Abstract
AIM
To investigate the effects of intravitreal injection of bevacizumab-chitosan nanoparticles on pathological morphology of retina and the expression of vascular endothelial growth factor (VEGF) protein and VEGF mRNA in the retina of diabetic rats.
METHODS
Seventy-two 3-month aged diabetic rats were randomly divided into 3 groups, each containing 24 animals and 48 eyes. Both eyes of the rats in group A were injected into the vitreous at the pars plana with 3µL of physiological saline, while in groups B and C were injected with 3µL (75µg) of bevacizumab and 3µL of bevacizumab-chitosan nanoparticles (containing 75µg of bevacizumab), respectively. Immunohistochemistry was used to assess retinal angiogenesis, real-time PCR assay was used to analyse the expression of VEGF mRNA, and light microscopy was used to evaluate the morphology of retinal capillaries.
RESULTS
Real-time PCR assay revealed that the VEGF mRNA expression in the retina before injection was similar to 1 week after injection in group A (P>0.05), while the VEGF mRNA expression before injection significantly differed from those 4 and 8 weeks after injection (P<0.05). Retinal expression of VEGF protein and VEGF mRNA was inhibited 1 week and 4 weeks after injection (P<0.05) in group B, and the expression of VEGF protein and VEGF mRNA was obviously inhibited until 8 weeks after injection (P<0.05) in group C. Using multiple comparisons among group A, group B, and group C, the VEGF expression before injection was higher than at 1, 4 and 8 weeks after injection (P<0.05). The amount of VEGF expression was higher 8 weeks after injection than 1 week or 4 weeks after injection, and also higher 1 week after injection compared with 4 weeks after injection (P<0.05). No toxic effect on SD rats was observed with bevacizumab-chitosan nanoparticles injection alone.
CONCLUSION
The results offer a new approach for inhibiting angiogenesis of diabetic retinopathy and indicate that the intravitreal injection of bevacizumab inhibits VEGF expression in retina, and bevacizumab-chitosan nanoparticles have a longer duration of action.
Keywords: bevacizumab, nanoparticles, diabetic retinopathy, intravitreal injection
INTRODUCTION
Diabetic retinopathy is one of the most common and serious complications of diabetes, and retinal neovascularization is the most characteristic and dangerous key pathological change of diabetic retinopathy. The vascular endothelial growth factor (VEGF) level is closely associated with the pathological process of the occurrence and development of retinal and choroidal neovascularization. A monoclonal antibody targeting VEGF (rhMAb VEGF, bevacizumab) or its fragment (ranibizumab) is shown to inhibit angiogenesis and be efficacious for treatment of age-related macular degeneration, which provides a novel idea and evidence for treatment of diabetic retinopathy. The effectiveness of bevacizumab or ranibizumab for treatment of diabetic retinopathy has been widely recognized, revealed by either basic experimental studies or clinical studies[1]-[3]. However, repeated multiple intravitreal injection may cause severe complications during the whole treatment process[4]-[7]. The present study was therefore, designed to solve the problem of exerting the function of bevacizumab and avoiding repeated intravitreal injection.
It is proved that use of nanoparticles as the carriers for peptide and protein drugs could effectively protect the drugs from inactivation, achieve sustained-release, controlled-release and targeted drug delivery, significantly enhance the bioavailability and reduce the side effects. Such a drug delivery system enables highly effective and predictable release of drugs and reduces drug delivery time, thereby increasing the safety, effectiveness and reliability of drug therapy. It is reported that chitosan and chitosan derivatives exhibit many biological activities beneficial for the human body, such as anti-tumor effect, immune adjuvant effect, promotion of tissue repair and hemostatic effect, which enables chitosan and chitosan derivatives to be an ideal sustained-release material for drugs, and the controlled-release drug delivery system based on chitosan nanoparticles has become a widely used sustained-release form currently[8]. The drug molecule is dissolved and wrapped into the particle, or adherent to the particle surface through absorption, which regulates drug diffusion through controlling the polymer properties and degradation time. Chitosan nanoparticle is mainly characterized by ultra-micro size, direction function on cells, increases in the stability of protein and peptide drugs, reduction of side effects, prolongation of drug efficacy and enhancement of bioavailability. Intravitreal injection of chitosan nanoparticles decreases injection times, which reduces the complications of invasive drug delivery. The present study prepared bevacizumab-chitosan nanoparticles using chitosan as a carrier, and assessed the effects of the nanoparticles on enhancement of bioavailability of bevacizumab and reduction in number of intravitreal injection time, in a animal model, through inhibiting the expression of VEGF protein and VEGF mRNA in the retina of diabetic rats, so as to provide experimental evidence for effective treatment of diabetic retinopathy.
MATERIALS AND METHODS
Materials
Seventy-two 3-month aged diabetic rats of the Sprague-Dawley (SD) strain, each weighing 190±26g, were purchased from the Laboratory Animal Center of Second Military Medical University (Shanghai, China). This study was approved by the Ethics Review Committee of Second Military Medical University and was in adherence to the tenets of the Declaration of Helsinki.
Methods
Seventy-two 3-month aged diabetic rats of the SD rats were randomly assigned into 3 groups (A, B and C), of 24 animals and 48 eyes each group. Intravitreal injections were performed just posterior to the pars plana with a 5µL syringe (Hamilton, Reno, NV, USA) and a 33-gauge needle. Both eyes of the rats were injected into the vitreous at the pars plana with 3µL of physiological saline, 3µL (75µg) of bevacizumab and 3µL of bevacizumab-chitosan nanoparticles (containing 75µg of bevacizumab) in group A, group B and group C, respectively. Rats with any kind of postoperative complication (e.g. cataract) were excluded from analysis. Six animals were sacrificed at four time points (before injection, 1 week after injection, 4 weeks after injection and 8 weeks after injection). Typically, six animals were operated on in each session and 12 eyeballs were collected. The eyeballs were then fixed, embedded, cut into sections and stained with hematoxylin and eosin (HE), and the pathological morphology of retina was observed. The expression of VEGF protein and VEGF mRNA was determined using immunohistochemistry and real-time PCR technique.
Bevacizumab-chitosan nanoparticles preparation
Bevacizumab-chitosan nanoparticles (NPs) were prepared with a emulsification evaporation method. Anti-VEGF chitosan NPs were washed three times to removed unbound antibody. The average numbers of chitosan groups per NP were 9 500. The mean diameters bevacizumab-chitosan NPs (n=3/group) as determined with photon correlation spectroscopy was 88.9±106.7nm. The larger diameters of bevacizumab-chitosan NPs indicate that the antibody is successfully linked to the NPs. The zeta potential values for chitosan NPs, bevacizumab-chitosan NPs were 23.03±4.6mv, 21.63±2.4mv, respectively. The similar zeta potential values of the two NPs suggest that the antibody linkage has little effect on their surface charge. No aggregation of these NPs was observed with transmission electronic microscopy in blood plasma after up to 4 h of incubation.
HE staining of paraffin-embedded sections and immunohistochemical determination of VEGF protein
The diabetic rats in each group underwent general anesthesia through peritoneal injection of 100g/L of chloral hydrate at four time points (before injection, 1 week after injection, 4 weeks after injection and 8 weeks after injection), and six rats were randomly sampled from each group. The samples of 12 eyeballs were collected from the rats in each group, and an incision was punctured on the central cornea. The eyeball samples were then fixed in 10% neutral formalin for 48h, dehydrated in series of ethanol, embedded in paraffin and cut into sections of 5-µm thickness, which were used for immunohistochemical determination of VEGF in retina. The paraffin-embedded sections were stained with HE.
Vascular endothelial growth factor immunohistochemistry
Immunostaining was performed using frozen sections. Dissected posterior segments of eyes were embedded in optimal cutting temperature media, and 8-mm serial retinal sections were processed for immunolabelling for VEGF. In brief, sections were blocked in phosphate-buffered saline (PBS) containing 1% horse serum, 1% bovine serum albumin (BSA) and 0.05% Triton X-100 for 40min. Sections were subsequently treated with an anti-VEGF primary antibody, which was diluted in PBS containing 1% horse serum, 1% BSA and 0.3% Triton X-100, and incubated at room temperature for 1h. For VEGF, a monoclonal mouse anti-VEGF (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) primary antibody was used. Sections were washed with PBS and incubated with the secondary antibody for all primary antibodies, a biotinylated goat IgG anti-mouse IgG (supplied ready to use by Zymed, Histostain-Plus Bulk Kits; Zymed, South San Francisco, CA, USA), prepared in PBS containing 1% horse serum, 1% BSA and 0.3% Triton X-100, for 1h at ambient room temperature. Slides were washed with PBS, coverslipped with mounting media containing DAPI and evaluated using a light microscope (Olympus BX51, Tokyo, Japan). Some sections were incubated in blocking solution without the primary antibody and were used as negative controls.
Real-time PCR assay determines VEGF mRNA expression in retina
The other 6 eyeballs were collected at the given time points, and the retinal tissues were rapidly bluntly separated on ice under an animal surgical microscope. The tissues were then stored in liquid nitrogen for the subsequent real-time PCR amplification. The specific primers were designed according the known sequences in NCBI database using the software Primer Premier version 6.0. The forward primer for VEGF gene (NM_031836) with amplification length of 177bp was 5′-AGAAAGCCCATGAAGTGGTG-3′, and the reverse primer was 5′-ACTCCAGGGCTTCATCATTG-3′. The forward primer of the internal control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was 5′-AACGACCCCTTCATTGAC-3′, and the reverse primer was 5′-TCCACGACATACTCAGCAC-3′. The primers designed were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Total RNA was extracted from the tissues using the TransZOL Up reagents according to the manufacturer's instructions (TransGen Biotech, Inc.; Beijing, China). The purity of RNA was determined using electrophoresis on 10g/L agarose gels with the horizontal electrophoresis system (Bio-Rad, Hercules, CA, USA), and the RNA concentration was determined using the Backman 721 ultraviolet-visible spectrophotometer (Beckman Coulter, Inc.; Schaumburg, IL, USA). The cDNA was synthesized from VEGF mRNA using the reverse transcription reagents (TransGen Biotech, Inc.; Beijing, China) in 20µL of reaction system containing 8µL of total RNA, 1µL of primers, 1µL of reverse transcriptase and 10µL of reaction mixture under a reaction condition of at 42°C for 30min, at 85°C for 5min and at 4°C for 5min. The cDNA obtained was stored at -20°C for the subsequent experiment. PCR amplification of the target DNA fragment was performed in a reaction system with total volume of 20µL containing 10µL of reaction mixture, 0.5µL of forward and reverse primers, 1µL of template cDNA and 8µL of double-distilled water. Three replicates were done for each sample. PCR amplification was performed using the MyiQ fluorescence quantitative real-time detection system (Bio-Rad; Hercules, CA, USA) under 40 cycles of at 95°C for 30s, at 95°C for 5s and at 60°C for 30s. After amplification, melting curve analysis was performed since 60°C to validate the specificity of the amplification product. After the completion of reaction, the baseline value and threshold value were set, and the threshold cycle (Ct) value was obtained. The △Ct and △△Ct values were calculated using the following formula, △Ct=Ct value of the target gene-Ct value of the internal reference gene, △△Ct=△Ct of the experimental group-△Ct of the control group. The 2−△△Ct value was calculated, which indicated the times of the target gene expression in the experimental group corresponding to that in the control group.
Statistical Analysis
All results were expressed as mean±standard deviation (SD), and all statistical analyses were performed using the statistical software SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was employed to test the difference among groups. P<0.05 was considered significant.
RESULTS
HE Staining of Rat Retina
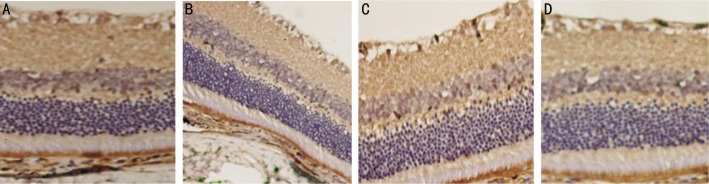
HE staining revealed that the morphology of retina before injection was not significantly different from those 1 week and 4 weeks after injection in group A, while the pathological changes, such as edema and vacuole in the diabetic retina were observed. At 8 weeks after injection, the tissues at different retinal layers became thin, the structure was loose and the cells appeared vacuole-like (arrow) changes (Figure 1). In group B, HE staining showed that the morphological changes were observed in the retinal tissues at the time of before injection, 1 week and 4 weeks after injection. Tissue edema, loose structure and vacuole-like changes in cells were observed in the retina of rats before injection, while the structure of the retinal tissues tended to be intact after injection. However, Tissue edema and loose structure were found in the retina 8 weeks after injection (Figure 2). In group C, the morphology of the retinal tissues before injection was significantly different from those of 1, 4 and 8 weeks after injection. All samples displayed a tissue edema, loose structure and vacuole-like changes in cells before injection, while the structure of the diabetic retinal tissues tended to be intact, the retinal layers were clear, the cells in the ganglion cell layer were arranged regularly without loss, and the cells in the inner and outer nuclear layers, as well as the cone and rod cells were arranged regularly (Figure 3) at the time of 1 week, 4 and 8 weeks after injection.
Figure 1. HE staining of retina from rats in group A.
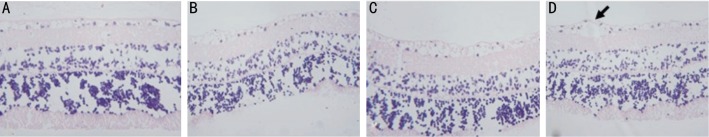
A: Before injection; B: 1 week after injection; C: 4 weeks after injection; D: 8 weeks after injection. (HE×100). The cells appeared vacuole-like (arrow) changes.
Figure 2. HE staining of retina from rats in group B.
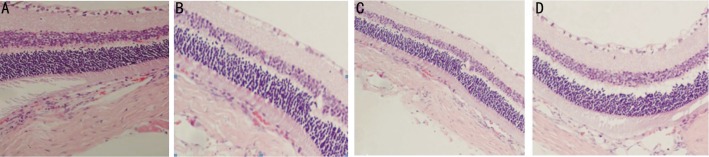
A: Before injection; B: 1 week after injection; C: 4 weeks after injection; D: 8 weeks after injection. (HE×100)
Figure 3. HE staining of retina from rats in group C.
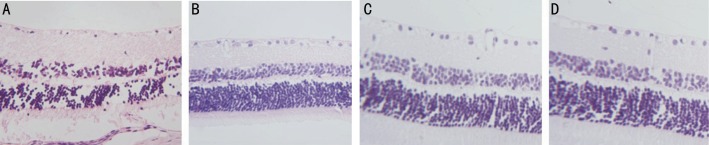
A: Before injection; B: 1 week after injection; C: 4 weeks after injection; D: 8 weeks after injection. (HE×100)
VEGF protein expression in retina determined by immunohistochemistry
The expression of VEGF was indicated in the tissue by brown staining. Immunohistochemistry showed that VEGF protein was mainly located in the retinal pigment epithelium layer (black row), ganglion cell layer(red arrow) and inner nuclear layer, with a scattered granular distribution in the diabetic rats from group A (Figure 4), and the VEGF-positive cell counts increased in the retinal tissues with the prolongation of time.
Figure 4. Immunohistochemistry of VEGF in retina of rats from group A.
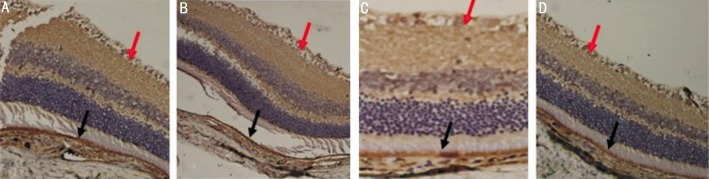
A: Before injection; B: 1 week after injection; C: 4 weeks after injection; D: 8 weeks after injection. VEGF protein was mainly located in the retinal pigment epithelium layer (black row), ganglion cell layer (red arrow).
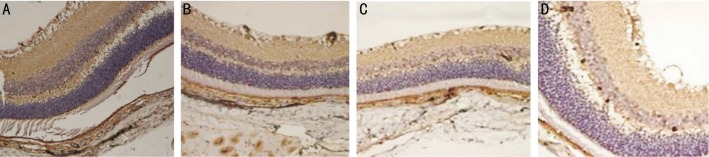
A large number of VEGF-positive cells were found in the retinal tissues of diabetic rats from group B, and the positive reaction at weeks 1 and 4 post-intravitreal bevacizumab injection was weakened as compared to that before injection, while the positive reaction significantly increased at week 8, which was similar to that before injection (Figure 5).
Figure 5. Immunohistochemistry of VEGF in retina of rats from group B.
A large number of VEGF-positive cells were found in the retinal tissues of group B. A: Before injection; B: 1 week after injection; C: 4 weeks after injection; D: 8 weeks after injection. At weeks 1 and 4 the positive reaction was weakened as compared to that before injection, at week 8 positive reaction significantly increased, which was similar to that before injection.
In group C, VEGF expression decreased at any time of post-intravitreal bevacizumab injection, the positive reaction at weeks 1, 4 and 8 after injection was weakened as compared to that before injection, while the positive reaction was slightly higher at week 8 than that at week 4 (Figure 6).
Figure 6. Immunohistochemistry of VEGF in retina of rats from group C.
A: Before injection; B: 1 week after injection; C: 4 weeks after injection; D: 8 weeks after injection. VEGF expression decreased at weeks 1, 4 and 8 after injection as compared to that before injection.
VEGF mRNA expression in retina determined by real-time PCR assay
Real-time PCR assay revealed that the VEGF mRNA expression in the retina before injection was not significantly different from that 1 week after injection in group A (P>0.05), while the VEGF mRNA expression before injection significantly differed from those 4 and 8 weeks after injection (P<0.05) (Table 1). In group B, the VEGF mRNA expression before injection was significantly different from those 1 week and 4 weeks after injection (P<0.05), however, the VEGF mRNA expression 8 weeks after injection was not significantly different from that before injection (P>0.05). The VEGF mRNA expression 8 weeks after injection significantly differed from that 4 weeks after injection (P<0.05) (Table 1). In group C, the VEGF mRNA expression before injection was significantly different from those 1 week, 4 and 8 weeks after injection (P<0.05), while the VEGF mRNA expression 8 weeks after injection was not significantly different from that 4 weeks after injection (P>0.05) (Table 1).
Table 1. Comparison of VEGF mRNA expression in retina of rats from group A, groupB and group C at different time points.
| Time point | n | VEGF mRNA expression |
P |
||||
| Group A | Group B | Group C | Group A | Group B | Group C | ||
| Before injection | 6 | 1.0±0 | 1.0±0 | 1.0±0 | - | - | - |
| 1 week after injection | 6 | 1.163±0.078 | 0.607±0.019 | 0.577±0.033 | >0.05 | <0.05a | <0.05a |
| 4 weeks after injection | 6 | 1.545±0.014 | 0.317±0.019 | 0.320±0.014 | <0.05a | <0.01b | <0.01b |
| 8 weeks after injection | 6 | 1.881±0.022 | 0.822±0.015 | 0.375±0.046 | <0.05c | <0.05c | <0.05a |
Group A: aP<0.05, cP<0.05 vs before injection; GroupB: aP<0.05, bP<0.01 vs before injection, cP<0.05 vs 4 weeks after injection; Group C: aP<0.05, bP<0.01 vs before injection.
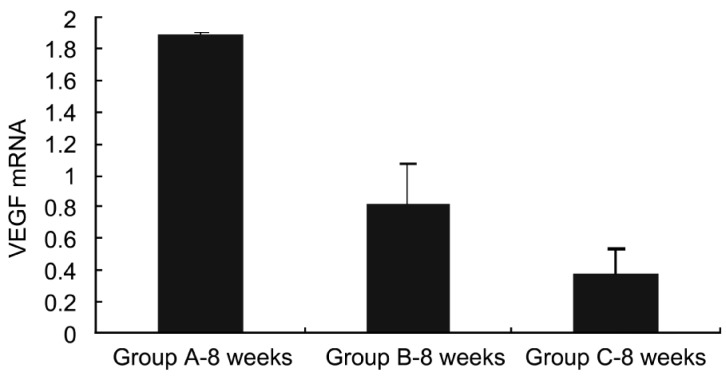
Obviously, the VEGF mRNA was expressed at equal levels in the three groups before injection, and no significant difference was observed. There were significant differences in VEGF mRNA expression between group A and groups B and C 1 week after injection (P<0.05), while no significant difference was found between group B and group C (P>0.05) (Figure 7). At week 4 after injection, there were significant differences in VEGF mRNA expression between group A and groups B and C (P<0.01), while no significant difference was found between groups B and C (P>0.05) (Figure 8). At week 8 after injection, there were significant differences in VEGF mRNA expression between group A and groups B and C (P<0.05), and significant difference was observed between group B and group C (P<0.05) (Figure 9), indicating that bevacizumab-chitosan nanoparticles exhibited a longer duration of action.
Figure 7. VEGF mRNA expression in retina of rats from groups A, B and C 1 week after injection.
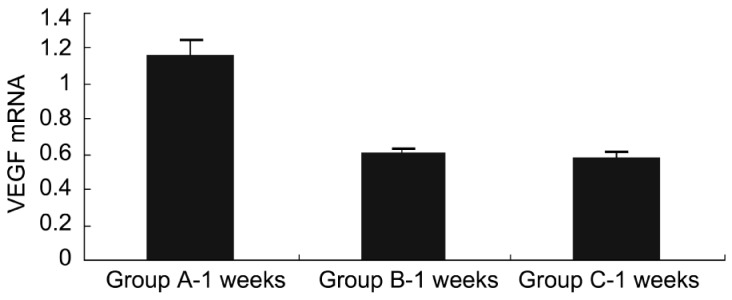
Figure 8. VEGF mRNA expression in retina of rats from groups A, B and C 4 weeks after injection.
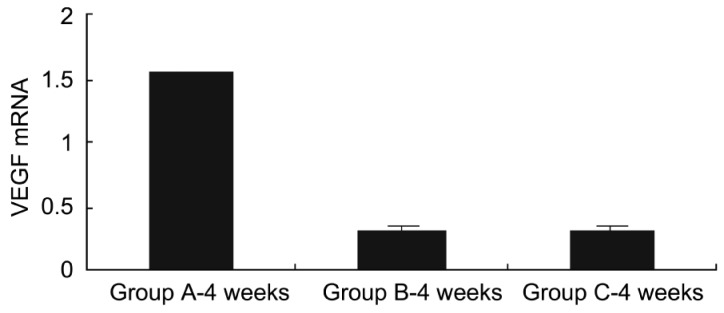
Figure 9. VEGF mRNA expression in retina of rats from groups A, B and C 8 weeks after injection.
DISCUSSION
Diabetic retinopathy is one of the most common and serious complications of diabetes, and it has becomes the third most common blinding eye disease in the world. It has been indicated that the occurrence and development of diabetic retinopathy is attributed to microcirculation structure damage-induced retinal hypoxia, angiogenesis and formation of abnormal blood vessels, which causes the pathological changes, such as vitreous hemorrhage and retinal detachment, thereby resulting blindness. Retinal hypoxia causes elevated expression of VEGF mRNA and VEGF protein, and promotes structure damage of the retinal microcirculation and angiogenesis. It is proved that VEGF level is closely associated with the pathological process of the occurrence and development of retinal microcirculation damage and neovascularization. Therefore, the strategy to inhibit neovascularization through inhibiting VEGF pathway and vascular endothelial growth factor receptor (VEGFR) has become a hot topic in clinical and basic researches. A monoclonal antibody targeting VEGF (rhMAb VEGF, bevacizumab, bevacizumab) is shown to inhibit retinal microcirculation damage and neovascularization, and currently use of the antibody is a novel strategy for treatment of neovascular eye diseases[10],[11].
In various types of angiogenesis inhibitors targeting VEGF and VEGFR, bevacizumab and ranibizumab are the types with the fastest progress in research. As a novel humanized anti-VEGF monoclonal antibody, they function through neutralizing VEGF and inhibiting the binding of VEGF to the receptor on endothelial cells. Bevacizumab is the first drug approved by U.S. Food and Drug Administration (FDA) for treatment of cancer through inhibiting angiogenesis. Intravitreal administration of anti-VEGF monoclonal antibodies was shown to effectively inhibit choroidal neovascularization, thereby effectively treating neovascular age-related macular degeneration[12],[13]. However, such type of monoclonal antibody suffers from the problems of poor stability, short half-life and limited duration of effective concentration in vitreous body, and in general, repeated intravitreal injection should be performed during the treatment process to maintain a stable effective drug concentration[14]. Unfortunately, repeated intravitreal injection may cause a series of severe complications such as retinal tear, intraocular hemorrhage, endophthalmitis and proliferative vitreoretinopathy[4]-[7]. Therefore, the purpose of this study was to observe the role of bevacizumab -chitosan nanoparticles as inhibiting angiogenesis and avoiding repeated intravitreal injection
It has been demonstrated that many preparations can inhibit the progression of diabetic retinopathy, and the mechanisms of these drugs are clear, in which VEGF inhibitors exhibit positive therapeutic efficacy[1]-[3],[15]-[19]. Therefore, bevacizumab, the current most widely used and effective drug in clinical practices, was selected as the object of the present study. In previous studies, bevacizumab has been successfully prepared into nanoparticles. The current study selected this preparation of nanoparticle as the sustained-release drug delivery system, and employed a single intravitreal injection to inhibit diabetic retinal vascular permeability in rat models, so as to assess whether the bioavailability of bevacizumab was significantly enhanced and diabetic retinopathy was effectively treated.
The results showed that bevacizumab could effectively inhibit VEGF expression, and bevacizumab-chitosan nanoparticles had a longer duration of action than bevacizumab alone. Such a finding was similar to a previous study[20]. The current study preliminarily proves the sustained-release effect of bevacizumab-chitosan nanoparticles, which is beneficial for designing the drug delivery system for posterior segment of the eye. It is undoubted that the VEGF inhibitors like bevacizumab exhibit positive therapeutic efficacy. In future studies, the preparation of nanoparticle should be optimized to achieve the goal of reducing intravitreal injection and the occurrence of complications, which exhibits wide practical values in clinical practices.
Acknowledgments
Foundation: National Natural Science Foundation of China (No.81270979); Shanghai Committee of Science and Technology Foundation (No. 08411962300)
Conflicts of interest: Lu Y, None; Zhou N, None; Huang X, None; Cheng JW, None; Li FQ, None; Wei RL, None; Cai JP, None.
REFERENCES
- 1.Lee SG, Kim JL, Lee HK, Ryu GW, Hur DY, Yun IH, Yang JW, Kim HW. Simvastatin suppresses expression of angiogenic factors in the retinas of rats with streptozotocin-induced diabetes. Graefes Arch Clin Exp Ophthalmol. 2011;249(3):389–397. doi: 10.1007/s00417-010-1496-5. [DOI] [PubMed] [Google Scholar]
- 2.Mason JO, 3rd, Nixon PA, White MF. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142(4):685–688. doi: 10.1016/j.ajo.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 3.Khurana RN, Do DV, Nguyen QD. Anti-VEGF therapeutic approaches for diabetic macular edema. Int Ophthalmol Clin. 2009;49(2):109–119. doi: 10.1097/IIO.0b013e31819fd8b5. [DOI] [PubMed] [Google Scholar]
- 4.Gelisken F, Ziemssen F, Voelker M, Bartz-Schmidt KU, Inhoffen W. Retinal pigment epithelial tears after single administration of intravitreal bevacizumab for neovascular age-related macular degeneration. Eye (Lond) 2009;23(3):694–702. doi: 10.1038/sj.eye.6703098. [DOI] [PubMed] [Google Scholar]
- 5.Jonas JB, Spandau UH, Rensch F, Von Baltz S, Schlichtenbrede F. Infectious and noninfectious endophthalmitis after intravitreal bevacizumab. J Ocul Pharmacol Ther. 2007;23(3):240–242. doi: 10.1089/jop.2006.0146. [DOI] [PubMed] [Google Scholar]
- 6.Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y, Sawa M, Tsujikawa M, Kusaka S, Tano Y. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol Scand. 2008;86(4):372–376. doi: 10.1111/j.1600-0420.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB, Spandau UH, Schlichtenbrede F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye. 2008;22(4):590–591. doi: 10.1038/eye.2008.10. [DOI] [PubMed] [Google Scholar]
- 8.Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, Wang SL. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;6:765–774. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fante RJ, Durairaj VD, Oliver SC. Diabetic retinopathy: An update on treatment. Am J Med. 2010;123(3):213–216. doi: 10.1016/j.amjmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Bahar I, Kaiserman I, McAllum P, Rootman D, Slomovic A. Subconjunctival bevacizumab injection for corneal neovascularization. Cornea. 2008;27(2):142–147. doi: 10.1097/ICO.0b013e318159019f. [DOI] [PubMed] [Google Scholar]
- 11.Colucciello M. Intravitreal bevacizumab and triamcinolone acetonide combination therapy for exudative neovascular age-related macular degeneration: short-term optical coherence tomography results. J Ocul Pharmacol Ther. 2008;24(1):15–24. doi: 10.1089/jop.2007.0080. [DOI] [PubMed] [Google Scholar]
- 12.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, ANCHOR Study Group Ranibizumab versusverteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 14.Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW, Jr, Esquiabro M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Kim JH, Yu YS, Min BH, Kim KW. Protective effect of clusterin on blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(3):1659–1665. doi: 10.1167/iovs.09-3615. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Gorbey S, Pfister F, Höger S, Dorn-Beineke A, Krügel K, Berrone E, Wu L, Korff T, Lin J, Busch S, Reichenbach A, Feng Y, Hammes HP. Long-term treatment with suberythropoietic Epo is vaso- and neuroprotective in experimental diabetic retinopathy. Cell Physiol Biochem. 2011;27(6):769–782. doi: 10.1159/000330085. [DOI] [PubMed] [Google Scholar]
- 17.Rangasamy S, Srinivasan R, Maestas J, McGuire PG, Das A. A potential role for angiopoietin 2 in the regulation of the blood-retinal barrier in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(6):3784–3791. doi: 10.1167/iovs.10-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maharjan S, Lee S, Agrawal V, Choi HJ, Maeng YS, Kim K, Kim NJ, Suh YG, Kwon YG. Sac-0601 prevents retinal vascular leakage in a mouse model of diabetic retinopathy. Eur J Pharmacol. 2011;657(1–3):35–40. doi: 10.1016/j.ejphar.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Abdallah W, Fawzi AA. Anti-VEGF therapy in proliferative diabetic retinopathy. Int Ophthalmol Clin. 2009;49(2):95–107. doi: 10.1097/IIO.0b013e31819fd84a. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Zhu T, Tang X, Ye P, Zhang Z. Effect of an intravitreal injection of bevacizumab on the expression of VEGF and CD34 in the retina of diabetic rats. Clin Experiment Ophthalmol. 2010;38(9):875–884. doi: 10.1111/j.1442-9071.2010.02370.x. [DOI] [PubMed] [Google Scholar]