverage (29). Given documented associations between health insurance coverage and thyroid cancer incidence (10,23), it is of interest to examine socioeconomic trends in thyroid cancer incidence in Texas inasmuch as SES-based incidence differences attributable to detection biases might be particularly pronounced in this state. Moreover, because these relations may operate differently within racial/ethnic groups due to differing access to care issues (e.g., uninsurance rates in Texas are 37% for Hispanics, 21.4% for blacks, and 13.5% for whites) (30), it is also of interest to examine socioeconomic-based trends in thyroid cancer incidence among Texans by race/ethnicity. The examination of socioeconomic trends in cancer incidence by race/ethnicity in a single (but relatively large) state such as Texas is particularly appropriate given recent findings citing interactions of race/ethnicity and geographic region in thyroid cancer survival outcomes using the 17-region national SEER database (31), which may also be relevant to thyroid cancer incidence.
The current study was designed to examine trends in thyroid cancer incidence in Texas from 1995 to 2008 by SES, for the population as a whole and by racial/ethnic group, using census-tract-level data as a proxy for individual-level SES. First, we were interested in better understanding whether increases in incidence over time appeared attributable to a true increase in disease rates, which might be suggested by similarly increasing incidence among low and high SES groups, or to a potential detection bias whereby increases in incidence over time were largely attributable to the high SES group. Second, we were interested in how racial/ethnic trends in incidence by SES might further enhance our understanding of rising incidence. A better understanding of the potential factors underlying increasing thyroid cancer incidence is important, as resulting information can help to guide future efforts toward delineating and preventing disease-related causal factors (in the case of true disease increases) or focusing clinical attention on avoiding overdiagnosis among certain segments of the population (in the case of a detection bias).
Materials and Methods
Human subjects study approval
The University of Texas MD Anderson Cancer Center Institutional Review Board and the Texas Department of State Health Services Institutional Review Board approved this study.
Data sources and variables
Thyroid cancer incidence data were provided by the Texas Department of State Health Services from the Texas Cancer Registry, which has achieved an annual “gold” certification from the North American Association of Central Cancer Registries based on their standards for data completeness, accuracy, and timeliness. Provided data were based on the International Classification of Diseases for Oncology code C73.9. These data were available for each year beginning in 1995 and concluding in 2008. Data provided from this source included the following information: race/ethnicity, sex, age at diagnosis, cancer identification information (histologic type), stage, and identification information regarding the census tract of residence at the time of diagnosis. In addition, tumor size data were made available, but approximately half of the cases were missing these data. Previous research suggests that the rising incidence in thyroid cancers is largely attributable to the papillary type (1,2,6,10,13). However, we elected to include all histologic types in the current study because of the potential for detection disparities to affect all histologic types, to account for any potential histologic misclassification, and to maximize the sample size. A similar approach has been taken in previous literature (12).
The individual-level SES of thyroid cancer cases was not part of registry information, and had to be estimated based on area-level information. The U.S. census tract of residence at the time of diagnosis was used to procure the relevant SES information. First, all tracts in Texas were ranked based on median household income from the 2000 U.S. census. The 2000 U.S. census information was used because it was the only decennial census data collection that was conducted during the span of time relevant to the cancer incidence data (1995–2008). Next, we categorized each tract as a “low SES” or “high SES” tract. The low SES tracts represented those in the lowest quartile of median household income (range=$0–$27,803; mean [SD]=$21,761 [$5280]), whereas the high SES tracts represented the remaining tracts (i.e., those tracts within the top 75% of median household income; range=$27,803–$200,001; mean [SD]=$47,658 [$20,946]). This procedure is similar to other work in the area (12), and is intended to target tracts that are particularly socioeconomically disadvantaged. The use of area-level median household income as an indicator of SES is supported by its relation with thyroid incidence in previous work (10,12). The designation of census tracts as low or high SES was then linked back to the incidence data based on the residential census tract identifier for each incidence case.
Data preparation
To prepare data for analysis, we calculated age-standardized incidence by race/ethnicity based on 2000 U.S. census tract-level population estimates per recommendations in the literature (32). Age adjustments were made based on 18 age groups (0–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, ≥85 years). There were 134 cases missing information on race/ethnicity, and an additional four cases that could not be matched to viable census tracts. These cases were excluded from these calculations and subsequent analyses.
Data analyses
Incidence calculations, descriptive statistics, and preliminary analyses were performed using SAS v9.3 (SAS Institute Inc., Cary, NC). Preliminary analyses included chi-square tests to examine for significant differences in demographic and clinical variables by SES group. Joinpoint regression analyses were used to examine incidence trends over time by SES for the whole study population and by race/ethnicity. Joinpoint regression examines the statistical significance of trends as well as the annual percentage change (APC) across segments with 95% confidence intervals (CI). Joinpoint analyses were performed using the NCI's Joinpoint Regression Program v3.5.4 (33). Results were examined for the presence of joinpoints (adjacent segments denoting significant changes in incidence over time) and for significant APCs by SES. p-Values of ≤0.05 were considered statistically significant.
Several exploratory analyses were conducted to follow up the main analyses as described above. First, in order to explore temporal trends in a different way, we also broke the study years into two equal time periods—T1 1995–2001, and T2 2002–2008—and reran main joinpoint analyses to examine trends within each time period. Next, because readers might be interested in how relations between SES and incidence trends varied by sex and by cancer histology, we reran the main analyses: (i) stratified by sex, and (ii) for papillary histology cases only (presentation by other histology type was precluded due to small case numbers).
Results
Study population
There were 22,390 thyroid cancer cases from 1995 to 2008. As expected, the majority of patients were female (75.7%) and non-Hispanic white (65.0%). Approximately 24.3% of patients were Hispanic, and 6.7% were non-Hispanic black. The remaining patients (4.1%) comprised Asian, American Indian, and several other racial/ethnic groups. Patients resided within 4076 of the 4388 census tracts within Texas, with the number of cases per tract ranging from 1 to 49. The majority of cases (84.3%) were from high SES tracts.
When cancer cases were categorized by histological category, 85.9% (18,397) were papillary (ICD codes 8050, 8052, 8130, 8260, 8340–8344, 8450, 8452), 10.7% (2291) were follicular (ICD codes 8290, 8330–8332, 8335), 2.5% (528) were medullary (8345, 8346, 8510), and 0.9% (190) were anaplastic (8021). In addition to those falling into the four major histological categories, there were 984 additional thyroid cancer cases of other histological types. Unfortunately, there was a significant amount of missing data for tumor size (50.4%), limiting our ability to examine racial/ethnic trends in incidence by SES over time based on these factors, which is an issue not uncommon among other state-based incidence studies (10). When cases from each year were collapsed into a single group, chi-square tests indicated statistically significant differences in age, sex, race/ethnicity, tumor size, and tumor stage by SES (see Table 1 for participant characteristics and differences by SES). Due to the relatively low incidence of thyroid cancer among Asian/other races, all racial/ethnic analyses included only the non-Hispanic white, non-Hispanic black, and Hispanic cases.
Table 1.
Characteristics of Patients with Thyroid Cancer—Texas Cancer Registry Data, 1995–2008
| Characteristics | Total cases [n (%)] | SES-low cases [n (%)] | SES-high cases [n (%)] | SES differences (p-value) |
|---|---|---|---|---|
| Age | <0.001 | |||
| <45 years | 9464 (42.3) | 1365 (38.9) | 8099 (42.9) | |
| ≥45 years | 12,926 (57.7) | 2148 (61.1) | 10,778 (57.1) | |
| Sex | <0.001 | |||
| Male | 5448 (24.3) | 728 (20.7) | 4720 (25.0) | |
| Female | 16,942 (75.7) | 2785 (79.3) | 14,157 (75.0) | |
| Race/Ethnicity | <0.001 | |||
| Non-Hispanic white | 14,460 (65.0) | 1054 (30.1) | 13,406 (71.5) | |
| Non-Hispanic black | 1484 (6.7) | 465 (13.3) | 1019 (5.4) | |
| Hispanic | 5400 (24.3) | 1920 (54.8) | 3480 (18.6) | |
| Asian/other | 912 (4.1) | 65 (1.9) | 847 (4.5) | |
| Thyroid tumor size | <0.001 | |||
| ≤1 cm | 3773 (34.0) | 488 (28.6) | 3285 (34.9) | |
| >1–2 cm | 3008 (27.1) | 451 (26.5) | 2557 (27.2) | |
| >2–4 cm | 2963 (26.7) | 468 (27.5) | 2495 (26.5) | |
| >4 cm | 1361 (12.3) | 297 (17.4) | 1064 (11.3) | |
| Missing tumor size | 0.158 | |||
| No | 11,105 (49.6) | 1704 (48.5) | 9401 (49.8) | |
| Yes | 11,285 (50.4) | 1809 (51.5) | 9476 (50.2) | |
| Thyroid tumor stage | <0.001 | |||
| In situ/local | 14,080 (68.7) | 2022 (63.7) | 12,058 (69.6) | |
| Regional | 5334 (26.0) | 880 (27.7) | 4454 (25.7) | |
| Distant | 1096 (5.3) | 273 (8.6) | 823 (4.8) | |
| Missing tumor stage | 0.004 | |||
| No | 20,510 (91.6) | 3175 (90.4) | 17,335 (91.8) | |
| Yes | 1880 (8.4) | 338 (9.6) | 1542 (8.2) | |
| Whole sample | 22,390 | 3513 (15.7) | 18,877 (84.3) |
Socioeconomic status (SES) was calculated based on quartiles of median household income at the census tract level using 2000 U.S. census data. SES-low represents case representation in the first quartile of statewide median household income, whereas SES-high represents case representation in the remaining three quartiles. Significant differences between SES groups were assessed using chi-square tests.
Main analyses
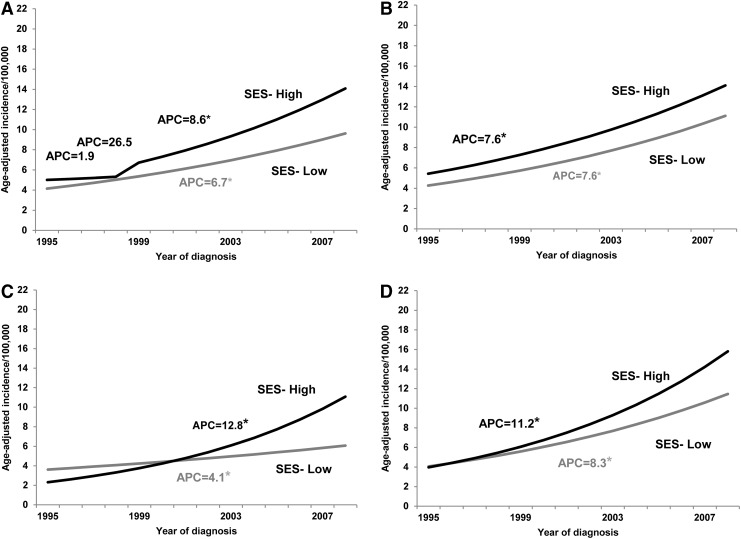
The study population (which included Asian/other cases) demonstrated an expected increase in thyroid cancer incidence over the study period among cases from both low and high SES areas, as indicated by positive incidence slopes in both groups. Among the low SES group, thyroid cancer incidence increased from 4.7 per 100,000 [CI 4.0–5.4] in 1995 to 8.9 per 100,000 [CI 8.0–9.9] in 2008. Incidence among the high SES group increased from 5.0 per 100,000 [CI 4.6–5.3] in 1995 to 13.8 per 100,000 [CI 13.2–14.3] in 2008. There were no joinpoints among the low SES cases, indicating a steady increase over time (APC=6.7%, p<0.05). However, two joinpoints emerged among the high SES cases between 1995 and 1999. Specifically, the APC between 1995 and 1998 was 1.9% (p>0.05), which jumped to 26.5% (p>0.05) from 1998 to 1999, and returned to a less pronounced but statistically significant steady 8.6% (p<0.05) increase in incidence from 1999 to 2008. A parallelism test indicated that the joinpoint projected incidence trends between low and high SES groups were significantly different from one another (p=0.047).
There was an increase in thyroid cancer incidence within each of the racial/ethnic-specific SES subgroups as well. Incidence among the low SES groups per 100,000 in 1995 and 2008 respectively by racial/ethnic subgroup were as follows: non-Hispanic white 6.4 [CI 4.8–7.9] and 9.3 [CI 7.4–11.2], non-Hispanic black 3.7 [CI 2.3–5.1] and 4.6 [CI 3.0–6.2], and Hispanic 4.2 [CI 3.3–5.2] and 10.8 [CI 9.2–12.3]. Among the high SES groups, incidence per 100,000 in 1995 and 2008 respectively by racial/ethnic subgroup were as follows: non-Hispanic white 5.6 [CI 5.2–6.1] and 13.9 [CI 13.2–14.7], non-Hispanic black 2.6 [CI 1.6–3.5] and 11 [CI 9–13], and Hispanic 4.7 [CI 3.8–5.6] and 15.3 [CI 13.7–16.9]. Age-adjusted incidence rates by SES and race/ethnicity for all study years are summarized in Table 2. No joinpoints emerged among the non-Hispanic white, non-Hispanic black, or Hispanic cases in either the low or the high SES groups. However, the results indicate that whereas non-Hispanic whites experienced a steady increase in incidence over time among both high and low SES groups (combined APC=7.6%, p<0.05), non-Hispanic blacks and Hispanics of higher SES had a much more pronounced increase in incidence over time relative to non-Hispanic blacks (black low SES APC=4.1% vs. high SES APC=12.8%, p<0.05) and Hispanics of lower SES (Hispanic low SES APC=8.3% vs. high SES APC=11.2%; p<0.05). Parallelism tests indicate that the joinpoint projected incidence trends between low and high SES groups were significantly different from one another in the non-Hispanic black (p<0.001) and Hispanic (p=0.004) subgroups, but not in the non-Hispanic white subgroup (p=0.064).
Table 2.
Age-Adjusted Incidence Rate per 100,000 for Thyroid Cancer by Socioeconomic Status and Race/Ethnicity, 1995–2008
| SES-low | SES-high | |||||
|---|---|---|---|---|---|---|
| Year | White AAIR [CI] | Black AAIR [CI] | Hispanic AAIR [CI] | White AAIR [CI] | Black AAIR [CI] | Hispanic AAIR [CI] |
| 1995 | 6.37 [4.81–7.92] | 3.68 [2.28–5.08] | 4.23 [3.29–5.17] | 5.61 [5.16–6.07] | 2.56 [1.58–3.53] | 4.73 [3.81–5.65] |
| 1996 | 5.55 [4.07–7.03] | 3.03 [1.73–4.33] | 3.52 [2.68–4.37] | 5.99 [5.52–6.46] | 2.19 [1.38–3.00] | 4.52 [3.66–5.37] |
| 1997 | 5.94 [4.40–7.47] | 3.79 [2.35–5.22] | 5.41 [4.33–6.50] | 5.72 [5.26–6.18] | 3.07 [2.04–4.10] | 4.22 [3.40–5.03] |
| 1998 | 4.93 [3.50–6.36] | 3.47 [2.11–4.84] | 5.08 [4.05–6.12] | 6.14 [5.66–6.62] | 3.37 [2.32–4.42] | 4.40 [3.55–5.26] |
| 1999 | 6.07 [4.49–7.65] | 4.24 [2.72–5.76] | 6.45 [5.27–7.62] | 7.15 [6.64–7.67] | 3.62 [2.52–4.72] | 6.73 [5.68–7.78] |
| 2000 | 5.28 [3.82–6.73] | 5.86 [4.09–7.63] | 5.41 [4.37–6.46] | 8.03 [7.48–8.57] | 3.59 [2.47–4.70] | 6.70 [5.67–7.73] |
| 2001 | 7.01 [5.36–8.67] | 4.73 [3.11–6.35] | 6.63 [5.45–7.81] | 8.08 [7.53–8.62] | 4.22 [2.99–5.46] | 6.84 [5.80–7.88] |
| 2002 | 6.69 [5.05–8.33] | 3.93 [2.47–5.40] | 7.79 [6.48–9.11] | 9.38 [8.79–9.97] | 6.02 [4.57–7.47] | 8.98 [7.73–10.23] |
| 2003 | 6.56 [4.97–8.16] | 5.05 [3.39–6.71] | 6.61 [5.40–7.82] | 9.69 [9.09–10.29] | 6.62 [5.14–8.10] | 9.54 [8.28–10.79] |
| 2004 | 9.04 [7.18–10.89] | 5.10 [3.43–6.77] | 7.81 (6.53–9.10) | 10.72 [10.09–11.35] | 6.96 [5.42–8.49] | 10.74 [9.37–12.12] |
| 2005 | 9.49 [7.56–11.43] | 7.33 [5.31–9.35] | 9.30 [7.87–10.74] | 11.77 [11.11–12.43] | 8.94 [7.16–10.72] | 11.15 [9.77–12.53] |
| 2006 | 8.29 [6.54–10.04] | 5.02 [3.35–6.68] | 10.26 [8.74–11.77] | 12.41 [11.73–13.08] | 9.52 [7.70–11.33] | 13.59 [11.99–15.19] |
| 2007 | 9.03 [7.18–10.87] | 5.67 [3.91–7.43] | 11.06 [9.51–12.60] | 13.04 [12.36–13.73] | 7.78 [6.21–9.36] | 13.89 [12.32–15.45] |
| 2008 | 9.30 [7.40–11.20] | 4.63 [3.04–6.21] | 10.75 [9.23–12.27] | 13.94 [13.22–14.65] | 10.96 [8.97–12.95] | 15.28 [13.67–16.90] |
AAIR, age-adjusted incidence rate per 100,000; CI, 95% confidence interval.
Trends over time in thyroid cancer incidence by SES for the whole study population and by race/ethnicity are displayed in Figure 1.
FIG. 1.
Results from joinpoint regression models on thyroid cancer incidence trends in Texas, 1995–2008, by socioeconomic status (SES; high [black], low [gray]) for (A) the whole study population, and within three major racial/ethnic groups: (B) non-Hispanic white, (C) non-Hispanic black, and (D) Hispanic. *Statistically significant annual percent change (APC) at p<0.05 in the joinpoint regression analyses.
Exploratory analyses
Exploratory analyses included an examination of joinpoint incidence trends in T1 (1995–2001) and T2 (2002–2008). For the whole population, results indicated a steady increase in incidence over time among both high and low SES groups during both time periods (T1 combined APC=7.9%, p<0.05; T2 combined APC=7.6%, p<0.05), and the incidence trends between groups were considered parallel (p≥0.31). Likewise, for non-Hispanic blacks, results indicated a steady increase in incidence over time among both high and low SES groups during both time periods (T1 combined APC=9.2%, p<0.05; T2 combined APC=7.2%, p<0.05), with parallel incidence trends (p≥0.45). However, trends for non-Hispanic whites were not parallel between SES groups (p≤0.014), with the low SES APC=0.5% (p>0.05) and the high SES 7.5% (p<0.05) during T1 and the low SES APC=5.1% (p<0.05) and the high SES 7.2% (p<0.05) during T2. Among Hispanics, the low SES group had a steady increase of 9.0% (p<0.05) during T1, whereas the high SES group had three joinpoints (1995–1998 APC=3.0%; 1998–1999 APC=58.6%; 1998–2001 APC=0.3%; p>0.05). These trends were not parallel (p<0.02). However, trends were parallel during T2 for Hispanic cases (p=0.46), with a combined APC of 9.1% (p<0.05) among both the high and low SES groups.
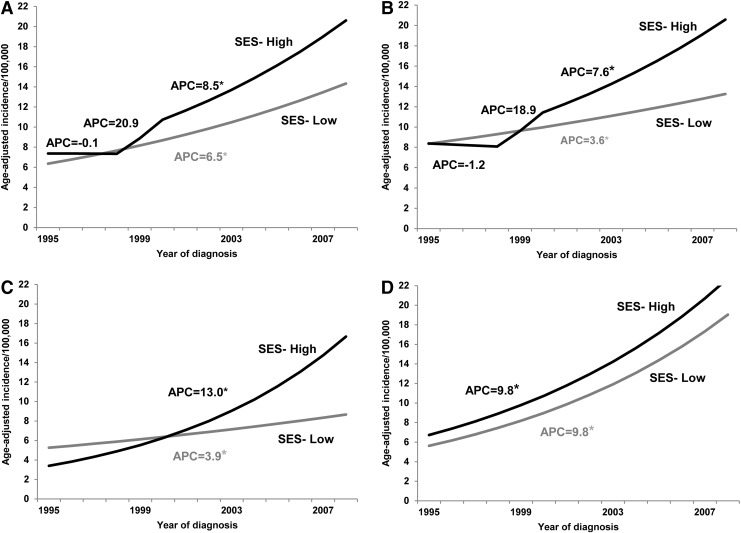
Sex-stratified analyses also reflected the increase in thyroid cancer incidence over the study period among cases from both low and high SES areas. Age-adjusted incidence rates by SES and sex for all study years are summarized in Table 3. Among males, there were no joinpoints in either the low or high SES groups (APC=7.8%, p<0.05), and the incidence trends between groups were considered parallel (p=0.33). Among females, there were no joinpoints among the low SES cases, indicating a steady increase over time (APC=6.5%, p<0.05). However, two joinpoints emerged among the high SES cases between 1995 and 2000. Specifically, the APC between 1995 and 1998 was −0.1% (p>0.05), which jumped to 20.9% (p>0.05) from 1998 to 2000, and returned to a less pronounced but statistically significant steady 8.5% (p<0.05) increase in incidence from 2000 to 2008. A parallelism test indicated that the joinpoint projected incidence trends between low and high SES groups of female cases were significantly different from one another (p=0.048).
Table 3.
Age-Adjusted Incidence Rate per 100,000 for Thyroid Cancer Incidence by Socioeconomic Status and Sex, 1995–2008
| SES-low | SES-high | |||||
|---|---|---|---|---|---|---|
| By sex | By sex | |||||
| Year | All AAIR [CI] | Male AAIR [CI] | Female AAIR [CI] | All AAIR [CI] | Male AAIR [CI] | Female AAIR [CI] |
| 1995 | 4.71 [4.02–5.39] | 1.79 [1.16–2.41] | 7.41 [6.22–8.59] | 4.96 [4.61–5.31] | 2.62 [2.24–3.00] | 7.25 [6.66–7.84] |
| 1996 | 4.02 [3.39–4.66] | 2.09 [1.41–2.78] | 5.92 [4.85–6.99] | 5.20 [4.84–5.56] | 2.76 [2.36–3.15] | 7.65 [7.05–8.25] |
| 1997 | 5.04 [4.33–5.76] | 2.32 [1.59–3.05] | 7.67 [6.45–8.90] | 5.09 [4.73–5.44] | 3.19 [2.77–3.61] | 6.97 [6.40–7.55] |
| 1998 | 4.54 [3.86–5.22] | 1.64 [1.05–2.23] | 7.18 [5.99–8.36] | 5.41 [5.05–5.78] | 3.43 [3.00–3.87] | 7.43 [6.83–8.02] |
| 1999 | 5.72 [4.95–6.48] | 2.45 [1.68–3.21] | 8.97 [7.64–10.30] | 6.56 [6.16–6.97] | 4.09 [3.62–4.56] | 9.04 [8.39–9.70] |
| 2000 | 5.33 [4.60–6.05] | 2.38 [1.64–3.11] | 8.23 [6.97–9.48] | 7.34 [6.91–7.76] | 3.86 [3.40–4.31] | 10.78 [10.06–11.49] |
| 2001 | 6.47 [5.65–7.29] | 3.82 [2.88–4.75] | 8.99 [7.66–10.32] | 7.52 [7.09–7.95] | 4.15 [3.67–4.62] | 10.80 [10.09–11.52] |
| 2002 | 6.42 [5.61–7.23] | 2.90 [2.09–3.71] | 9.63 [8.26–10.99] | 8.91 [8.44–9.38] | 4.62 [4.11–5.14] | 13.20 [12.41–13.99] |
| 2003 | 6.20 [5.40–6.99] | 2.62 [1.84–3.39] | 9.59 [8.22–10.96] | 9.31 [8.83–9.79] | 4.75 [4.23–5.27] | 13.81 [13.00–14.62] |
| 2004 | 7.56 [6.68–8.43] | 3.04 [2.20–3.87] | 11.82 [10.31–13.34] | 10.24 [9.73–10.74] | 5.71 [5.14–6.28] | 14.72 [13.88–15.56] |
| 2005 | 8.67 [7.72–9.62] | 5.14 [4.03–6.25] | 12.18 [10.63–13.73] | 11.30 [10.77–11.83] | 6.26 [5.67–6.84] | 16.19 [15.31–17.07] |
| 2006 | 8.37 [7.44–9.29] | 3.88 [2.94–4.83] | 12.48 [10.92–14.03] | 12.14 [11.59–12.69] | 5.75 [5.19–6.32] | 18.28 [17.35–19.22] |
| 2007 | 9.31 [8.34–10.29] | 3.96 [3.01–4.91] | 14.25 [12.59–15.91] | 12.75 [12.19–13.32] | 6.81 [6.19–7.43] | 18.56 [17.62–19.51] |
| 2008 | 8.92 [7.96–9.87] | 4.04 [3.08–4.99] | 13.49 [11.87–15.11] | 13.76 [13.17–14.34] | 7.15 [6.52–7.78] | 20.19 [19.20–21.17] |
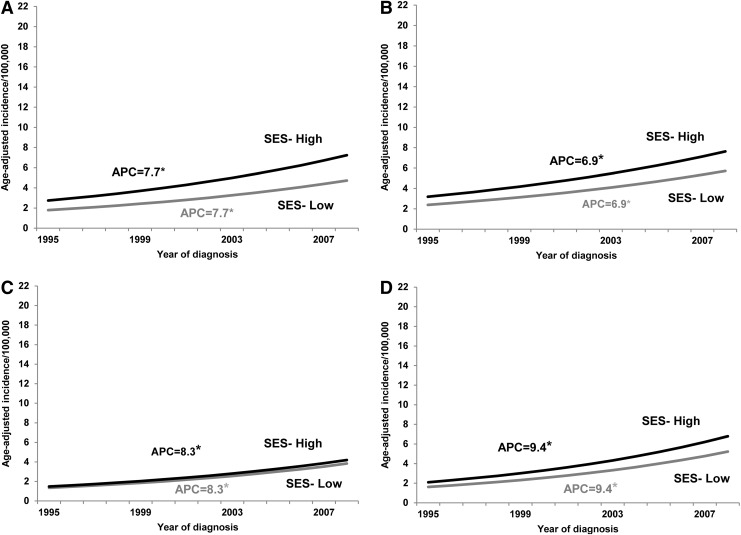
In sex-stratified, racial/ethnic subgroup analyses, no joinpoints emerged for males of any race/ethnicity in either the low or the high SES groups (white APC=6.9%, p<0.05; black APC=8.4%, p<0.05; Hispanic APC=9.4%, p<0.05), and the incidence trends between groups were considered parallel (p≥0.09). Likewise, there were no joinpoints among Hispanic females of either SES group (APC=9.8%, p<0.05; trends were parallel, p>0.05). Among non-Hispanic white females, however, there were no joinpoints among the low SES cases (APC=3.6%, p<0.05), but two joinpoints among the high SES cases (APC 1995–1998=−1.2%, p<0.05; APC 1998–2000=18.9%, p>0.05; APC 2000–2008=7.0%, p<0.05; trends not parallel, p<0.05). Whereas trends among non-Hispanic black females were devoid of joinpoints, those of higher SES had a much more pronounced increase in incidence over time relative to those of lower SES (high SES APC=13.0% vs. low SES APC=3.9%, p<0.05). A parallelism test indicated that the joinpoint projected incidence trends between low and high SES non-Hispanic black females were significantly different from one another (p<0.001). Sex-stratified trends over time in thyroid cancer incidence by SES for the whole study population and by race/ethnicity are displayed in Figures 2 and 3. Incidence rate ratios of thyroid cancer by SES, sex, and race/ethnicity are displayed in Table 4.
FIG. 2.
Results from sex-stratified joinpoint regression models on thyroid cancer incidence trends among females in Texas, 1995–2008, by SES (high [black], low [gray]) for (A) the whole study population, and within three major racial/ethnic groups: (B) non-Hispanic white, (C) non-Hispanic black, and (D) Hispanic. *Statistically significant APC at p<0.05 in the joinpoint regression analyses.
FIG. 3.
Results from sex-stratified joinpoint regression models on thyroid cancer incidence trends among males in Texas, 1995–2008, by SES (high [black], low [gray]) for (A) the whole study population, and within three major racial/ethnic groups: (B) non-Hispanic white, (C) non-Hispanic black, and (D) Hispanic. *Statistically significant APC at p<0.05 in the joinpoint regression analyses.
Table 4.
Incidence Rate Ratios of Thyroid Cancer by Socioeconomic Status, Sex, and Race/Ethnicity
| Year | Female/male IRR [CI] | Black/white IRR [CI] | Hispanic/white IRR [CI] | White female/male IRR [CI] | Black female/male IRR [CI] | Hispanic female/male IRR [CI] |
|---|---|---|---|---|---|---|
| SES-low | ||||||
| 1995 | 4.15 [2.83–6.09] | 0.58 [0.37–0.91] | 0.67 [0.48–0.93] | 3.68 [2.04–6.63] | 3.42 [1.29–9.06] | 5.27 [2.77–10.01] |
| 1996 | 2.83 [1.95–4.11] | 0.55 [0.33–0.90] | 0.63 [0.44–0.91] | 2.95 [1.62–5.39] | 2.67 [0.97–7.31] | 3.08 [1.67–5.66] |
| 1997 | 3.30 [2.32–4.70] | 0.64 [0.40–1.01] | 0.91 [0.66–1.26] | 3.30 [1.82–6.00] | 4.75 [1.64–13.76] | 2.93 [1.76–4.86] |
| 1998 | 4.39 [2.95–6.52] | 0.70 [0.43–1.15] | 1.03 [0.72–1.47] | 3.66 [1.80–7.44] | 4.14 [1.42–12.08] | 4.55 [2.60–7.96] |
| 1999 | 3.67 [2.59–5.19] | 0.70 [0.45–1.09] | 1.06 [0.77–1.46] | 3.81 [2.04–7.13] | 6.94 [2.09–23.03] | 2.86 [1.79–4.56] |
| 2000 | 3.46 [2.45–4.88] | 1.11 [0.74–1.67] | 1.03 [0.73–1.44] | 6.00 [2.79–12.87] | 2.92 [1.40–6.12] | 2.67 [1.66–4.29] |
| 2001 | 2.36 [1.77–3.13] | 0.67 [0.44–1.02] | 0.95 [0.70–1.27] | 2.57 [1.52–4.35] | 1.50 [0.72–3.11] | 2.69 [1.78–4.06] |
| 2002 | 3.32 [2.43–4.55] | 0.59 [0.38–0.92] | 1.17 [0.87–1.57] | 3.45 [1.92–6.19] | 3.18 [1.19–8.47] | 3.39 [2.21–5.20] |
| 2003 | 3.66 [2.64–5.10] | 0.77 [0.51–1.16] | 1.01 [0.74–1.36] | 3.64 [2.03–6.53] | 2.04 [0.97–4.28] | 4.55 [2.72–7.62] |
| 2004 | 3.89 [2.87–5.27] | 0.56 [0.38–0.83] | 0.86 [0.66–1.12] | 3.12 [1.94–5.02] | 5.47 [1.91–15.62] | 3.97 [2.52–6.24] |
| 2005 | 2.37 [1.84–3.05] | 0.77 [0.55–1.09] | 0.98 [0.76–1.27] | 1.77 [1.16–2.70] | 2.09 [1.10–3.96] | 2.95 [1.99–4.38] |
| 2006 | 3.21 [2.44–4.23] | 0.61 [0.41–0.90] | 1.24 [0.96–1.60] | 2.10 [1.33–3.30] | 3.12 [1.34–7.26] | 4.08 [2.70–6.15] |
| 2007 | 3.60 [2.75–4.70] | 0.63 [0.43–0.91] | 1.22 [0.96–1.57] | 2.24 [1.45–3.48] | 3.84 [1.69–8.72] | 5.49 [3.60–8.36] |
| 2008 | 3.34 [2.56–4.35] | 0.50 [0.33–0.74] | 1.16 [0.90–1.48] | 2.68 [1.70–4.24] | 4.58 [1.76–11.88] | 3.89 [2.69–5.62] |
| SES-high | ||||||
| 1995 | 2.76 [2.34–3.27] | 0.46 [0.31–0.67] | 0.84 [0.68–1.04] | 2.80 [2.32–3.38] | 2.70 [1.06–6.84] | 2.85 [1.70–4.78] |
| 1996 | 2.77 [2.35–3.27] | 0.37 [0.25–0.53] | 0.75 [0.61–0.93] | 2.66 [2.22–3.19] | 1.55 [0.73–3.30] | 5.67 [2.99–10.77] |
| 1997 | 2.19 [1.87–2.55] | 0.54 [0.38–0.76] | 0.74 [0.60–0.91] | 1.95 [1.64–2.31] | 3.42 [1.58–7.41] | 4.53 [2.75–7.48] |
| 1998 | 2.16 [1.86–2.51] | 0.55 [0.40–0.76] | 0.72 [0.58–0.88] | 2.10 [1.77–2.48] | 7.07 [2.65–18.90] | 2.38 [1.52–3.71] |
| 1999 | 2.21 [1.93–2.53] | 0.51 [0.37–0.69] | 0.94 [0.79–1.12] | 2.09 [1.79–2.44] | 2.37 [1.11–5.08] | 3.28 [2.26–4.77] |
| 2000 | 2.79 [2.44–3.20] | 0.45 [0.32–0.61] | 0.83 [0.71–0.99] | 2.74 [2.35–3.21] | 1.93 [0.90–4.12] | 3.30 [2.20–4.95] |
| 2001 | 2.60 [2.28–2.97] | 0.52 [0.39–0.71] | 0.85 [0.72–1.00] | 2.44 [2.09–2.83] | 4.29 [2.15–8.53] | 2.88 [1.96–4.23] |
| 2002 | 2.85 [2.52–3.24] | 0.64 [0.50–0.82] | 0.96 [0.82–1.12] | 2.83 [2.45–3.28] | 1.67 [0.97–2.86] | 3.09 [2.10–4.55] |
| 2003 | 2.91 [2.57–3.29] | 0.68 [0.54–0.86] | 0.98 [0.85–1.14] | 2.87 [2.48–3.31] | 3.57 [1.98–6.45] | 3.28 [2.33–4.61] |
| 2004 | 2.58 [2.30–2.89] | 0.65 [0.52–0.82] | 1.00 [0.87–1.15] | 2.32 [2.04–2.64] | 5.91 [3.08–11.32] | 3.41 [2.41–4.84] |
| 2005 | 2.59 [2.32–2.88] | 0.76 [0.62–0.93] | 0.95 [0.83–1.08] | 2.38 [2.11–2.70] | 4.29 [2.48–7.41] | 4.07 [2.92–5.68] |
| 2006 | 3.18 [2.84–3.55] | 0.77 [0.63–0.94] | 1.10 [0.96–1.25] | 2.98 [2.62–3.39] | 3.19 [1.98–5.15] | 3.62 [2.67–4.91] |
| 2007 | 2.73 [2.46–3.03] | 0.60 [0.48–0.74] | 1.06 [0.94–1.21] | 2.59 [2.30–2.92] | 3.31 [1.94–5.62] | 3.27 [2.46–4.35] |
| 2008 | 2.82 [2.55–3.12] | 0.79 [0.65–0.95] | 1.10 [0.97–1.23] | 2.84 [2.52–3.20] | 3.52 [2.27–5.46] | 2.75 [2.13–3.56] |
IRR, incidence rate ratio.
Finally, histology-stratified analyses (papillary) were also conducted. As with the whole population, no joinpoints emerged among the low SES cases (APC=7.4%, p<0.05). Also similarly, two joinpoints emerged among the high SES cases between 1995 and 1999. Specifically, the APC between 1995 and 1998 was 1.5% (p>0.05), which increased markedly to 29.3% (p>0.05) from 1998 to 1999, and returned to a less pronounced but statistically significant steady 9.5% (p<0.05) increase in incidence from 1999 to 2008. A parallelism test indicated that the joinpoint projected incidence trends between low and high SES groups were significantly different from one another (p<0.006).
Racial/ethnic-specific SES subgroup analyses were also conducted for papillary thyroid cancer. No joinpoints emerged among the non-Hispanic black or Hispanic cases in either the low or the high SES groups. Specifically, those of higher SES had a more pronounced increase in papillary thyroid cancer incidence over time relative to those of lower SES in both racial/ethnic minority subgroups (black low SES APC=6.4% vs. high SES APC=14.5%; Hispanic low SES APC=8.5% vs. high SES APC=12.0%; p<0.05). This pattern of results was similar to those indicated earlier for analyses including all histology types. Among the non-Hispanic white papillary cases, however, two joinpoints emerged among both the low and high SES groups. For the low SES papillary cases, the APC between 1995 and 1999 was −3.5% (p>0.05), which increased markedly to 10.2% (p<0.05) from 1999 to 2005, which decreased to −3.0% (p>0.05) from 2005 to 2008. For the high SES papillary cases, the APC between 1995 and 1998 was 0.9% (p>0.05), which increased markedly to 26.3% (p>0.05) from 1998 to 1999, and returned to a less pronounced but statistically significant steady 8.5% (p<0.05) increase in incidence from 1999 to 2008. Parallelism tests indicated that the joinpoint projected incidence trends between low and high SES groups were significantly different from one another in the non-Hispanic black (p<0.003), Hispanic (p=0.031), and non-Hispanic white subgroups (p=0.024).
Discussion
The current study is the first to examine SES-based trends in thyroid cancer incidence over time in Texas using economic information generated at the census tract level. The goal of the study was to examine the impact of SES on thyroid cancer detection from 1995 to 2008 in this state in order to understand the basis of rising incidence rates better. Moreover, we also investigated the nature of these relations within three major racial/ethnic groups (non-Hispanic white, non-Hispanic black, and Hispanic) to characterize further the nature of SES-based trends in rising thyroid cancer incidence.
With regard to SES-based incidence trends within the whole study population, we found steady annual incidence increases of 6.7% from 1995 to 2008 among the low SES, whereas trends among higher SES cases were more variable (from 1995 to 1998) but ultimately steeper with annual incidence increases of 8.6% from 1999 to 2008. The relatively steeper increase among the high SES group relative to the low SES group suggests that higher SES Texans may experience a greater likelihood of thyroid cancer diagnosis than lower SES Texans. The development and use of more sophisticated diagnostic technologies for thyroid cancer began in the late 1990s through early 2000s. This shift to the use of ultrasound with fine needle aspiration biopsies enabled the detection of smaller, nonsymptomatic thyroid carcinomas than was previously possible via palpitation alone, and several studies have indicated the increased incidence of smaller tumors in recent years (1,2,12,16). Because the gaps between incidence in the high SES and low SES groups seem to widen with time, particularly among non-Hispanic white and non-Hispanic black women, these results suggest the potential of a detection bias whereby higher SES Texans have better access to enhanced diagnostic technologies than their lower SES counterparts. As many smaller tumors may be initially detected incidentally as part of other medical procedures (34), part of that gap may reflect that higher SES patients may be more likely to seek (or have access to) healthcare services in general as compared to those of lower SES. Unlike many cancers, however, early detection is controversial, and there is currently debate in the field about the necessity of intervention with the small, asymptomatic tumors that may be significant contributors to rising thyroid cancer rates (35). Because thyroid cancer treatment carries health risks and may ultimately impact patients' quality of life, it is important that clinicians follow treatment guidelines promulgated by the American Thyroid Association and National Comprehensive Cancer Network. More research is necessary to inform these guidelines further to prevent potentially unnecessary treatment of tumors that are small, asymptomatic, and detected serendipidously or secondarily to other presenting problems.
Although results indicate that higher SES Texans are diagnosed with thyroid cancer at higher rates than lower SES Texans, there was a steadily higher incidence of thyroid cancer seen within both SES groups over time. Similar patterns between low and high SES groups (defined by county-level SES) have been found using SEER registry data focused on other parts of the country (12). The current study adds to this literature using a potentially more representative measure of SES (tract-level SES), and extends findings to Texas, which is not represented in SEER registry data. These converging data appear to support the ubiquity of rising incidence rates. Several prior studies have reviewed potential reasons for these increases, including increased environmental or hormonal exposures (8,12–16). However, identifiable contributors to true, significant increases in thyroid cancer incidence remain elusive. Given the lack of concomitant increases in thyroid cancer mortality in recent years (1,17,21), and the relatively high level of subclinical occult papillary thyroid cancers identified in previous autopsy research (18,36), the most parsimoneous explanation for rising incidence rates appears to be enhanced detection rather than true increases in disease rates (19). Nevertheless, additional research focused on better understanding the basis for these steady and significant increases in incidence among all segments of the population is needed, as thyroid cancer is little understood and fairly unique among carcinomas in this regard.
The current study also allowed examination of the potential for racial/ethnic differences in the rates of increasing incidence trends between low and high SES groups over time. The results indicate that whereas non-Hispanic whites experienced a steady 7.6% annual increase in incidence over time among both high and low SES groups, Hispanics of lower SES had a less pronounced increase in incidence over time relative to Hispanics of higher SES (8.3% vs. 11.2%). These differences were even more marked among the non-Hispanic blacks, with a 4.1% APC among the lower SES group versus a 12.8% APC among the higher SES group. These results suggest that enhanced detection among non-Hispanic blacks and Hispanics of higher SES are substantial contributors to the higher incidence rates seen among higher SES Texans among the population as a whole. Previous research suggested a lack of disparities in clinical presentation of thyroid cancer (e.g., tumor size) between whites and blacks when both groups had equal access to healthcare (24). However, the high rate of uninsurance has been cited as one relevant factor that limits the ability for low SES minorities to access healthcare (30), and the particularly low incidence of thyroid cancers among low SES non-Hispanic blacks and Hispanics may reflect this issue. A previous study, for example, attributed approximately 50% of the thyroid cancer incidence gap between blacks and whites to racial disparities in health insurance coverage (23). However, other factors may also be relevant. For example, research in other areas of the country has linked residential adjacency to metropolitan areas, where access to screening and medical care may be better, with steeper increases in thyroid cancer incidence trends relative to nonadjacency (12). Therefore, it may also be that patterns of residential racial/ethnic segregation may impact access to healthcare or health-related resources among low SES minority groups due to a greater likelihood of nonadjacency to those resources, resulting in lower thyroid incidence among lower SES minority groups. Although thyroid cancer mortality rates are generally low (especially for papillary, follicular, and medullary thyroid cancers) (21) and some studies have failed to support racial differences in mortality (23), the literature is mixed on this point. For example, a recent study cited a recent 51% increase in mortality among black patients versus white patients with papillary and follicular thyroid cancers (31). Therefore, although the incidence of thyroid cancers is relatively low among racial/ethnic minority populations (particularly non-Hispanic blacks) compared to non-Hispanic white populations, it seems there is a delicate balance to be struck between avoiding overdiagnosis among high SES racial/ethnic minority patients and failing to diagnose and/or treat potentially aggressive tumors among lower SES racial/ethnic minority patients due to healthcare access issues (cf. 19).
Analyses of SES-based incidence trends in thyroid cancer incidence by tumor size could help to shed additional light on the potential contribution of enhanced detection to rising thyroid cancer incidence. If enhanced detection was influencing increases, increased incidence of smaller tumor sizes (e.g., ≤2 cm or ≤1 cm) would be expected, especially beginning in the late 1990s with the emergence of new detection technology. On the other hand, the incidence of large tumors (>4 cm) might decrease over time, assuming that diagnostic improvements would have led to the earlier detection of tumors in recent years (i.e., their detection at smaller sizes). Unfortunately, one limitation of our registry data is that half of the cases were missing tumor size data. Using the data available to us, however, we conducted a post hoc investigation of SES-based trends in the proportion of incidence cases by tumor size (see Table 5). In general, the results suggest that enhanced detection (as evidenced by increasing proportion of smaller tumors and decreasing proportion of larger tumors) is contributing to observed patterns in this disease among the population as a whole. However, an increase in the proportional detection of tumors ≤2 cm among high and low SES non-Hispanic blacks over time was not significant, nor was the increase in the proportional detection of tumors ≤1 cm among low SES non-Hispanic blacks. In addition, decreases in the proportional detection of large tumors among Hispanics over time were not significant. These findings may suggest that racial/ethnic minority groups are not experiencing the effects of enhanced diagnostic technologies for thyroid cancer in the same way that non-Hispanic whites are. However, because missing tumor data might not be missing at random, and because of a small number of low SES cases for some racial/ethnic subgroups, these results should be interpreted with caution. The increased incidence of smaller tumors has been supported in previous research (1,2,12,16). Contrary to our findings, however, some studies indicate increases in the incidence of large tumors too (21), though results tend to vary between studies regarding the extent to which this growth is contributing to overall incidence trends (1,16). More research, particularly on racial/ethnic SES-based differences in thyroid cancer incidence by tumor size, is needed to inform the literature.
Table 5.
Trends in the Proportion of Incidence Cases by Tumor Size, 1997–2008
| Annual percentage change | |||
|---|---|---|---|
| Tumor size ≤1 cm | Tumor size ≤2 cm | Tumor size >4 cm | |
| Population | |||
| SES-low | 2.0* | 2.0* | −3.1* |
| SES-high | 2.0* | 2.0* | −3.1* |
| Non-Hispanic white | |||
| SES-low | 4.5* | 2.6* | −3.9* |
| SES-high | 4.5* | 2.6* | −3.9* |
| Non-Hispanic black | |||
| SES-low | 4.0a | 2.7 | −3.7* |
| SES-high | 6.2a* | 2.7 | −3.7* |
| Hispanic | |||
| SES-low | 4.7* | 1.5* | −0.2 |
| SES-high | 4.7* | 1.5* | −0.2 |
Table reflects results from joinpoint analyses examining trends in the proportion of incidence by tumor size, based on available data. For example, the first column of data refers to changes in the proportion of tumors size ≤1 cm relative to all other tumor sizes diagnosed from 1997 to 2008. In all population and racial/ethnic subgroup comparisons, the annual percentage change (APC) was the same in the low and high SES groups (i.e., joinpoint regression lines were parallel). The years 1995 and 1996 had to be excluded from these analyses due to too few cases.
The year 1997 had to be additionally excluded from these analyses due to too few cases.
Statistically significant APC at p<0.05 in the joinpoint regression analyses.
Finally, exploratory analyses included an examination of SES and racial/ethnic trends by sex and histology. Not surprisingly, given that the sample comprised 76% females and 86% papillary cancers, results from the main analyses were largely driven by the females in the population and by the papillary cancers. However, it is important to note that incidence rates were steadily increasing over time among males as well as females in the study population, and that incidence rates among high SES non-Hispanic black females demonstrated the most marked increases over time (13%), whereas those among low SES non-Hispanic black females showed the smallest rate of increase of any group (3.9%). These results suggest that high SES non-Hispanic black females may be at particular risk for overdiagnosis.
Limitations of the current study include the lack of information on several individual-level variables including socioeconomic and health-related data (e.g., insurance coverage status), which are common to registry-based studies. Previous studies have used county-level estimates of insurance coverage in analyses. However, we were unaware of any available insurance coverage data on the scale used in the present study (i.e., tract level). Also, the use of SES and population data from a single point in time (2000 U.S. census) is a limitation, given that incidence spanned over a decade. As a result, incidence may be over- or underestimated in some years due to population changes. The inability to examine SES-based trends in cancer incidence among Asian and other races is also a limitation, especially given the relatively high incidence found among Asians (particularly Filipinos) in previous studies (21). Finally, lower incidence of follicular, medullary, and anaplastic thyroid cancers prohibited SES and race/ethnicity-based analyses by these histologic categories. Future studies with greater sample sizes (e.g., using national data) may be used to understand better how associations reported here might vary by histology.
Similar to several recent studies in the field, our results also support the rising incidence of thyroid cancer over recent years (1–6,12). Comparing our results to those of previous studies (10,12,21), however, it is of interest that thyroid cancer incidence in Texas appears to be rising more steeply than in other areas of the country. For example, a previous study using similar methodology but at the county-level in SEER 9 registries found 4.0% annual incidence increases from 1980 to 2008 among low SES groups (vs. 6.7% in the current study) and 6.6% annual incidence increases from 1997 until 2008 among high SES groups (vs. 8.6% in the current study) (12). Likewise, racial/ethnic-specific trends in incidence were also higher in the current sample than in previous studies (20). The reasons behind these potential incidence trend differences are not clear and beyond the scope of the current study. However, geographic variations in thyroid cancer incidence, and factors that might underlie these variations, might be worthy of future investigation.
In summary, the current study is the first to examine census-tract-level socioeconomic trends in thyroid cancer incidence in Texas to understand better the causes of rising incidence from 1995 to 2008. Results of the current study suggest SES-based detection biases, whereby higher SES and particularly higher SES non-Hispanic blacks and Hispanics in Texas, may have increased access to new diagnostic technologies relative to their lower SES counterparts. The current study used a potentially more representative measure of SES to add to a growing literature elucidating the connection between SES and thyroid cancer incidence trends, and further delineated the nature of those relations in racial/ethnic specific analyses. Although true underlying causal processes cannot be depicted via the analysis of SES-based trends in incidence, the relatively more indirect methodology in this study is commonly used in the field to explore potential contributors to incidence trends and for the purposes of hypothesis-generation (12,23). Additional research is needed to confirm whether the findings reported in this study can be replicated in other areas of the nation. However, results suggest that a dual focus on delineating and preventing known disease-related causal factors (e.g., radiation exposure) and avoiding overdiagnosis among certain populations (e.g., high SES) may be advisable approaches to address thyroid cancer in Texas. Clinicians are encouraged to adhere to American Thyroid Association and National Comprehensive Cancer Network guidelines when choosing patients for thyroid ultrasound, selecting which nodules to examine, and deciding which patients should proceed to biopsy.
Acknowledgments
Cancer incidence data have been provided by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, TX. We would like to express our gratitude to the Texas Cancer Registry for providing these data and enabling their analysis. The content of this study, however, is solely the responsibility of the authors and does not necessarily represent the official views of the Texas Cancer Registry or the Texas Department of State Health Services.
This manuscript was supported by the National Institutes of Health through The University of Texas MD Anderson's Cancer Center Support Grant (CA016672), the National Institute of Dental and Craniofacial Research (U01 DE019765-01 to A.K. El-Naggar, E.M.S.—Project 2 Leader), and The University of Texas MD Anderson Cancer Center start-up funds (to L.R.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or The University of Texas MD Anderson Cancer Center.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Davies L, Welch HG.2006Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM.2009Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 3.Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, Evers BM.2007Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg 204:764–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell I, Livingston EH, Chang AY, Holt S, Snyder WH, 3rd, Lingvay I, Nwariaku FE.2007Trends in thyroid cancer demographics and surgical therapy in the United States. Surgery 142:823–828 [DOI] [PubMed] [Google Scholar]
- 5.Zhu C, Zheng T, Kilfoy BA, Han X, Ma S, Ba Y, Bai Y, Wang R, Zhang Y.2009A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid 19:1061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albores-Saavedra J, Henson DE, Glazer E, Schwartz AM.2007Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol 18:1–7 [DOI] [PubMed] [Google Scholar]
- 7.Baker SR, Bhatti WA.2006The thyroid cancer epidemic: is it the dark side of the CT revolution? Eur J Radiol 60:67–69 [DOI] [PubMed] [Google Scholar]
- 8.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y.2009International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 20:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Semenciw R, Ugnat AM, Mao Y.2001Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer 85:1335–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sprague BL, Warren Andersen S, Trentham-Dietz A.2008Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control 19:585–593 [DOI] [PubMed] [Google Scholar]
- 11.Kent WD, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA.2007Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ 177:1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM.2013Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980–2008. Thyroid 23:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulla ZD, Margo CE.2000Primary malignancies of the thyroid: epidemiologic analysis of the Florida Cancer Data System registry. Ann Epidemiol 10:24–30 [DOI] [PubMed] [Google Scholar]
- 14.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS.2009Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leux C, Guenel P.2010Risk factors of thyroid tumors: role of environmental and occupational exposures to chemical pollutants. Rev Epidemiol Sante Publique 58:359–367 [DOI] [PubMed] [Google Scholar]
- 16.Morris LG, Myssiorek D.2010Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 200:454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surveillance, Epidemiology and End Results Program 2011SEER*Stat Database: SEER 9 Registry Research Data (1973–2008) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch. Available at: www.seer.cancer.gov (accessed January3, 2013)
- 18.Arem R, Padayatty SJ, Saliby AH, Sherman SI.1999Thyroid microcarconoma: prevalence, prognosis, and management. Endocr Pract 5:148–156 [DOI] [PubMed] [Google Scholar]
- 19.Welch HG, Black WC.2010Overdiagnosis in cancer. J Natl Cancer Inst 102:605–613 [DOI] [PubMed] [Google Scholar]
- 20.Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS.2011Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 21:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu GP, Li JC, Branovan D, McCormick S, Schantz SP.2010Thyroid cancer incidence and survival in the national cancer institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid 20:465–473 [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute (NCI) 2011 Age-adjusted SEER incidence rates by race/ethnicity thyroid, all ages, both sexes, 1975–2009 (SEER 9). Available at: http://seer.cancer.gov/faststats/selections.php?run=runit&output=1&data=1&statistic=1&cancer=80&year=201201&sex=1&age=1&series=race&race=2;3 (accessed January3, 2013)
- 23.Morris LG, Sikora AG, Myssiorek D, DeLacure MD.2008The basis of racial differences in the incidence of thyroid cancer. Ann Surg Oncol 15:1169–1176 [DOI] [PubMed] [Google Scholar]
- 24.Brown SR, Lee S, Brown TA, Waddell BE.2010Effect of race on thyroid cancer care in an equal access healthcare system. Am J Surg 199:685–689 [DOI] [PubMed] [Google Scholar]
- 25.U.S. Census Bureau 2010Geographic areas reference manual. Available at: www.census.gov/prod/2010pubs/p60-238.pdf (accessed January3, 2013)
- 26.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R.2003Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: the Public Health Disparities Geocoding Project (US). J Epidemiol Community Health 57:186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV.2003Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the Public Health Disparities Geocoding Project. Am J Public Health 93:1655–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarts MJ, van der Aa MA, Coebergh JW, Louwman WJ.2010Reduction of socioeconomic inequality in cancer incidence in the south of the Netherlands during 1996–2008. Eur J Cancer 46:2633–2646 [DOI] [PubMed] [Google Scholar]
- 29.The Henry J Kaiser Family Foundation 2011Health insurance coverage of adults 19–64, states (2010–2011). Available at: www.statehealthfacts.org/comparetable.jsp?ind=130&cat=3 (accessed January3, 2013)
- 30.Texas Medical Association 2011The uninsured in Texas. Available at: www.texmed.org/template.aspx?id=5517 (accessed January3, 2013)
- 31.Johnston LE, Tran Cao HS, Chang DC, Bouvet M.2012Sociodemographic predictors of survival in differentiated thyroid cancer: results from the SEER database. ISRN Endocrinol 2012:384707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle P, Parkin D.1991Statistical methods for registries. In: Cancer Registration: Principles and Methods. IARC Scientific Publications No. 95, International Agency for Research on Cancer, Lyon, pp. 126–158 [PubMed] [Google Scholar]
- 33.Joinpoint Regression Program 2011Statistical methodology and applications branch and data modeling branch, Surveillence Research Program, National Cancer Institute. Available at: http://surveillance.cancer.gov/joinpoint (accessed November1, 2011)
- 34.Kahn C, Simonella L, Sywak M, Boyages S, Ung O, O'Connell D.2012Pathways to the diagnosis of thyroid cancer in New South Wales: a population-based cross-sectional study. Cancer Causes Control 23:35–44 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhu Y, Risch HA.2006Changing incidence of thyroid cancer. JAMA 296:1350. [DOI] [PubMed] [Google Scholar]
- 36.Harach HR, Franssila KO, Wasenius V.1985Occult papillary carcinoma of the thyroid: a “normal” finding in Finland. A systematic autopsy study. Cancer 56:531–538 [DOI] [PubMed] [Google Scholar]