Abstract
AIM
To evaluate the expression of dendritic cell-associated C-type lectin-1 (dectin-1) in human corneal epithelial (HCE) cells infected by fungus.
METHODS
A total of 20 cases of healthy donor corneas were group A, and 20 patients (20 eyes) suffered from fungal keratitis (FK) composed group B. Real-time qPCR and immunohistochemistry were applied to detect dectin-1 expression in corneal epithelium of both groups. HCE cells were cultured with aspergillus fumigatus (AF) antigens in vitro. The expression of dectin-1 mRNA was measured by real-time qPCR at the stimulation of 0, 4, 8 and 24h separately. Dectin-1 protein was detected by immunocytochemistry at 0 and 24h separately.
RESULTS
Dectin-1 expressed in corneal epithelium of normal persons and FK patients. Vitro cellular experiment showed that the expression of dectin-1 mRNA in HCE cells began to increase after stimulation of AF antigens at 4h, and dectin-1 protein expression increased after stimulation at 24h.
CONCLUSION
Dectin-1 expressed in corneal epithelium of normal persons. AF antigens stimulation can elevate the expression of dectin-1 in HCE cells in vitro.
Keywords: dectin-1, corneal epithelial cells, fungal keratitis, human
INTRODUCTION
Fungal keratitis (FK) is a severe corneal disease with high rate of blindness[1]. Human innate immune system which mainly through pattern recognition receptors (PRRs) to recognize highly conserved structure of pathogenic microorganism called pathogen associated molecular pattern (PAMPs) is the first line to resist fungal antigens in cornea[2]. Dendritic cell-associated C-type lectin-1 (dectin-1) which was firstly found in dendritic cell is a C-type lectin. Dectin-1 recognizes fungal β-glucans. It has been discovered that dectin-1 contributed to innate immune response for variety of fungi inflammation as PRRs[3]. As far as we see, there was no detailed report on whether dectin-1 expressed in human corneal epithelial (HCE) cells or its antifungal function in HCE cells. The present study mainly observed the expression of dectin-1 in corneal epithelium of normal persons and FK patients. We also used aspergillus fumigatus (AF) antigens to stimulate HCE cells to evaluate the antifungal function of dectin-1 in HCE cells.
MATERIALS AND METHODS
Materials
Aspergillus fumigatus strains (NO3.0772) was bought from China General Microbiological Culture Collection Center; Sabouroud culture was purchased from American Sigma company; Rabbit anti-human dectin-1 multi-clonal antibody, Histostain® PLUS kit and DAB kit were purchased from Beijing Biosynthesis Biotechnology Co., LTD; Trizol Reagent was purchased from Invitrigen; PrimeScript® RT reagent Kit With gDNA Eraser (Perfect Real Time) was purchased from TaKaRa; Primers and probes were purchased from TaKaRa.
Clinical Specimen
A total of 20 cases of healthy donor corneas were group A. Healthy donor corneas were used as corneal graft and the rest of the peripheral corneal tissues were used for experiment. Twenty FK patients (20 eyes) who underwent penetrating keratoplasty at the Affiliated Hospital of Medical College, Qingdao University from January 2011 to December 2011 composed group B. The diagnosis of FK was based on the case history, clinical features, identification and cultivation of fungal elements from corneal ulcer scrapings, and the detection of corneal confocal microscope, etc. There were 12 cases of fusarium, 6 cases of aspergillus, and 2 cases of other fungal genus. There were 12 males and 8 females, with averaged age of (54.50±11.37) years (ranged 36-72 years). After penetrating keratoplasty, half of corneal epithelium around lesion was scraped, by avoiding necrosis tissue and purulent secretion. The scrapings were put in 500µL RNAiso and cryopreserved at -80°C refrigerator. Immunohistochemical staining for dectin-1 was carried out on the left region. Managements of specimen were obtained the agreements of patients and their families and informed consents were signed. The experiment had the approval of hospital's Ethics Committee.
Vitro Experiment
Aspergillus fumigatus antigens
AF grew in Sabouroud medium, 28°C for 5d; Physiological saline flushed the fungi surface; Collected the fluid; 3 000r/min centrifugal 5min; Inactivated 30min in 70% alcohol; Washed three times by PBS[4]. High glucose medium was used as solution for AF antigens. The above antigenic stimulation liquid was saved in -20°C and should be used in 2 weeks.
Human corneal epithelial cells culture
HCE cells were cultured in high glucose medium, 37°C, 5%CO2. Near 80% confluence, the cells were cultured in serum free dulbecco's modified eagle medium (DMEM) for 24h. Cells were used for real-time qPCR and immunocytochemistry.
Stimulation of aspergillus fumigatus antigens
HCE cells were cultured with AF antigenic stimulation liquid after discarding the medium. The expression of dectin-1 mRNA in HCE cells were detected by real-time qPCR at the stimulation of 0, 4, 8 and 24h. Dectin-1 protein expression in HCE cells were detected by immunocytochemistry at 0 and 24h.
Real-time qPCR
Samples obtained at different time were extracted total RNA, determined RNA concentration, reverse transcripted and conducted real-time qPCR at the same time to minimize the experimental errors. The primer and probe sequences of dectin-1 were as follows: Sense AAGGATCGTGTGCTGCATCTC; Antisense TGGTACCCAGGACCACAGCTA; Probe TCCTTGGCGCCTCATTGCTGTAATTTT; Sequences of β-actin were: Sense GACTACCTCATGAAGATCCTCACC; Antisense TCTCCTTAATGTCACGCACGATT; Probe CGGCTACAGCTTCACCACCACGGC. Applied recommend steps in PrimeScript RT reagent Kit With gDNA Eraser as reverse transcription steps. Forty cycles were performed. Each cycle reaction ended with recording the fluorescence values. The 40 cycles formed amplification curve, and reported the number of cycles that reached the threshold fluorescence value (Ct value).
Immunochemistry
Immunochemistry was performed by usage of the streptavidin-peroxidase method. After fixation, dehydration, transparent, baptist wax and embedding processing, corneal lesions of fungal keratitis and normal corneas were cut into 5µm sections and then dewaxing and hydration, and distilled water for 3 times. After getting rid of endogenous peroxidase with 3% hydrogen peroxide, slices reacted with goat blocking antibody at 37°C for 20min. After that, slices reacted with dectin-1 antibody (dilution of 1:100) at 4°C overnight, then with a biotin-conjugated anti-rabbit secondary antibody at 37°C for 40min, and followed by a peroxidase-conjugated streptavidin for 10-15min. Slices were developed with diaminobenzidine. The reacting time was controlled under microscope. Then slices were counterstained with hematoxylin for 1min, dehydrated, and finalized with neutral balsam. HCE cells were cultured on slides. Near 80% confluence, cells were exposed to AF antigenic stimulation liquid at 0 and 24h. After fixed in 4% paraformaldehyde for 30min, the cells were used for immunocytochemistry (rabbit polyclonal antibody for dectin-1, dilution of 1:300). Isotype control cells were performed using rabbit IgG instead of primary antibodies.
Statistical Analysis
All data were presented as mean±SD. The data were analyzed using SPSS 17.0. The differences were analyzed by one-way ANOVA and LSD test in groups. P<0.05 was considered as statistically difference.
RESULTS
Real-time qPCR
Clinical specimen
Stable β-actin expression could be detected in corneal epithelium. Amplification curve had flat baseline level, clear inflection point, obvious exponential phase and well overall parallel. Confirmed that intact cDNA fragments and successful PCR reactions. Twenty cases of normal human and 20 cases of FK patients expressed dectin-1 mRNA in corneal epithelium (Table 1).
Table 1. The Ct value of dectin-1 mRNA in corneal epithelium of normal persons and patients with fungal keratitis (Cycles).
| Cases | Group A |
Group B |
||
| β-actin | dectin-1 | β-actin | dectin-1 | |
| 1 | 23.13 | 32.61 | 25.03 | 35.71(UT) |
| 2 | 26.44 | 37.48 | 23.61 | 35.01(UT) |
| 3 | 23.54 | 33.89 | 22.15 | 33.71(UT) |
| 4 | 23.8 | 33.69 | 23.09 | 34.16(UT) |
| 5 | 23.19 | 34.05 | 22.15 | 32.76(UT) |
| 6 | 23.74 | 33.04 | 26.03 | 36.93(UT) |
| 7 | 22.93 | 32.77 | 21.44 | 31.81(UT) |
| 8 | 24.81 | 34.48 | 22.72 | 32.76 |
| 9 | 21.8 | 32.19 | 25.21 | 35.64 |
| 10 | 22.03 | 32.38 | 23.12 | 35.17 |
| 11 | 22.71 | 32.72 | 23.71 | 34.22 |
| 12 | 22.21 | 32.21 | 24.09 | 34.66 |
| 13 | 21.89 | 32.12 | 22.51 | 33.88 |
| 14 | 21.76 | 32.41 | 22.36 | 33.35 |
| 15 | 22.61 | 32.52 | 24.52 | 35.99 |
| 16 | 22.28 | 33.67 | 25.2 | 35.03 |
| 17 | 21.58 | 32.74 | 23.21 | 34.75 |
| 18 | 23.22 | 33.28 | 20.38 | 30.9 |
| 19 | 23.25 | 33.05 | 20.12 | 30.71 |
| 20 | 21.64 | 31.33 | 20.74 | 30.67 |
UT: Untreated.
Vitro experiment
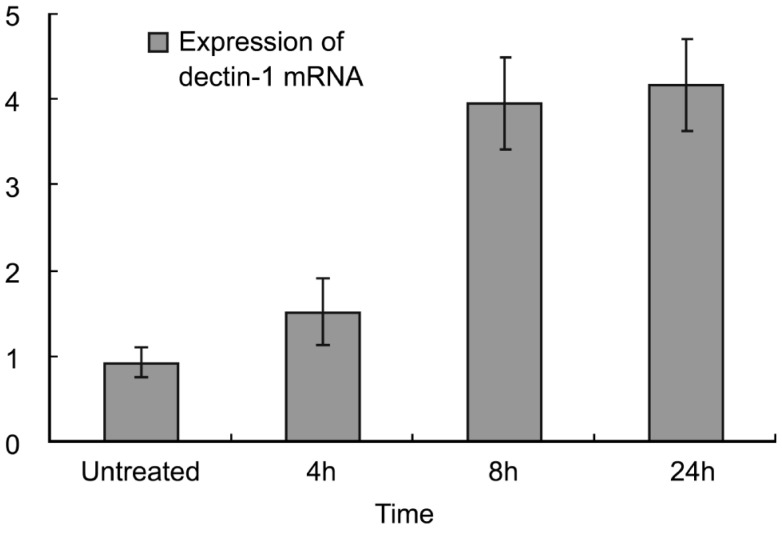
HCE cells could be detected the stable expression of β-actin. Confirmed that successful PCR reactions. The expression of dectin-1 mRNA was detected at each time point in HCE cells (Figure 1). LSD test were used between every two time points, and the results showed that there were statistically differences between 0 and 4, 8, 24h (P<0.05), there was no statistically difference between 8 and 24h (P=0.409).
Figure 1. The expression of dectin-1 mRNA in untreated group and AF antigens stimulated groups (x±s).
Immunochemistry
Clinical specimen
Dectin-1 protein can be detected in 20 cases of normal corneas and 20 cases of FK corneal epithelium. Dectin-1 protein expressed lowly in normal corneal epithelium. Stronger staining was observed in FK corneal epithelium (Figure 2).
Figure 2. Dectin-1 expression in corneal epithelium by immunohistochemical staining.
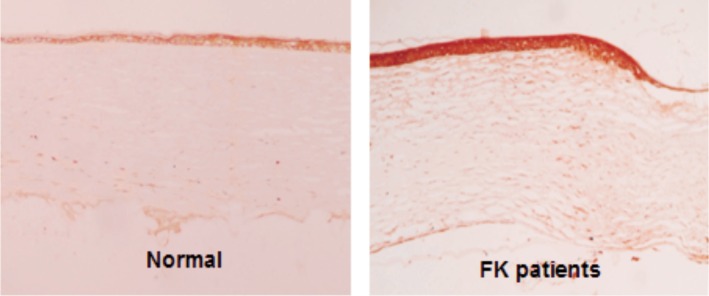
Dectin-1 protein expressed lowly in normal corneal epithelium. Stronger staining was observed in FK corneal epithelium (×100).
Vitro experiment
HCE cells were cultured on slides. Immuocytochemical staining showed that positive dectin-1 expression of brown particles located in the nucleus and cytoplasm (Figure 3). Dectin-1 protein was lowly produced by the normal HCE cells. Stronger staining throughout the cytoplasm was observed in the cells exposed to AF spores for 24h.
Figure 3. Dectin-1 expression in cultured HCE cells evaluated by immunocytochemical staining.
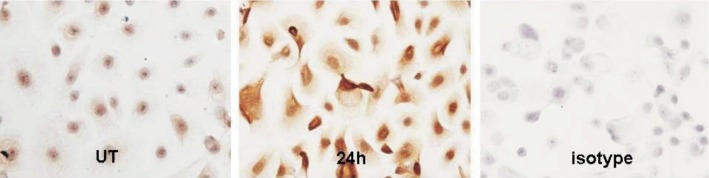
The cultured HCE cells incubated with AF antigens stimulation liquid for 0 (UT: untreated) and 24h. Dectin-1 protein was lowly produced by the normal HCE cells. Stronger staining was observed in the cells exposed to AF spores for 24h (×200).
DISCUSSION
Being a developing country, China owns a majority of population that engages in industry and agriculture production. Corneal trauma especially plant trauma is common, which provide a chance of FK. Due to the lack of broad-spectrum, low toxicity, effective anti-fungal drugs, shortages of corneal donor, recurrence and complications of operation, treatment of FK is difficult. Many patients have vision loss, even eyeballs removed ultimately. Because of high blindness rate and great clinical harm, FK has become one of the research priorities in infectious corneal diseases[5].
Up to now, the pathogenesis of FK is not clear. Along with the development of immunology, molecular biology and related research, people begin to pay close attention to immune mechanisms in FK. As the first line of defense microbial infection, innate immune inhibit the reproduction and invasion of fungi in the host through immune cells and immune mechanisms which constitute important components for resisting fungal infection in cornea. Innate immune become more and more important in the immune mechanism of fungal infection. PRRs are keys of the innate immune[2]. To recognize PAMPs and mediate rapid innate immune responses, then induce lasting specific adaptive immune responses to recognize and remove pathogenic fungi.
As one of the C-type lectin receptors, dectin-1 is a type II transmembrane receptor which was found in recent years[6]. Dectin-1 plays important role in defending fungal infection. It can specific recognize β-glucan structure that exists in cell wall of yeast, candida, penicillium, aspergillus and other fungus. It can cause a variety of cellular responses, including respiratory burst, ligand endocytosis and phagocytosis, maturation of dendritic cells, generation of cytokine and chemokine (including TNF, IL-1 β, CXCL2, CCL3, IL-23, IL-6, IL-10, IL-2, GM-CSF, G-CSF)[7]. Studies have shown that dectin-1 was up-regulated in fungal infected corneas, while infected dectin-1-/- corneas have diminished cellular infiltration and fungal clearance compared with control mice[3]. Dectin-1 is required for neutrophil activation in response to conidia and macrophage-mediated phagocytosis of conidia[8].
The expression of dectin-1 was considered in macrophages, dendritic cells, neutrophils, mast cells, eosinophils and other immune cells in the past. Recent studies suggest that human skin keratinocytes, epithelial cells, endothelial cells, fibroblasts and other structural cells also have certain immune cell activity, performed that the cells could express dectin-1 and other PRRs, upregulate the expression of PRRs after the binding with PAMPs ligand, secret cytokines and chemokines subsequently[9]-[12]. We detected the expression of dectin-1 in corneal epithelium of normal human and FK patients and found that normal corneal epithelial cells can synthesize dectin-1, there also existed structural involvement of dectin-1 after fungal infection. The expression of dectin-1 of corneal epithelial layers can be confirmed by cytological experiments. The results indicate that not only toll-like receptor 2 and 4(TLR2/4)[13], but dectin-1 acts as PRRs in human corneal epithelium.
In order to further confirm whether dectin-1 in HCE cells involve in innate immunity and against fungal infections as PRRs, we used AF hyphae to produce fungal antigenic stimulation fluid and stimulated cultured HCE cells. We detected the expression of dectin-1 in HCE cells after stimulation of AF antigens. Results showed that after stimulation of 4h, the expression of dectin-1 mRNA increased gradually and continued to rise in 24h. The expression of dectin-1 protein increased at 24h. These results suggest that the AF infection of HCE cells can induce up-regulation of dectin-1. As non-immune cells, corneal epithelial cells can express dectin-1, and dectin-1 expression is elevated after fungal infection, suggesting dectin-1 expressed in corneal epithelial may play the same role as discussed before. Dectin-1 of the corneal epithelial cells may participate in innate immune resistance to fungal infection.
As one kind of the most important barrier of cornea to the outside world, corneal epithelium plays an important role in resistance of fungal infection. The preliminary study on the relationship between the expression of dectin-1 in corneal epithelial cells and fungal infections made a further understanding to the role of PRRs in antifungal innate immunity. The related functional studies will reveal the idiographic role of dectin-1 in resistance to fungal infection in innate immune stage as PRRs.
Acknowledgments
Foundation: Supported by National Natural Science Foundation of China (No.81170825; No.81300730)
Conflicts of Interest: Li C, None; Zhao GQ, None; Che CY, None; Li N, None; Lin J, None; Xu Q, None; Wang Q, None; Liu Y, None; Qiu S, None.
REFERENCES
- 1.Xie LX, Zhong WX, Shi WY, Sun SY. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Li N, Zhao GQ. Mechanism of the immune response to keratomycosis. Zhonghua Yake Zazhi. 2011;47(4):378–381. [PubMed] [Google Scholar]
- 3.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6(7):e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun. 2009;15(3):155–168. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]
- 5.Xie LX. Advances in basic and clinical corneal research. Zhonghua Yanke Zazhi. 2010;46(10):883–887. [PubMed] [Google Scholar]
- 6.Huysamen C, Brown GD. The fungal pattern recognition receptor, Dectin-1, and the associated cluster of C-type lectin-like receptors. FEMS Microbiol Lett. 2009;290(2):121–128. doi: 10.1111/j.1574-6968.2008.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HM, Yuk JM, Shin DM, Jo EK. Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J Clin Immunol. 2009;29(6):795–805. doi: 10.1007/s10875-009-9319-3. [DOI] [PubMed] [Google Scholar]
- 8.Leal SM, Jr, Vareechon C, Cowden S, Cobb BA, Latgé JP, Momany M, Pearlman E. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest. 2012;122(7):2482–2498. doi: 10.1172/JCI63239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Koning HD, Rodijk-Olthuis D, van Vlijmen-Willems IM, Joosten LA, Netea MG, chalkwijk J, Zeeuwen PL. A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: upregulation of dectin-1 in psoriasis. J Invest Dermatol. 2010;130(11):2611–2620. doi: 10.1038/jid.2010.196. [DOI] [PubMed] [Google Scholar]
- 10.Marakalala MJ, Kerrigan AM, Brown GD. Dectin-1: a role in antifungal defense and consequences of genetic polymorphisms in humans. Mamm Genome. 2011;22(1–2):55–65. doi: 10.1007/s00335-010-9277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang PP, Xu XY, Zhang F, Shi Y. The changes of Dectin-1 and TLR2 in human umbilical vein endothelial cells stimulated by Aspergillus fumigatus. Zhonghua Jiehe He Huxi Zazhi. 2011;34(2):91–94. [PubMed] [Google Scholar]
- 12.Zhang C, Wang SH, Liao CP, Shao S, Lasbury ME, Durant PJ, Lee CH. Downregulation of PU.1 leads to decreased expression of Dectin-1 in alveolar macrophages during Pneumocystis pneumonia. Infect Immun. 2010;78(3):1058–1065. doi: 10.1128/IAI.01141-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun. 2009;15(3):155–168. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]