Abstract
AIM
To evaluate the influence of an intravitreal injection of bevacizumab and fasudil on the retinal vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNFα), and caspase 3 levels in a diabetic rabbit model.
METHODS
The study included 6 healthy rabbits (Group 1), 6 rabbits with experimentally induced diabetes mellitus (DM) (Group 2), 7 rabbits with experimentally induced DM to which intravitreal bevacizumab was administered (Group 3), and 7 rabbits with experimentally induced DM to which intravitreal fasudil was administered (Group 4). An intravitreal injection of 1.25mg/50µL bevacizumab in the right eye of rabbits in Group 3 and an intravitreal injection of 0.0064mg/50µL fasudil in the right eye of rabbits in Group 4 were administered on day 21 after the induction of DM. The studied eyes of the rabbits were enucleated three days after the intravitreal injection. The TNFα, VEGF, and caspase 3 levels were determined using the ELISA method.
RESULTS
There was a statistically significant difference in the VEGF and caspase 3 levels between groups (P=0.005 and P =0.013, respectively), but the TNFα level did not differ significantly between groups (P=0.792). It was found that VEGF levels were significantly lower in Group 1 and Group 3 than in Group 2 using the Mann-Whitney U test with the Bonferroni correction (P=0.004 for both comparison). There was no statistically significant difference between other groups with regard to VEGF levels (the P value ranged between 0.015 and 0.886). Although the P values of the caspase 3 levels were 0.015 for Group 1 and Group 4, 0.038 for Group 2 and Group 3, and 0.018 for Group 3 and Group 4, these P values remained above the threshold P value of 0.0083, which was the statistically significant level for post hoc tests.
CONCLUSION
An intravitreal injection of bevacizumab decreased both the VEGF level, which plays a role in angiogenesis, and the caspase 3 level, which plays a role in apoptosis. Although not as effective as bevacizumab, fasudil had a beneficial effect on the VEGF levels but significantly increased the caspase 3 levels.
Keywords: bevacizumab, caspase 3, experimental diabetes, fasudil, tumor necrosis factor alpha, vascular endothelial growth factor
INTRODUCTION
The growing prevalence of diabetic retinopathy, the common ocular complication of diabetes mellitus (DM), is a critical problem for global public health[1]. While visual acuity is not always affected in early stages, progression of the diabetic retinopathy leads to severe vision loss[2].
Vascular endothelial growth factor (VEGF) has been shown to play a major role in macular edema and retinal neovascularization[3],[4]. Activation of the VEGF-receptor pathway triggers a network of signaling processes that promotes endothelial cell growth, migration, survival from preexisting vessels, differentiation, and mobilization of endothelial progenitor cells from the bone marrow into the peripheral circulation[5]-[7]. Furthermore, VEGF increases vessel permeability, leading to the deposition of proteins in the interstitium that facilitate the process of angiogenesis[8].
Bevacizumab is a complete full-length humanized antibody that binds to all subtypes of VEGF and is successfully used in tumor therapy as a systemic drug[5]. Recent studies have demonstrated the usefulness of an intravitreal injection of bevacizumab in the reduction of macular edema secondary to central retinal vein occlusion, vascular permeability, and proliferative diabetic retinopathy[9],[10].
The Rho/Rho-associated protein kinase (ROCK) pathway promotes leukocyte adhesion to the microvasculature by affecting the expression and function of adhesion molecules, including intercellular adhesion molecules (ICAMs)-1 and integrins[11]-[13]. Moreover, ROCK causes firm adhesion through the activation of ezrin, radixin, and moesin in endothelial cells, which jointly form the anchoring structures for leukocyte integrins[14],[15]. These findings suggest that the elevated activity of the Rho-ROCK pathway is involved in the pathogenesis of diabetic microvasculopathy mediated through leukocyte adhesion[2].
The specific Rho-kinase inhibitor fasudil is relatively safe and effective in cardiovascular disease[16]. Fasudil prevent development of diabetic nephropathy in diabetic rats[17]. The therapeutic potential of fasudil for ocular angiogenic diseases through the blockade not only of Rho-kinase signaling but also of extracellular signal-related kinase and Akt signaling is demonstrated in vitro study[18]. Intravitreal fasudil protects the vascular endothelium by inhibiting neutrophil adhesion and reducing neutrophil-induced endothelial injury in diabetic rats[19].
During the acute stages of diabetes, retinal neuropathy symptoms, including retinal neural function disorder, increases in the apoptosis of retinal ganglion cells and caspase 3 activity, and abnormal metabolic changes in retinal glial cells, have been observed[20],[21]. VEGF is elevated in diabetic retinopathy and causes both proliferation and increased permeability in vascular endothelial cells[4]. Tumor necrosis factor alpha (TNFα) is an important pro-inflammatory factor in retinal and central nervous system neurodegenerative diseases, such as diabetic retinopathy and Parkinson's disease[22],[23].
We evaluated the effect of intravitreal bevacizumab and fasudil on retinal VEGF, TNFα, and caspase 3 levels in a diabetic rabbit model.
MATERIALS AND METHODS
Materials
Thirty-six male albino New Zealand rabbits weighing 2.0-2.5kg were included in the study. They were housed in a room held at 22±1°C and 66%±3% humidity with a 24-hour light-dark cycle. They were handled in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. The study was carried out with approval from the Committee on the Ethics of Animal Experiments of Abant Izzet Baysal University.
Methods
Animals were randomly assigned to the groups. Diabetes was induced by a single intraperitoneal injection of 150mg/kg streptozotocin (Calbiochem, Massachusetts, USA) dissolved in freshly prepared citrate buffer (0.1mol/L citrate and 0.2mol/L sodium phosphate, pH4.5) under intraperitoneal 50mg/kg ketamine and 5mg/kg xylazine anesthesia[24]. Two days after the streptozotocin injection, streptozotocin-injected rabbits were considered diabetic when the fasting glucose level was found to be >145mg/dL with an Accu-Chek Go (Roche, Basel, Switzerland) by puncturing the marginal vein of the rabbit's ear. The fasting blood glucose level was monitored weekly. We excluded 9 rabbits from the study (2 rabbits died before the initiation of the study, 1 rabbit died after the day of streptozotocin injection, 5 rabbits did not become diabetic after the streptozotocin injection, and 1 diabetic rabbit had a normal blood glucose level during the follow-up period). The rabbits were divided into 4 groups. Group 1 comprised 6 untreated, healthy rabbits; Group 2 comprised 6 diabetic rabbits; Group 3 comprised 7 diabetic rabbits treated with single intravitreal bevacizumab injection (1.25mg/50µL); and Group 4 comprised 7 diabetic rabbits treated with single intravitreal fasudil injection [0.0064mg (180µmol/L)/50µL], which was dissolved with intraocular irrigating solution (Bausch&Lomb, Waterford, Ireland).
Bevacizumab and fasudil were purchased from Roche Pharma AG (Basel, Switzerland) and Calbiochem (Massachusetts, USA), respectively. The diabetic rabbits were kept under diabetic conditions for 21d, after which intravitreal bevacizumab and intravitreal fasudil were injected over a period of 1min intravitreally to rabbits in Group 3 and Group 4 with a sterile 30-gauge needle from 1mm of the limbus. After 3d of single intravitreal injection, rabbits were sacrificed by an intraperitoneal injection of 200mg/kg sodium pentobarbital. The eyes were enucleated, and the retina was dissected, and samples were prepared and stored according to the manufacturer's instructions until the ELISA was performed. The VEGF and TNFα levels were estimated with kits from Cusabio Biotech Co., Ltd. (Wuhan, China), and the caspase 3 levels were estimated with a kit from BioVision Inc. (California, USA) according to the manufacturers' instructions. We performed all measurements in duplicate, and the tissue sample concentration was calculated from a standard curve.
Statistical Analysis
All values were expressed as the mean±standard deviation. The statistical analysis was performed using the Kruskal Wallis test and the Mann-Whitney U test, using the Bonferroni adjustment as the post hoc test. Differences were accepted as significant for P<0.05.
RESULTS
We did not encounter any clinically visible gross complication in any group due to diabetes or any intervention during the study. The blood glucose levels measured during the study are presented in Table 1. The levels of VEGF, TNFα, and caspase 3 in all groups are presented in the Figures (Figures 1–3).
Table 1. Fasting blood glucose levels of the groups.
| Groups | Fasting blood glucose level (mg/dL) |
||||
| Initial | 2nd day | 9th day | 16th day | 23rd day | |
| Group 1 | 87±8.07 | 86±9.13 | 88±8.62 | 82±10.02 | 90±9.06 |
| Group 2 | 90±7.92 | 238±17.13 | 230±17.13 | 224±22.34 | 236±23.04 |
| Group 3 | 84±8.24 | 229±21.18 | 223±21.18 | 220±18.54 | 230±21.73 |
| Group 4 | 91±8.62 | 230±18.65 | 231±19.42 | 224±20.22 | 226±21.38 |
x±s
Figure 1. Retinal vascular endothelial growth factor levels of the groups.
The box represents 50% of the sample. The remaining 50% of the sample is contained within the areas between the box and the whiskers, with some exceptions (outliers). Single line inside the box represents median.
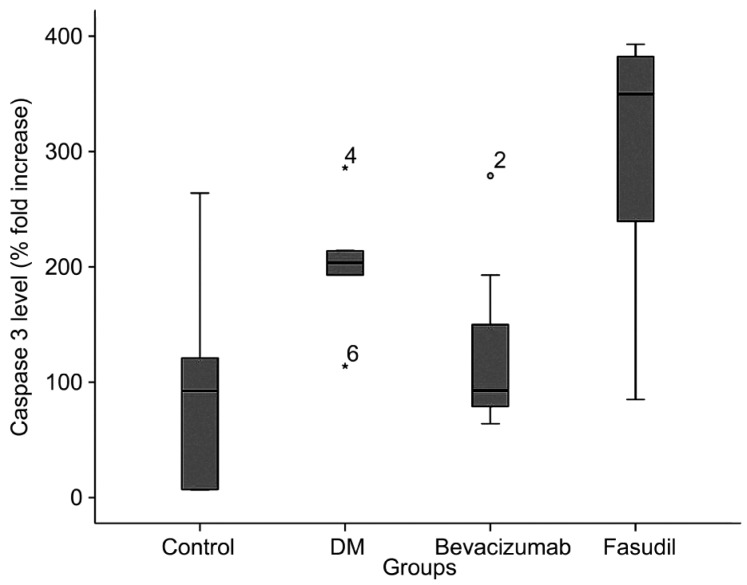
Figure 3. Retinal caspase 3 levels of the groups.
The box represents 50% of the sample. The remaining 50% of the sample is contained within the areas between the box and the whiskers, with some exceptions (outliers). Single line inside the box represents median. Small circle and asterisk represents the outlier with its number in bevacizumab group and diabetes group, respectively.
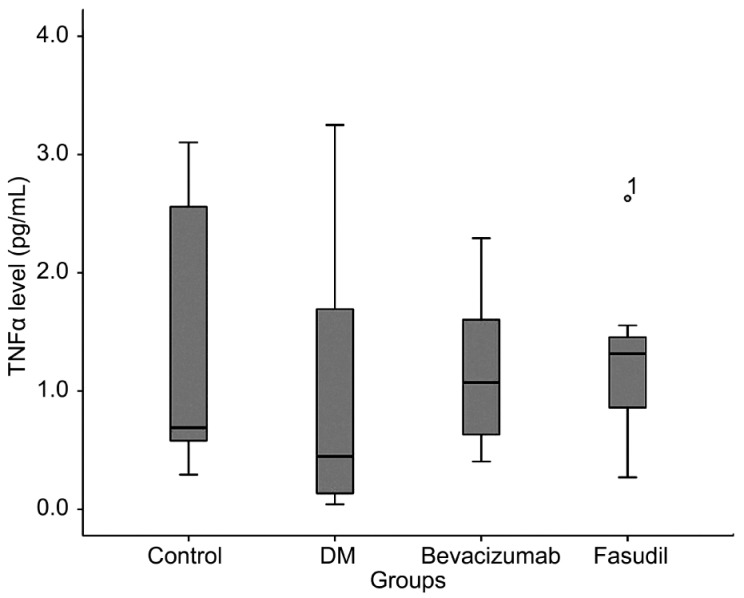
Figure 2. Retinal tumor necrosis factor alpha levels of the groups.
The box represents 50% of the sample. The remaining 50% of the sample is contained within the areas between the box and the whiskers, with some exceptions (outliers). Single line inside the box represents median. Small circle represents the outlier with its number in fasudil group.
There was a statistically significant difference between the groups for the VEGF levels (Table 2). The Mann-Whitney U test using the Bonferroni adjustment revealed significant differences between Group 1 and Group 2 and between Group 2 and Group 3 (the P values were 0.004 for both). The significance of the difference between Group 2 and Group 4 was 0.015, which was over the significance level of 0.0083 obtained by the Bonferroni adjustment. There was no statistically significant difference between the groups for the TNFα levels (Table 2). There was a statistically significant difference between the groups for the caspase 3 levels (Table 2). The Mann-Whitney U test using the Bonferroni adjustment did not reveal a significant difference between groups (the P values were over 0.0083).
Table 2. VEGF, TNFα and caspase 3 levels of the groups.
| Group 1 | Group 2 | Group 3 | Group 4 | 1P | |
| VEGF (pg/mL)±SD | 0.814±0.568 | 2.910±1.032 | 1.025±0.633 | 1.403±0.551 | 0.005 |
| Caspase 3 (%)±SD | 97.33±96.26 | 202.33±54.87 | 127.71±79.15 | 296.00±128.87 | 0.013 |
| TNFα (pg/mL)±SD | 1.319±1.194 | 1.003±1.251 | 1.177±0.698 | 1.264±0.739 | 0.792 |
1Kruskal Wallis test. TNF: Tumor necrosis factor; VEGF: Vascular endothelial growth factor.
DISCUSSION
Inflammation, angiogenesis, and apoptosis play a role in the development of diabetic retinopathy and its complications. VEGF, also known as vascular permeability factor, has been demonstrated to increase retinal vessel permeability and plays a critical role in the pathogenesis of diabetic retinopathy[25]. Because VEGF has been shown to play a major role in macular edema and retinal neovascularization, anti-VEGF treatments have been used for the treatment of diabetic retinopathy[3]. Bevacizumab is a complete full-length humanized antibody that binds to all subtypes of VEGF. Ma et al[26] reported that a single intraocular injection of bevacizumab (25mg/mL) may be beneficial as a therapy for preventing retinal vascular endothelial cell growth in the eyes of diabetic rats. In the current study, VEGF level decreased nearly to control group level in diabetic eyes after a single intravitreal bevacizumab injection.
The ROCK pathway plays an important role in pathological vascular conditions. The ROCK pathway promotes leukocyte adhesion to the microvasculature by affecting the expression and function of adhesion molecules. ROCK activation dephosphorylates endothelial nitric oxide synthase and decreases NO, which is an antiapoptotic factor[27]-[28]. Fasudil is a potent ROCK inhibitor, and it has a therapeutic potential for lung cancer by inhibiting cell proliferation, migration, invasion, matrix metalloproteinase activity, and down-regulating the expression of RhoA and VEGF[29]. Kuno et al[30] reported that fasudil inhibits TGF-beta-stimulated VEGF synthesis in osteoblast-like cell cultures. As a first study of intravitreal fasudil injection, Arita et al[19] applied intravitreal fasudil injections after diabetes onset and reported that the ROCK pathway plays a critical role in diabetic retinal microvasculopathy[19]. They repeated the fasudil injection every 3d after diabetes onset for 2 weeks due to the low half-life of fasudil. In the current study, we applied a single injection and studied the retinal parameters after 72h of injection. The VEGF level in the fasudil-injected group was lower than that in the diabetic group, but this difference was not statistically significant, most likely due to the small study group. The VEGF level of the fasudil-injected group was slightly higher than the VEGF levels of the control and bevacizumab-injected groups. Future studies are needed to determine the appropriate intravitreal dosage and form of fasudil due to the short half-life of fasudil and the limited number of studies.
TNFα contributes to the development of inflammation and the secretion of other cytokines. TNFα leads to the loss of retinal microvascular cells and plays a role in the early pathogenesis of diabetic retinopathy[31]. The widespread distribution of TNFα and the nature of the adhesion molecules expressed by vascular endothelial cells in proliferative diabetic retinopathy membranes suggest that the local activation of TNFα and the enhanced expression of vascular cell adhesion molecules may play an important role in the development of the proliferative phase of diabetic retinopathy[32]. TNFα and other inflammatory factors are expressed at significantly high levels under high glucose in human mesangial cells, and fasudil pretreatment decreases the TNFα secretion[33]. We investigated the effects of bevacizumab and fasudil on retinal TNFα levels in an experimental diabetes model. We did not determine a significant difference between the groups. This was most likely due to the shorter study period in the current study; the duration was not long enough for the development of the neovascularization process.
Li et al[34] reported that there is a significant increase in caspase 3 protein expression and that localization occurs in the nerve fiber layer, ganglion cell layer, and inner plexiform layer of the retina 2 weeks after the induction of diabetes. The intravitreal injection of 1.25mg of intravitreal bevacizumab does not result in structural changes in a porcine retina or changes in retinal caspase 3 levels[35]. The ROCK inhibitor prevented hepatocyte damage in acute liver injury in rats and merits consideration as a hepatocyte-protective agent in liver injury, considering its direct antiapoptotic effect on hepatocytes in vitro by decreasing caspase 3[36]. In the current study, caspase 3 was significantly increased in the diabetic group. We determined that there was a significant difference in the caspase 3 levels of the groups (P=0.013). The caspase 3 level in the bevacizumab-applied group decreased nearly to control group levels, but the caspase 3 level in the fasudil-applied group exceeded the levels of caspase 3 in the diabetic group. According to the results of this study, we can propose that bevacizumab has an antiapoptotic effect but fasudil increases apoptosis at this dosage when applied as an intravitreal injection. Future studies investigating the dosage and application model of fasudil are needed.
There are some critical limitations to this study. This study was designed to investigate short-term changes. We induced an experimental diabetes model in rabbits using streptozotocin, which is not preferred in studies investigating long-term diabetes effects; in those studies, alloxan is frequently preferred. Alloxan produces severe diabetes; streptozotocin is less toxic, and the mortality rate of animals is lower for streptozotocin than for alloxan. Therapeutic and toxic dosages of intravitreal fasudil injections have not yet been determined. The dosage injected in the current study decreased the VEGF level but increased the caspase 3 level. Further research using test with higher specificites (western blot), detecting apoptosis by other method (terminal deoxynucleotidyl transferase dUTP nick end labeling) and studying more cytokines (interleukins) are needed to elucidate the effect of intravitreal fasudil injection.
Bevacizumab decreased VEGF and caspase 3 levels as a standard intravitreal agent. For the injected route and dosage, fasudil decreased VEGF levels but not as effectively as bevacizumab. However, fasudil increased the caspase 3 level, and this effect might alter either the dosage or application route of fasudil. The ROCK pathway plays a role in the pathogenesis of diabetes, and applications of the ROCK inhibitor may play a potential therapeutic role in diabetic retinopathy. Future research is required to fully determine the presumed effect of fasudil on diabetic retinopathy.
Acknowledgments
Foundation: Supported by the Scientific Research Project Coordination Unit of Abant Izzet Baysal University.
Conflicts of Interest: Çelik F, None; Ulaş F, None; Özünal ZG, None; Fırat T, None; Çelebi S, None; Doğan Ü, None.
REFERENCES
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Arita R, Hata Y, Ishibashi T. ROCK as a therapeutic target of diabetic retinopathy. J Ophthalmol. 2010;2010:175163. doi: 10.1155/2010/175163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 6.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 7.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 9.Arevalo JF, Garcia-Amaris RA, Roca JA, Sanchez JG, Wu L, Berrocal MH, Maia M. Primary intravitreal bevacizumab for the management of pseudophakic cystoid macular edema: pilot study of the Pan-American Collaborative Retina Study Group. J Cataract Refract Surg. 2007;33(12):2098–2105. doi: 10.1016/j.jcrs.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 10.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26(3):275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Lin CI, Liao JJ, Lee YW, Yang HY, Lee CY, Hsu HY, Wu HL. Lysophospholipids increase ICAM-1 expression in HUVEC through a Gi- and NF-kappaB-dependent mechanism. Am J Physiol Cell Physiol. 2004;287(6):C1657–1666. doi: 10.1152/ajpcell.00172.2004. [DOI] [PubMed] [Google Scholar]
- 12.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145(6):1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giagulli C, Scarpini E, Ottoboni L, Narumiya S, Butcher EC, Constantin G, Laudanna C. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity. 2004;20(1):25–35. doi: 10.1016/s1074-7613(03)00350-9. [DOI] [PubMed] [Google Scholar]
- 14.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157(7):1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay DJ, Esch F, Furthmayr H, Hall A. Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138(4):927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28(6):296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Gojo A, Utsunomiya K, Taniguchi K, Yokota T, Ishizawa S, Kanazawa Y, Kurata H, Tajima N. The Rho-kinase inhibitor, fasudil, attenuates diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2007;568(1–3):242–247. doi: 10.1016/j.ejphar.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Hata Y, Miura M, Nakao S, Kawahara S, Kita T, Ishibashi T. Antiangiogenic properties of fasudil, a potent Rho-Kinase inhibitor. Jpn J Ophthalmol. 2008;52(1):16–23. doi: 10.1007/s10384-007-0487-5. [DOI] [PubMed] [Google Scholar]
- 19.Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58(1):215–226. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 21.Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001;276(35):32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- 22.Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17(3):463–471. doi: 10.1017/s0952523800173122. [DOI] [PubMed] [Google Scholar]
- 23.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. FASEB J. 2002;16(11):1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- 24.Grover JK, Vats V, Rathi SS, Dawar R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J Ethnopharmacol. 2001;76(3):233–238. doi: 10.1016/s0378-8741(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Zhu T, Tang X, Ye P, Zhang Z. Effect of an intravitreal injection of bevacizumab on the expression of VEGF and CD34 in the retina of diabetic rats. Clin Experiment Ophthalmol. 2010;38(9):875–884. doi: 10.1111/j.1442-9071.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- 27.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22(24):8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossig L, Haendeler J, Hermann C, Malchow P, Urbich C, Zeiher AM, Dimmeler S. Nitric oxide down-regulates MKP-3 mRNA levels: involvement in endothelial cell protection from apoptosis. J Biol Chem. 2000;275(33):25502–25507. doi: 10.1074/jbc.M002283200. [DOI] [PubMed] [Google Scholar]
- 29.Zhu F, Zhang Z, Wu G, Li Z, Zhang R, Ren J, Nong L. Rho kinase inhibitor fasudil suppresses migration and invasion though down-regulating the expression of VEGF in lung cancer cell line A549. Med Oncol. 2011;28(2):565–571. doi: 10.1007/s12032-010-9468-5. [DOI] [PubMed] [Google Scholar]
- 30.Kuno M, Takai S, Matsushima-Nishiwaki R, Minamitani C, Mizutani J, Otsuka T, Harada A, Adachi S, Kozawa O, Tokuda H. Rho-kinase inhibitors decrease TGF-beta-stimulated VEGF synthesis through stress-activated protein kinase/c-Jun N-terminal kinase in osteoblasts. Biochem Pharmacol. 2009;77(2):196–203. doi: 10.1016/j.bcp.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172(5):1411–1418. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limb GA, Chignell AH, Green W, LeRoy F, Dumonde DC. Distribution of TNF alpha and its reactive vascular adhesion molecules in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol. 1996;80(2):168–173. doi: 10.1136/bjo.80.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma DW, Wang QY, Ma XY, Li J, Guan QH, Fu Y. The effect of fasudil via Rho/ROCK signaling pathway on the inflammation and fibrosis in human mesangial cells in high glucose medium. Zhonghua Neike Zazhi. 2011;50(7):580–584. [PubMed] [Google Scholar]
- 34.Li YH, Zhuo YH, Lu L, Chen LY, Huang XH, Zhang JL, Li SY, Wang XG. Caspase-dependent retinal ganglion cell apoptosis in the rat model of acute diabetes. Chin Med J (Engl) 2008;121(24):2566–2571. [PubMed] [Google Scholar]
- 35.Iandiev I, Francke M, Makarov F, Hollborn M, Uhlmann S, Wurm A, Savvinov A, Kohen L, Reichenbach A, Wiedemann P, Pannicke T, Bringmann A. Effects of intravitreal bevacizumab (Avastin) on the porcine retina. Graefes Arch Clin Exp Ophthalmol. 2011;249(12):1821–1829. doi: 10.1007/s00417-011-1773-y. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda H, Kume Y, Tejima K, Tomiya T, Nishikawa T, Watanabe N, Ohtomo N, Arai M, Arai C, Omata M, Fujiwara K, Yatomi Y. Rho-kinase inhibitor prevents hepatocyte damage in acute liver injury induced by carbon tetrachloride in rats. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G911–917. doi: 10.1152/ajpgi.00210.2007. [DOI] [PubMed] [Google Scholar]