Abstract
AIM
To compare the efficacy of ranibizumab and bevacizumab for macular edema due to retinal vein occlusion (RVO).
METHODS
A retrospective study was conducted at a single academic institution. Eighty-one patients naïve to anti-VEGF therapy with RVO and macular edema were identified. Twenty-six eyes were treated with ranibizumab, 33 eyes with bevacizumab, and 22 eyes with bevacizumab then switched to ranibizumab (crossover). The main outcome was change in visual acuity at 3 months, 6 months, and final visit.
RESULTS
The mean visual acuity improved from 20/80 to 20/40 in the ranibizumab (R) group and from 20/125 to 20/60 in the bevacizumab (B) group (P=0.66). The mean change in central subfield thickness (CST) was -186 and -212µm, respectively (P=0.69). Mean time between injections was 94±21.1d in the R group and 103.8±10.5d in the B group (P=0.78). In the crossover group, mean initial visual acuity was 20/125, reached 20/60 at crossover, and remained 20/60 at conclusion (P=0.91).
CONCLUSION
Both ranibizumab and bevacizumab are effective for the treatment of RVO and appear to have similar visual and anatomic outcomes. Changing treatments from bevacizumab to ranibizumab did not result in further gains in visual acuity.
Keywords: macular edema, retinal vein occlusion, bevacizumab, ranibizumab, optical coherence tomography
INTRODUCTION
Retinal vein occlusions (RVOs) are the second most common form of retinal vascular disease with an estimated 15-year cumulative incidence in adults of 2.3%[1]. Complications from RVOs resulting in vision loss include macular edema, macular ischemia, and sequelae from neovascularization. The Collaborative Branch Vein Occlusion Study (BVOS) reported that grid argon laser photocoagulation was useful in the treatment of macular edema from branch RVO (BRVO), but the Central Vein Occlusion Study (CVOS) did not show a similar benefit in central RVO (CRVO)[2],[3]. More recent studies employing intravitreal injection of steroids have shown a benefit in patients with CRVO as well as BRVO[4]-[6]. Steroid formulations however have side effects such as elevated intraocular pressure and cataract formation. Vascular endothelial growth factor (VEGF) inhibitors have a more favorable safety profile and have been widely used for the treatment of age related macular degeneration. Several studies have shown that they are also effective for treating macular edema in RVOs. Ranibizumab was shown in several randomized prospective trials to be effective and was the first VEGF inhibitor to be FDA approved for use in RVOs[7]-[10]. Bevacizumab has also been shown to be effective in multiple trials and is currently being used off-label[11]-[15]. Although the recent Comparison of AMD Treatment Trials (CATT) study directly compared the efficacy of ranibizumab with bevacizumab for neovascular age-related macular degeneration, similar comparative studies in the setting of RVOs are lacking[16],[17]. The aim of this retrospective study is to compare the effectiveness of VEGF inhibitors in treating RVOs at a single center.
SUBJECTS AND METHODS
The principles of the Declaration of Helsinski were followed and Institutional Review Board approval was obtained from the Cleveland Clinic for this retrospective, comparative study. Patients were identified who received initial treatment between March 2008 and January 2012 at the Cole Eye Institute. Patients were included in the study if they met the following inclusion criteria: concurrent diagnoses based on ICD-9 codes of macular edema (362.53 or 362.83) and central/branch vein occlusion (362.35, 362.36, or 362.37) and treatment with anti-VEGF therapy. Exclusion criteria included duration of follow-up less than 120d (n=22), unknown onset of symptoms (n=3), the presence of active confounding retinal or ocular disease (e.g. diabetic retinopathy, exudative macular degeneration, pseudophakic macular edema) (n=11), or prior vision loss not due to RVO (e.g. trauma, keratoprosthesis and macular hole) (n=3). Patients were also excluded if they switched between the use of bevacizumab and ranibizumab more than once during follow up (n=4) or if they had previously been treated with anti-VEGF injections in the study eye (n=2). There were a total of 126 eyes identified, of which 45 were excluded. The 81 remaining eyes were divided into three groups consisting of patients who received ranibizumab for the treatment of macular edema (n=26), bevacizumab (n=33), and a crossover group that initially received bevacizumab and then switched to ranibizumab (n=22). Treatment was based on an as needed treatment protocol under the care of 7 retina specialists based on comprehensive ophthalmic examination and optical coherence tomography (OCT). Patients were followed at 4 to 6 week intervals and retreated based on OCT findings of persistent or recurrent intraretinal or subretinal fluid at the treating physician's discretion. Clinical variables including Snellen visual acuity, intraocular pressure, number of injections, and follow-up duration were recorded and analyzed. Cirrus HD-OCT (Carl Zeiss Meditec) parameters including central subfield thickness (CST) and presence of cystoid macular edema and/or subretinal fluid, measured from the 5 line raster scans, were recorded from each visit when available. If OCT data was missing on the final visit, the last observation carried forward method was used. Snellen visual acuity was converted to logMAR equivalent to facilitate statistical comparisons. Student's t-test, ANOVA, or Chi-square/Fisher's exact test were used where appropriate to compare visual acuity, OCT parameters, and clinical variables between the treatment groups. Standard errors are reported using pooled estimates of error variance. Post-hoc testing using the Tukey-Kramer HSD was applied where multiple comparisons were made; a significance level of P<0.05 was considered statistically significant.
RESULTS
Baseline Demographics and Clinical Characteristics
Patient baseline characteristics were relatively balanced between all three treatment groups as shown in Table 1. There was, however, a significant difference in follow-up duration between the three groups (P=0.007, ANOVA). Mean follow-up from initial treatment was 453d for the ranibizumab group (R), 423d for the bevacizumab group (B), and 638d for the crossover group (C). The R and B groups had significantly shorter follow-up compared to the C group (P=0.04 and P=0.007, respectively, Tukey HSD). There was no difference in follow-up duration between the R and B groups (P=0.89, Tukey HSD).
Table 1. Baseline variables.
| Parameters | Ranibizumab (n=26) | Bevacizumab (n=33) | Crossover (n=22) | P |
| Age (a) | 63.7±2.9 | 69.8±2.2 | 68.8±2.7 | 0.19 |
| Time to treatment (d) | 309.9±144.3 | 91.7±58.9 | 202.0±72.1 | 0.22 |
| logMAR acuity (Snellen equivalent) | 0.63±0.09 (20/80) | 0.77±0.08 (20/125) | 0.76±0.10 (20/125) | 0.51 |
| IOP (mmHg) | 16.8±0.7 | 16.6±0.6 | 16.8±0.8 | 0.98 |
| Follow-up (d) | 452.8±51.8 | 422.5±41.3 | 637.9±50.6 | 10.007 |
| Follow-up (range) | 140–1036 | 126–896 | 272–1078 | |
| Central subfield (µm) | 480±34 (n=23) | 524±33 (n=27) | 500±40 (n=18) | 0.66 |
| Cystoid macular edema | 21/22 (95%) | 25/27 (93%) | 18/18 (100%) | 0.50 |
| Subretinal fluid | 9/22 (41%) | 10/27 (37%) | 9/18 (50%) | 0.68 |
| CRVO | 13/26 (50%) | 13/33 (40%) | 8/22 (36%) | 0.60 |
| BRVO | 12/26 (46%) | 15/33 (45%) | 12/22 (55%) | 0.60 |
| HRVO | 1/26 (4%) | 5/33 (15%) | 2/22 (9%) | 0.60 |
| Diabetes | 6/26 (23%) | 12/33 (36%) | 6/22 (30%) | 0.52 |
| Hypertension | 21/26 (81%) | 27/33 (82%) | 12/22 (60%) | 0.05 |
| Glaucoma | 5 /26 (19%) | 11/33 (33%) | 3/22 (15%) | 0.20 |
1Statistically significant. There was no difference between the ranibizumab and bevacizumab groups, however there was a difference between the crossover group and the single treatment groups. See text for posthoc Tukey HSD test results. CRVO: Central retinal vein occlusion; BRVO: Branch retinal vein occlusion; HRVO: Hemiretinal vein occlusion; IOP: Intraocular pressure. The mean±SE is shown.
Comparison Between the Ranibizumab and Bevacizumab Groups
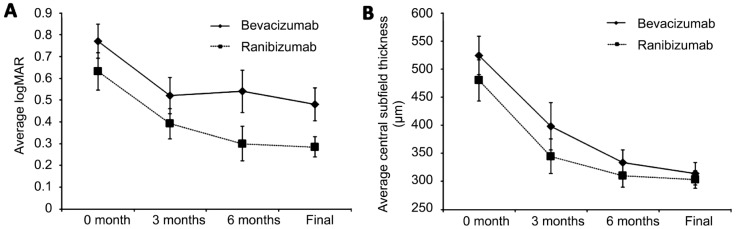
The mean baseline visual acuity was 20/80 or logMAR 0.63 in the R group and 20/125 or logMAR 0.77 in the B group (P=0.51). Both groups showed a significant mean change in logMAR vision of -0.35 (P<0.001, paired t-test) and -0.29 (P=0.008, paired t-test), corresponding to an improvement of 3.5 and 2.9 lines in the R and B groups, respectively. The mean final visual acuity was significantly improved to 20/40 or logMAR 0.28 in the R group and 20/60 or logMAR 0.48 in the B group (Figure 1). However, there was no significant difference in the change in visual acuity between the R and B groups (P=0.66, Table 2). The number of eyes gaining ≥3 lines or losing ≥3 lines were similar between the two groups (Table 2). Greater than 3 lines of vision improvement was seen in 50% of the R group and 48% of the B group (P=0.91). Reduction of 3 lines of vision was seen in 0% of the R group and in 6% of the B group (P=0.20).
Figure 1. Visual acuity and macular thickness improves after treatment with bevacizumab or ranibizumab.
A: The mean logMAR visual acuity decreases (improves) after treatment with bevacizumab or ranibizumab. There are no significant differences between the two groups; B: The mean central subfield thickness measured on SD-OCT also decreases with treatment with no significant differences between the two groups. Mean±SEM.
Table 2. Comparison between ranibizumab and bevacizumab groups.
| Parameters | Ranibizumab (n=26) | Bevacizumab (n=33) | P |
| Final logMAR (Snellen equivalent) | 0.28±0.24 (20/40) | 0.48±0.08 (20/60) | 0.07 |
| logMAR change | -0.35±0.08 | -0.29±0.09 | 0.66 |
| Equivalent lines/ETDRS letters gained | 3.5 lines/17.5 ETDRS letters | 2.9 lines/14.5 ETDRS letters | |
| Eyes gaining >3 lines | 13/26 (50%) | 16/33 (48%) | 0.91 |
| Eyes losing >3 lines | 0/26 | 2/33 (6%) | 0.20 |
| Central subfield (µm) change | -186±38 (n=22) | -212±40 (n=24) | 0.69 |
| Residual intraretinal fluid | 17/26 (65%) | 13/28 (46%) | 0.16 |
| Residual subretinal fluid | 0/26 | 4/28 (14%) | 10.14 |
| IOP (mmHg) change | 0.94±0.96 | -0.52±0.67 | 0.22 |
| Days between injections | 94±21.1 | 103.8±10.5 | 0.78 |
IOP: Intraocular pressure. 1Yate's correction was used due to low expected frequencies. The mean±SE is shown.
There was a significant improvement in central subfield thickness on OCT in the R group (-186µm, P<0.001) and B group (-212, P=0.008), however there was no difference in improvement between these groups (P=0.67) (Table 2). The mean final CST improved to 303µm in the R group (n=26) and 314µm in the B group (n=28) (Figure 1). There were no significant differences in residual cystoid macular edema (CME) (65% R vs 46% B, P=0.16) or subretinal fluid (SRF) (0% R vs 14% B, P=0.14) between the two groups.
There were no significant differences between the injection intervals between the R group with a mean of 94d compared with 104d in the B group (P=0.78, Table 2). Table 2 summarizes the results of the R and B groups.
Crossover Group
Patients in the crossover group were initially treated with bevacizumab and then switched to ranibizumab treatment. Patients initially received a mean of 5.7 bevacizumab injections over a mean duration of 302.5d, followed by a mean of 6.5 ranibizumab injections over a mean duration of 335.4d (Table 3). The mean difference in the number of injections after crossover was 0.77±0.95 (P=0.42, paired t-test) which was not significantly different from before crossover. There was a mean interval of 51.9±8.5d between the end of bevacizumab therapy and the initiation of ranibizumab. There was no significant difference between injection intervals before and after crossover (Table 3).
Table 3. Comparison of crossover treatment from bevacizumab to ranibizumab.
| Parameters | Before crossover bevacizumab (n=22) | After crossover ranibizumab (n=22) | P |
| Final logMAR (Snellen equivalent) | 0.52±0.09 (20/60) | 0.51=0.09 (20/60) | 0.91 |
| logMAR change | -0.24±0.07 | -0.02=0.07 | 10.04 |
| Equivalent lines/ETDRS letters gained | 2.4 lines/12 ETDRS letters | 0.2 lines/1 ETDRS letter | |
| Eyes gaining >3 lines | 9/22 (41%) | 4/22 (18%) | 0.10 |
| Eyes losing >3 lines | 2/22 (9%) | 2/22 (9%) | 1.00 |
| Central subfield (µm) change | -7±54 (n=17) | -72=49 (n=21) | 0.38 |
| Residual intraretinal fluid | 20/21 (95%) | 15/21 (71%) | 10.04 |
| Residual subretinal fluid | 7/21 (33%) | 9/21 (43%) | 0.53 |
| IOP (mmHg) change | 0.82=0.92 | -0.55=0.92 | 0.30 |
| Total injections | 5.7=0.84 | 6.5=0.84 | 0.52 |
| Days to final follow-up2 | 302.5=41.9 | 335.4=41.9 | 0.58 |
| Days between injections | 62.0=15.8 | 72.0=15.8 | 0.66 |
1Statistically significant; 2Prior to crossover, defined as the visit when a change in treatment was initiated; IOP: Intraocular pressure.
Of the 22 eyes in this group, 9 eyes (41%) showed ≥3 lines of visual improvement with bevacizumab before crossover, while 4 eyes (18%) showed improvement after switching to ranibizumab (P=0.10). Visual decrease ≥3 lines was noted in 2 eyes (9%) before and after crossover treatment (P=1.00).
The mean baseline Snellen visual acuity was 20/125. After initial treatment with bevacizumab, the mean visual acuity significantly improved to 20/60, corresponding to a mean logMAR change of -0.24 or -2.4 lines (P=0.01, paired t-test). Despite an improvement in vision, the mean change in CST was only -7µm (P=0.90, paired t-test). In this cohort, the baseline CME rate was 18/18 (100%), which did not significantly change at the crossover point where 20/21 (95%) still had CME. The baseline SRF rate of 8/18 (44%) decreased slightly to 7/21 (33%) at the crossover point. The mean follow-up prior to crossover was 302.5d (Table 3).
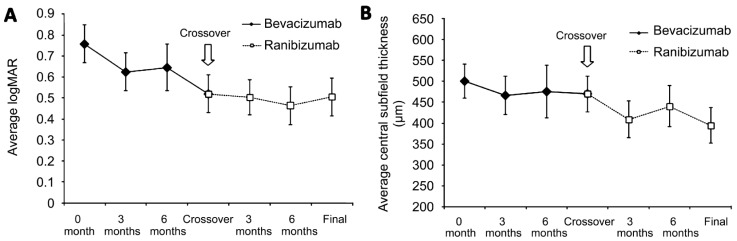
After switching treatments to ranibizumab, the visual acuity remained 20/60, corresponding to a mean logMAR change of -0.02 or -0.2 lines (P=0.73, paired t-test) and the mean change in CST was -72µm (P=0.16, paired t-test). Interestingly, the final rate of CME was 15/21 (71%), which is significantly lower (P=0.04) than before crossover (95%). The final rate of SRF was 9/21 (43%), which was not significantly different from before crossover (P=0.53). The mean follow-up after crossover treatment to ranibizumab was 335.4d. Table 3 summarizes the results of the crossover group and Figure 2 shows the change in visual acuity and CST before and after crossover.
Figure 2. Switching from bevacizumab to ranibizumab does not provide further improvements in visual acuity or macular thickness.
A: The mean logMAR visual acuity decreases after treatment with bevacizumab up to the crossover point shown by the arrow. After switching treatments to ranibizumab, no further change in logMAR visual acuity is seen; B: There is minimal change in central subfield thickness after treatment with bevacizumab. After switching to ranibizumab, there is some decrease in macular thickness, but it does not reach statistical significance. Mean±SEM.
DISCUSSION
Both intravitreal ranibizumab and bevacizumab are known to be effective in reducing macular edema secondary to RVOs. More recently, aflibercept dosed monthly has also been shown to be effective for CRVOs[18]. In our retrospective study, functional and anatomical outcomes were similar without a statistically significant difference in visual acuity improvement or macular thickness on OCT. There was a trend favoring ranibizumab at 6 months and also at the final visit, however this did not reach statistical significance. Both groups showed similar improvements in CST, rate of CME, and SRF (Table 2). Similar to CATT, there were no significant differences in visual outcomes between the B and R groups. Unlike CATT however, the reduction in macular thickness was similar between the two drugs, although the incidence of any intraretinal fluid at the final visit was higher in the R group compared to the B group[16],[17].
The in vivo half-life of intravitreal bevacizumab is longer than the half-life of ranibizumab, measured from several animal models[19]-[21]. In rabbits, the vitreous half-life of bevacizumab was 4.32d and for ranibizumab, it was 2.88d[19]. In humans, the half-life of bevacizumab was 6.7d[22]. The recommended dosing interval for ranibizumab for RVO is 4 weeks based on the BRAVO and CRUISE studies[7],[8]. The ideal interval for bevacizumab is unknown. Some clinicians extend this interval for bevacizumab to 6 weeks based on its longer measured half-life. Several studies have demonstrated that the VEGF load resulting from retinal vein occlusion is higher than both proliferative diabetic retinopathy and age-related macular degeneration[23],[24]. It is possible this higher VEGF load in retinal vein occlusion may uncover differences of anti-VEGF medications that may not be apparent in treatment of age-related macular degeneration or diabetic retinopathy. In our study, however, functional and anatomical outcomes were similar between the two treatment groups.
Changes in anti-VEGF injection frequency for RVO has not been well studied. In one prospective study, it was demonstrated that during OCT-guided per required need (PRN) treatment, the mean time interval from previous injection before recurrence of macular edema resulting from RVO ranged from 1.2 to 2.4 months[25]. This correlates with the average time period between injections in our study. In CATT, the differences in visual outcomes between a monthly dosing regimen and a less frequent PRN regimen was minimal (approximately half a line) and would not be detected in our study due to a much smaller sample size[17]. In our study, the time interval between injections depended on physician preference. Some patients were treated on a PRN basis and some monthly.
Although switching to ranibizumab improved anatomic results with decreased cystoid macular edema and a trend towards decreased macular thickness, the functional change was not significant. There was no strict crossover algorithm and some physicians might have switched based on patient preference; but presumably these patients were not responding well to bevacizumab, which likely portends a worse clinical responsiveness to any VEGF inhibitor, including ranibizumab.
The strengths of this study include the strict adherence to monotherapy in the ranibizumab and bevacizumab arms, treatment naïve patients, the strict exclusion of concurrent ocular disease, and the analysis of anatomic factors such as CME and SRF on OCT. There was also good balance in the baseline parameters between the three groups. The limitations of this study include a small sample size and its retrospective nature. The small sample size precluded meaningful analysis of patients separated by the type of RVO (central vs branch). Other limitations include missing OCT data from some of the visit dates, although OCT imaging was standard of care during the enrollment period and missing data was rare. When OCT data was missing from follow-up examinations, the last visit carried forward method was used. The retrospective nature of this study introduces several biases particularly related to selection of ranibizumab vs bevacizumab, as well as the reason for crossover. For example patients who responded well to bevacizumab would likely not have been switched to ranibizumab and would have been excluded from that group. Physician selection and treatment style also introduce bias particularly related to frequency of injections.
Our study suggests that differences between bevacizumab and ranibizumab for the treatment of macular edema in RVO are small and that the efficacy of these two medications is similar with regard to visual and anatomic outcomes. Larger, prospective studies will be needed to validate these findings.
Acknowledgments
Conflicts of Interest: Yuan A, None; Ahmad BU, None; Xu D, None; Martin DF, None; Sears JE, None; Schachat AP, None; Singh RP: consultant for Thrombogenics, Regeneron, Alcon, and Bausch and Lomb; Kaiser PK: consultant for Bayer, Regeneron, Genentech, Kanghong, Novartis, and Alcon; Ehlers JP: speaker for Regeneron, consultant and speaker for Thrombogenics.
REFERENCES
- 1.Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the beaver dam eye study. Arch Ophthalmol. 2008;126(4):513–518. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 2.The Branch Vein Occlusion Study Group Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98(3):271–282. doi: 10.1016/0002-9394(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 3.The Central Vein Occlusion Study Group M Report Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology. 1995;102(10):1425–1433. doi: 10.1016/s0161-6420(95)30849-4. [DOI] [PubMed] [Google Scholar]
- 4.Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, Chan CK, Gonzalez VH, Singerman LJ, Tolentino M, SCORE Study Research Group A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115–1128. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jacques ML, Jiao J, Li XY, Whitcup SM, OZURDEX GENEVA Study Group Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117(6) 2010;117(6):1134, 1146.e3. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, Singerman LJ, Tolentino M, Chan CK, Gonzalez VH, SCORE Study Research Group A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127(9):1101–1114. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, Adamis AP, Rubio RG, Murahashi WY. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594–1602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, Murahashi WY, Rubio RG. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041–2049. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 9.Spaide RF, Chang LK, Klancnik JM, Yannuzzi LA, Sorenson J, Slakter JS, Freund KB, Klein R. Prospective study of intravitreal ranibizumab as a treatment for decreased visual acuity secondary to central retinal vein occlusion. Am J Ophthalmol. 2009;147(2):298–306. doi: 10.1016/j.ajo.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Pieramici DJ, Rabena M, Castellarin AA, Nasir M, See R, Norton T, Sanchez A, Risard S, Avery RL. Ranibizumab for the treatment of macular edema associated with perfused central retinal vein occlusions. Ophthalmology. 2008;115(10):e47–54. doi: 10.1016/j.ophtha.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa MS, Contreras I, Noval S, Arruabarrena C. Results of bevacizumab as the primary treatment for retinal vein occlusions. Br J Ophthalmol. 2010;94(8):1052–1056. doi: 10.1136/bjo.2009.173732. [DOI] [PubMed] [Google Scholar]
- 12.Gregori NZ, Rattan GH, Rosenfeld PJ, Puliafito CA, Feuer W, Flynn HW, Jr, Berrocal AM, Al-Attar L, Dubovy S, Smiddy WE, Schwartz SG, Lee WH, Murray TG. Safety and efficacy of intravitreal bevacizumab (avastin) for the management of branch and hemiretinal vein occlusion. Retina. 2009;29(7):913–925. doi: 10.1097/IAE.0b013e3181aa8dfe. [DOI] [PubMed] [Google Scholar]
- 13.Jaissle GB, Leitritz M, Gelisken F, Ziemssen F, Bartz-Schmidt KU, Szurman P. One-year results after intravitreal bevacizumab therapy for macular edema secondary to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2009;247(1):27–33. doi: 10.1007/s00417-008-0916-2. [DOI] [PubMed] [Google Scholar]
- 14.Wu L, Arevalo JF, Roca JA, Maia M, Berrocal MH, Rodriguez FJ, Evans T, Costa RA, Cardillo J, Pan-American Collaborative Retina Study Group (PACORES) Comparison of two doses of intravitreal bevacizumab (avastin) for treatment of macular edema secondary to branch retinal vein occlusion: Results from the pan-american collaborative retina study group at 6 months of follow-up. Retina. 2008;28(2):212–219. doi: 10.1097/IAE.0b013e3181619bee. [DOI] [PubMed] [Google Scholar]
- 15.Costa RA, Jorge R, Calucci D, Melo LA, Jr, Cardillo JA, Scott IU. Intravitreal bevacizumab (avastin) for central and hemicentral retinal vein occlusions: IBeVO study. Retina. 2007;27(2):141–149. doi: 10.1097/IAE.0b013e31802eff83. [DOI] [PubMed] [Google Scholar]
- 16.CATT Research Group. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL, 3rd, Comparison f Age-related Macular Degeneration Treatments Trials (CATT) Research Group Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 18.Boyer D, Heier J, Brown DM, Clark WL, Vitti R, Berliner AJ, Groetzbach G, Zeitz O, Sandbrink R, Zhu X, Beckmann K, Haller JA. Vascular endothelial growth factor trap-eye for macular edema secondary to central retinal vein occlusion: Six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119(5):1024–1032. doi: 10.1016/j.ophtha.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (lucentis) Ophthalmology. 2007;114(12):2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (avastin) Ophthalmology. 2007;114(5):855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46(2):726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Q, Ziemssen F, Henke-Fahle S, Tatar O, Szurman P, Aisenbrey S, Schneiderhan-Marra N, Xu X, Tubingen Bevacizumab Study Group. Grisanti S. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115(10):1750–1755, 1755.e1. doi: 10.1016/j.ophtha.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. 2011;55(3):256–263. doi: 10.1007/s10384-011-0004-8. [DOI] [PubMed] [Google Scholar]
- 24.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 25.Karagiannis DA, Karampelas MD, Soumplis VM, Amariotakis C, Georgalas I, Kandarakis A. Recurrence of macular edema in retinal vein occlusions after treatment with intravitreal ranibizumab (lucentis) Can J Ophthalmol. 2011;46(6):486–490. doi: 10.1016/j.jcjo.2011.09.014. [DOI] [PubMed] [Google Scholar]