Abstract
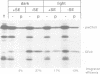
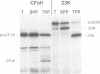
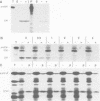
The CFoII subunit of the ATP synthase is an integral component of the thylakoid membrane which is synthesized in the cytosol with a bipartite, lumen-targeting presequence similar in structural terms to those of imported lumenal proteins such as plastocyanin. This presequence is shown to possess a terminal cleavage site for the thylakoidal processing peptidase, but no intermediate site for the stromal processing peptidase. The integration of CFoII into the thylakoid membrane of Pisum sativum has been analysed using in vitro assays for the import of proteins into intact chloroplasts or isolated thylakoids. Efficient integration into thylakoids is observed in the light and dark, and the integration process does not require the presence of either stromal extracts or nucleoside triphosphates. The uncoupler nigericin inhibits integration only very slightly, indicating that the thylakoidal delta pH does not play a significant role in the integration mechanism. In each of these respects, the requirements for CFoII integration differ notably from those determined for integration of the light-harvesting chlorophyll-binding protein of photosystem II. The integration mechanism also differs significantly from the two mechanisms involved in the translocation of lumenal proteins across the thylakoid membrane, since one of these processes requires the presence of stromal protein factors and ATP, and the other mechanism is dependent on the thylakoidal delta pH. This conclusion is reinforced by the finding that saturation of the translocation system for the precursor to the lumenal 23 kDa oxygen-evolving complex protein does not affect integration of CFoII into thylakoids.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


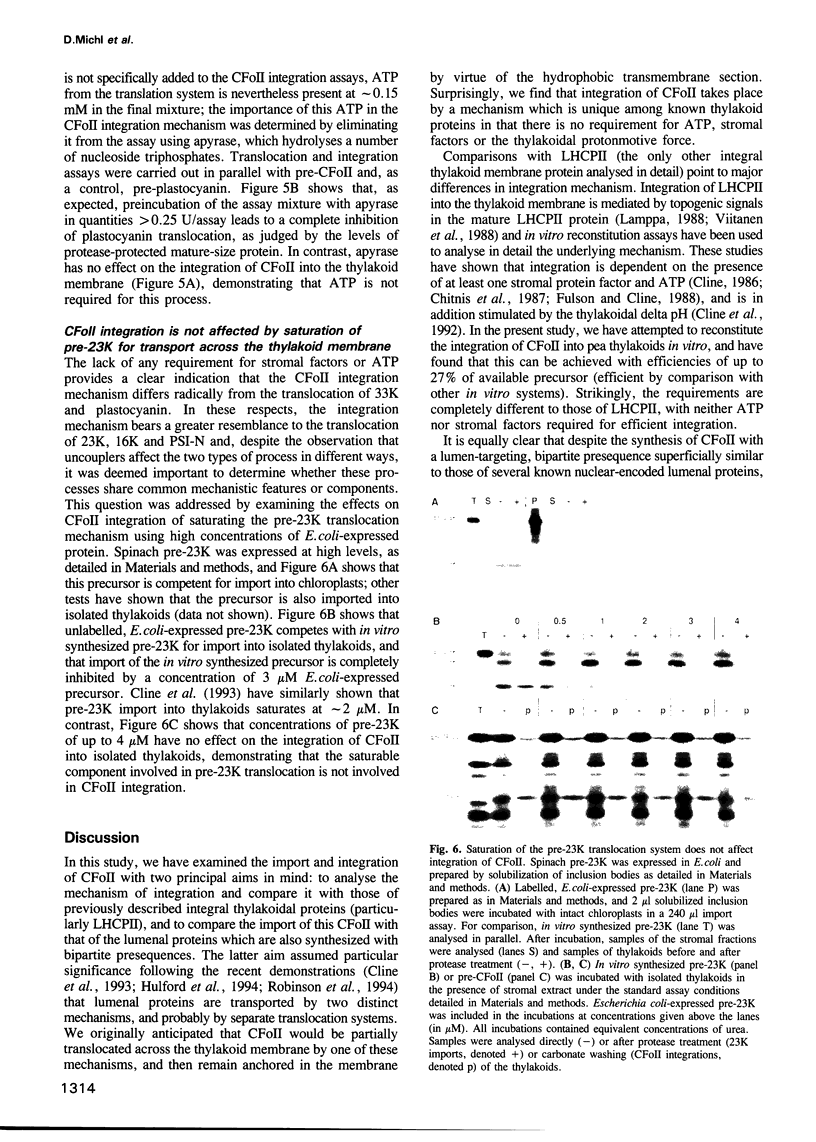
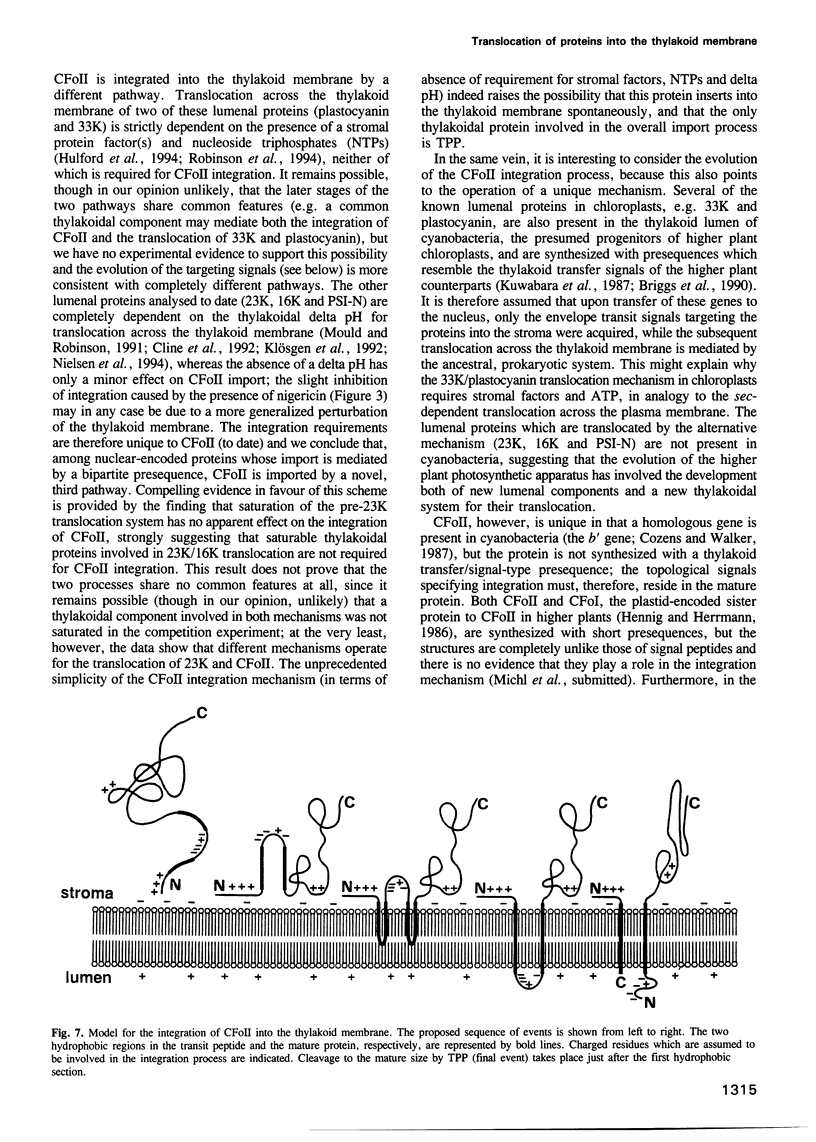


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs L. M., Pecoraro V. L., McIntosh L. Copper-induced expression, cloning, and regulatory studies of the plastocyanin gene from the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol Biol. 1990 Oct;15(4):633–642. doi: 10.1007/BF00017837. [DOI] [PubMed] [Google Scholar]
- Clausmeyer S., Klösgen R. B., Herrmann R. G. Protein import into chloroplasts. The hydrophilic lumenal proteins exhibit unexpected import and sorting specificities in spite of structurally conserved transit peptides. J Biol Chem. 1993 Jul 5;268(19):13869–13876. [PubMed] [Google Scholar]
- Cline K., Ettinger W. F., Theg S. M. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992 Feb 5;267(4):2688–2696. [PubMed] [Google Scholar]
- Cline K., Henry R., Li C., Yuan J. Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J. 1993 Nov;12(11):4105–4114. doi: 10.1002/j.1460-2075.1993.tb06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K. Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem. 1986 Nov 5;261(31):14804–14810. [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Fulson D. R., Cline K. A Soluble Protein Factor is Required in Vitro for Membrane Insertion of the Thylakoid Precursor Protein, pLHCP. Plant Physiol. 1988 Dec;88(4):1146–1153. doi: 10.1104/pp.88.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R. G., Steppuhn J., Herrmann G. S., Nelson N. The nuclear-encoded polypeptide Cfo-II from spinach is a real, ninth subunit of chloroplast ATP synthase. FEBS Lett. 1993 Jul 12;326(1-3):192–198. doi: 10.1016/0014-5793(93)81789-3. [DOI] [PubMed] [Google Scholar]
- James H. E., Bartling D., Musgrove J. E., Kirwin P. M., Herrmann R. G., Robinson C. Transport of proteins into chloroplasts. Import and maturation of precursors to the 33-, 23-, and 16-kDa proteins of the photosynthetic oxygen-evolving complex. J Biol Chem. 1989 Nov 25;264(33):19573–19576. [PubMed] [Google Scholar]
- Kirwin P. M., Elderfield P. D., Williams R. S., Robinson C. Transport of proteins into chloroplasts. Organization, orientation, and lateral distribution of the plastocyanin processing peptidase in the thylakoid network. J Biol Chem. 1988 Dec 5;263(34):18128–18132. [PubMed] [Google Scholar]
- Klösgen R. B., Brock I. W., Herrmann R. G., Robinson C. Proton gradient-driven import of the 16 kDa oxygen-evolving complex protein as the full precursor protein by isolated thylakoids. Plant Mol Biol. 1992 Mar;18(5):1031–1034. doi: 10.1007/BF00019226. [DOI] [PubMed] [Google Scholar]
- Ko K., Cashmore A. R. Targeting of proteins to the thylakoid lumen by the bipartite transit peptide of the 33 kd oxygen-evolving protein. EMBO J. 1989 Nov;8(11):3187–3194. doi: 10.1002/j.1460-2075.1989.tb08477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A., Wickner W., Kreil G. The cytoplasmic carboxy terminus of M13 procoat is required for the membrane insertion of its central domain. Nature. 1986 Jul 24;322(6077):335–339. doi: 10.1038/322335a0. [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Reddy K. J., Sherman L. A. Nucleotide sequence of the gene from the cyanobacterium Anacystis nidulans R2 encoding the Mn-stabilizing protein involved in photosystem II water oxidation. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8230–8234. doi: 10.1073/pnas.84.23.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G. K. The chlorophyll a/b-binding protein inserts into the thylakoids independent of its cognate transit peptide. J Biol Chem. 1988 Oct 15;263(29):14996–14999. [PubMed] [Google Scholar]
- Mould R. M., Robinson C. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J Biol Chem. 1991 Jul 5;266(19):12189–12193. [PubMed] [Google Scholar]
- Mould R. M., Shackleton J. B., Robinson C. Transport of proteins into chloroplasts. Requirements for the efficient import of two lumenal oxygen-evolving complex proteins into isolated thylakoids. J Biol Chem. 1991 Sep 15;266(26):17286–17289. [PubMed] [Google Scholar]
- Niesbach-Klösgen U., Guilley H., Jonard G., Richards K. Immunodetection in vivo of beet necrotic yellow vein virus-encoded proteins. Virology. 1990 Sep;178(1):52–61. doi: 10.1016/0042-6822(90)90378-5. [DOI] [PubMed] [Google Scholar]
- Pancic P. G., Strotmann H., Kowallik K. V. Chloroplast ATPase genes in the diatom Odontella sinensis reflect cyanobacterial characters in structure and arrangement. J Mol Biol. 1992 Mar 20;224(2):529–536. doi: 10.1016/0022-2836(92)91017-j. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Hartl F. U., Guiard B., Neupert W. Mitochondrial precursor proteins are imported through a hydrophilic membrane environment. Eur J Biochem. 1987 Dec 1;169(2):289–293. doi: 10.1111/j.1432-1033.1987.tb13610.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Cai D., Hulford A., Brock I. W., Michl D., Hazell L., Schmidt I., Herrmann R. G., Klösgen R. B. The presequence of a chimeric construct dictates which of two mechanisms are utilized for translocation across the thylakoid membrane: evidence for the existence of two distinct translocation systems. EMBO J. 1994 Jan 15;13(2):279–285. doi: 10.1002/j.1460-2075.1994.tb06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Theg S. M., Bauerle C., Olsen L. J., Selman B. R., Keegstra K. Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes. J Biol Chem. 1989 Apr 25;264(12):6730–6736. [PubMed] [Google Scholar]
- Viitanen P. V., Doran E. R., Dunsmuir P. What is the role of the transit peptide in thylakoid integration of the light-harvesting chlorophyll a/b protein? J Biol Chem. 1988 Oct 15;263(29):15000–15007. [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]