Abstract
AIM
To determine healthcare resource utilization and the economic burden associated with wet age-related macular degeneration (AMD) in Thailand
METHODS
This study included patients diagnosed with wet AMD that were 60 years old or older, and had best corrected visual acuity (BCVA) measured at least two times during the follow-up period. We excluded patients having other eye diseases. Two separate sub-studies were conducted. The first sub-study was a retrospective cohort study; electronic medical charts were reviewed to estimate the direct medical costs. The second sub-study was a cross-sectional survey estimating the direct non-medical costs based on face-to-face interviews using a structured questionnaire. For the first sub-study, direct medical costs, including the cost of drugs, laboratory, procedures, and other treatments were obtained. For the second sub-study, direct non-medical costs, e.g. transportation, food, accessories, home renovation, and caregiver costs, were obtained from face-to-face interviews with patients and/or caregivers.
RESULTS
For the first sub-study, sixty-four medical records were reviewed. The annual average number of medical visits was 11.1±6.0. The average direct medical costs were $3 604±4 530 per year. No statistically-significant differences of the average direct medical costs among the BCVA groups were detected (P=0.98). Drug costs accounted for 77% of total direct medical costs. For direct non-medical costs, 67 patients were included. Forty-eight patients (71.6%) required the accompaniment of a person during the out-patient visit. Seventeen patients (25.4%) required a caregiver at home. The average direct non-medical cost was $2 927±6 560 per year. There were no statistically-significant differences in the average costs among the BCVA groups (P=0.74). Care-giver cost accounted for 87% of direct non-medical costs.
CONCLUSION
Our study indicates that wet AMD is associated with a substantial economic burden, especially concerning drug and care-giver costs.
Keywords: age-related macular degeneration, health resource utilization, costs, Thailand
INTRODUCTION
Age-related macular degeneration (AMD) is well-recognized as the leading cause of blindness in elderly populations in western countries[1]. Traditionally, AMD has been considered as an eye disease of Caucasian populations because the prevalence of AMD in these populations was higher than that of Asian populations[2],[3]. However, some studies have suggested that the prevalence of AMD in Asian populations was comparable to that reported in Caucasian populations[4]-[8]. These studies indicated that pooled prevalence estimates of early and late AMD in Asian populations were 6.8% and 0.56% respectively, while corresponding prevalence estimated in Caucasian populations were 8.8% and 0.59% respectively[9]. The prevalence of AMD in Thailand was lower than that in other Asian and Caucasian populations. Data from the national survey in 2006-2007 indicated that 2.7% and 0.3% of persons aged more than 50 years suffered from early and late AMD, respectively. These estimates were projected to approximately 20 000 patients with wet AMD in Thailand[10].
AMD contributes to 80%-85% of blindness[11]. The treatment of wet AMD has been recognized as one of the major breakthroughs in the treatment of eye disease in recent years. The outcome of the treatment of this disease has been shifted-from stabilizing vision using laser photocoagulation or photodynamic therapy to improving vision using anti-vascular endothelial growth factor (anti-VEGF) medications[12]-[14]. Given the high prevalence of AMD worldwide, especially when a substantial growth in the number of elderly persons is expected in the next decade, the cost of the treatment for improving the vision of patients with wet AMD would be tremendous[15]. AMD constitutes a major public health priority for global health systems, but only a few studies focusing on economic assessment have been conducted. For example, Bonastre et al[16] estimated the economic burden of wet AMD in 105 patients and found that its annual costs were 3 660 Euros. As this study was conducted from the French payer perspective, the findings are unlikely to be applicable to countries in the Asia-Pacific region due to differences in patient behaviors, treatment patterns, and healthcare systems. For example, in many countries ranibizumab is the treatment of choice for treating wet AMD. In Thailand, bevacizumab is the most commonly chosen drug for treating AMD because bevacizumab's cost is covered under the national pharmaceutical benefit package. Furthermore, based on our knowledge and review, no previous study has been conducted to determine the economic burden of wet AMD in Asia.
In order to understand the overall economic burden associated with wet AMD, this study was conducted to estimate its direct medical and non-medical costs in Thailand. The cost estimates obtained from this study can be used as key input parameters in economic evaluations of interventions for patients with wet AMD for similar populations using similar healthcare systems.
SUBJECTS AND METHODS
This study estimated the costs of care of patients with wet AMD in Thailand using the societal perspective. Patients with wet AMD aged 60 years or older were included in this study. Only direct medical and non-medical costs were measured; we did not measure indirect costs because we included only patients aged 60 years or older and assumed that these patients had no productivity. In order to determine overall economic burden, this study was separated into two parts. The first sub-study was a retrospective cohort study; electronic medical charts were reviewed to estimate the direct medical costs. The second sub-study was a cross-sectional survey estimating the direct non-medical costs based on face-to-face interviews using a structured questionnaire. Both sub-studies were reviewed and approved by the ethic committees of Rajavithi Hospital, Maharaj Nakorn Chiang Mai Hospital, and Songklanakarin Hospital.
Patients and Study Design
Direct medical costs: In the retrospective cohort sub-study, patients with wet AMD that visited an ophthalmic clinic in an academic hospital in the southern region of Thailand during January 1, 2007 to May 31, 2010 were recruited. Our inclusion criteria were: 1) having a diagnosis of wet AMD [International classification of disease version 10 (ICD-10):H35.3 and confirmed by chart reviews]; 2) being 60 years old or older; 3) having best corrected visual acuity (BCVA) measured at least 2 times during the follow-up period because confirmation of the patients' continuing of care in the hospital was desired. Patients were excluded if they had other eye diseases.
Direct non-medical costs: a cross-sectional sub-study was conducted. A 30-item questionnaire covering three main domains, demographic, clinical, and cost domains, was used. This questionnaire was administered using a structured face-to-face interview. From March 2010 to November 2010, patients with wet AMD were recruited from two settings: an ophthalmic clinic of an academic hospital located in the north of Thailand and an ophthalmic clinic of a university-affiliated hospital in Bangkok. The same inclusion and exclusion criteria as in the direct medical cost sub-study were applied. Potential subjects were identified by ophthalmologists during routine services at the study sites.
Data Collection and Costs Estimation
Direct medical costs: patients were followed from when the diagnosis of wet AMD was made to May 31, 2010. Data included age, sex, health insurance, diagnosis, and the date of diagnosis. The BCVA of the better-seeing eye was used to determine the baseline BCVA of each patient. Charges including drugs, laboratory, medical procedures, the doctor's orders, and other treatments were also obtained. The drug charges included charges of AMD drugs, as with bevacizumab, ranibizumab, and verteporfin photodynamic therapy and other drugs. To estimate the direct medical costs, the ratio of cost to charge (RCC) was used to convert charges to cost value (0.77 for 2008, 0.90 for 2007, 2009 and 2010). The RCC was collected from a University-affiliated hospital. Costs were converted to the value in the year 2012 using an average consumer price index in the medical and personal care sector and then were converted to US$ using an exchange rate of 29.42 baht/US$[17]-[18]. The total direct medical annual costs were estimated. The costs were also calculated using the Snellen BCVA of the better-seeing eye (4 BCVA groups: ≥20/50, <20/50-20/160, >20/160-20/400, <20/400) because BCVA is an important factor affecting the costs of eye care[19]-[21].
Direct non-medical costs: direct non-medical costs were obtained from interviews with the patients with wet AMD or their caregivers. After the patients provided written informed consent, trained interviewers interviewed them or their care-givers for approximately 15min. The standard structured questionnaire was developed and tested in five patients with eye disease prior to actual data collection. The 30-item questionnaire covered three domains; namely, demographic, clinical, and cost domains. The demographic domain contained a patient identification code, sex, date of birth, education, occupation, and health insurance. The clinical domain contained a diagnosis of the AMD eye (left, right, or both eyes), type of diagnosis, and the BCVA of both eyes. Cost domain included data on the cost of transportations, food incurred during medical visits or hospitalizations, accessories required for the patients, house renovation associated with low vision from wet AMD, and caregivers. The data on the demographic and clinical domains were obtained by medical chart reviews, while the cost domain data were obtained by interviews. Specifically, the costs associated with the caregivers included time costs allocated for accompanying patients to seek medical care and employing a caregiver to take care of the patients. In the case of non-employment, the time that the relatives spent caring for the patients was calculated and converted to monetary value using the minimum wage rate at 245.00 baht, reported by the Ministry of Labour, Thailand in 2012[22]. Costs were converted to the value in the year 2012 and then were converted to US$ using an exchange rate of 29.42 baht/US$[18]. The total non-direct medical annual costs were estimated. The costs were also calculated using the Snellen BCVA of the better-seeing eye (4 BCVA groups: ≥20/50, <20/50-20/160, >20/160-20/400, <20/400).
Statistical Analysis Descriptive statistics were used to describe value patients' demographic characteristics and outcomes. Continuous variables were tested for normal distribution by visualized histograms. Mean and standard deviation were used when data were normally distributed, while median and inter-quartile ranges (IQR) were used when the data were not normally distributed. Discrete or dichotomous variables were presented as frequencies and percentages with a 95% confidence interval. Cost differences across the range of the Snellen BCVA of the better-seeing eyes (4 BCVA groups: ≥20/50, <20/50-20/160, >20/160-20/400, <20/400) were assessed using the Kruskal-Wallis test. STATA package version 11.0 (StataCorp®, College Station, TX, U.S.A.) was used for analysis.
RESULTS
Patient Characteristics
Direct medical costs: A total of 64 patients with wet AMD were included in the sub-study estimating direct medical costs. Of these, 59.4% were male, with a mean age of 71.9±7.8 years. Most patients were under the universal coverage insurance scheme (51.6%). About 17.2% of these patients were diagnosed with wet AMD for both eyes. About one-third (29.7%) had severe visual loss at baseline (BCVA <20/400). The average follow-up duration of each patient was 1.2 years. The demographic and clinical characteristics of the patients are presented in Table 1.
Table 1. Demographic characteristics of patients included in direct medical and non-medical cost sub-studies.
| Demographic characteristics | Direct medical costs sub-study (n=64) | Direct non-medical costs sub-study (n=67) |
| Age, mean (SD) | 71.9 (7.8) | 71.9 (6.8) |
| Gender | ||
| M | 38 (59.4) | 32 (47.8) |
| F | 26 (40.6) | 35 (52.2) |
| Health Insurance | ||
| Universal coverage | 33 (51.6) | 25 (37.3) |
| Civil servant medical benefit schemes | 30 (46.9) | 29 (43.3) |
| Social security schemes | 0 (0.0) | 1 (1.5) |
| Out-of-pocket | 1 (1.5) | 10 (14.9) |
| Others | 0 (0.0) | 2 (3.0) |
| Education | ||
| Lower than primary education | NR | 41 (61.2) |
| Primary education | NR | 4 (6.0) |
| Secondary education | NR | 13 (29.4) |
| Bachelor degree | NR | 9 (13.4) |
| AMD diagnosis | ||
| Only one eye | 53 (82.8) | 52 (77.6) |
| Both eyes | 11 (17.2) | 15 (22.4) |
| Baseline visual acuity1 | ||
| ≥20/50 | 12 (18.8) | 17 (25.8) |
| <20/50-20/160 | 18 (28.1) | 15 (22.7) |
| <20/160-20/400 | 15 (23.4) | 16 (24.2) |
| <20/400 | 19 (29.7) | 18 (27.3) |
AMD: Age-related macular degeneration; NR: Not reported, SD: Standard deviation. 1One patient did not have information about visual acuity (VA).
n (%)
Direct non-medical costs: A total of 67 patients with wet AMD were included. Forth-seven point eight percent were male, with a mean age of 71.9±6.8 years. Most patients (43.3%) were under the civil servant medical benefit scheme, and most patients (61.2%) had a lower primary school education or lower. About 22.4% of the patients were diagnosed with AMD for both eyes. About one-third (27.3%) had severe visual loss (BCVA <20/400). The demographic and clinical characteristics of the patients are also presented in Table 1.
Resource Utilizations and Costs
Direct medical costs: The average number of outpatient visits was 11.1±6.0 visits per patient per year. About 6.3% of patients were treated with best supportive care. About 6.3%, 14.1%, 25.0%, 6.3%, and 48.5% of these patients were treated with best supportive care, bevacizumab, ranibizumab, verteporfin photodynamic therapy, and combination therapy respectively”. On average, annual direct medical cost was $3 604±4 530. The median of annual direct medical cost with inter-quartile range was $1 584 (686-5 360) (Table 2).
Table 2. Resource utilization and direct medical and non-medical costs in Thailand.
| Resources utilization | Results (mean±SD) |
| Direct medical costs sub-study (n=64) | |
| Average number of outpatient visit per year (times /year) | 11.1±6.0 |
| Annual average costs in US$ (SD) | 3 604±4 530 |
| Costs of drug | 2 775±4 303 |
| Costs of laboratory | 1.8±3.9 |
| Costs of medical procedure | 762±552 |
| Other treatment and doctor order costs | 64±34 |
| Annual median of costs in US$ (IQR) | 1 584 (686-5 360) |
| Direct non-medical costs sub-study (n=67) | |
| Average number of outpatient visit per year (times/year) | 8.5±6.1 |
| Percentage of patients with an accompany during outpatient visit (95%CI) | 71.6% (65.9%-86.3%) |
| Average time spending for each outpatient visit; hours | 9.2±9.5 |
| Percentage of patients that need house renovation (95%CI) | 9.0% (2.1%-15.9%) |
| Percentage of patients that need caregiver services at home (95%CI) | 25.4% (15.0%-35.8%) |
| Average hour per day for assistance at home | 5.1±4.8 |
| Annual average costs in US$ (SD) | 2 927±6 560 |
| Costs of transportation | 205±339 |
| Costs of food incurred during medical visit or hospitalization | 77±237 |
| Costs of accommodation during medical visit or hospitalization | 20±110 |
| Costs of house renovation or accessory requirement | 83±555 |
| Costs associated with caregiver | 2 540±6 291 |
| Annual median of costs in US$ (IQR) | 207 (74-1 894) |
AMD: Age-related macular degeneration; IQR: Inter-quartile range; SD: Standard deviation; 95% CI: 95% confidence interval.
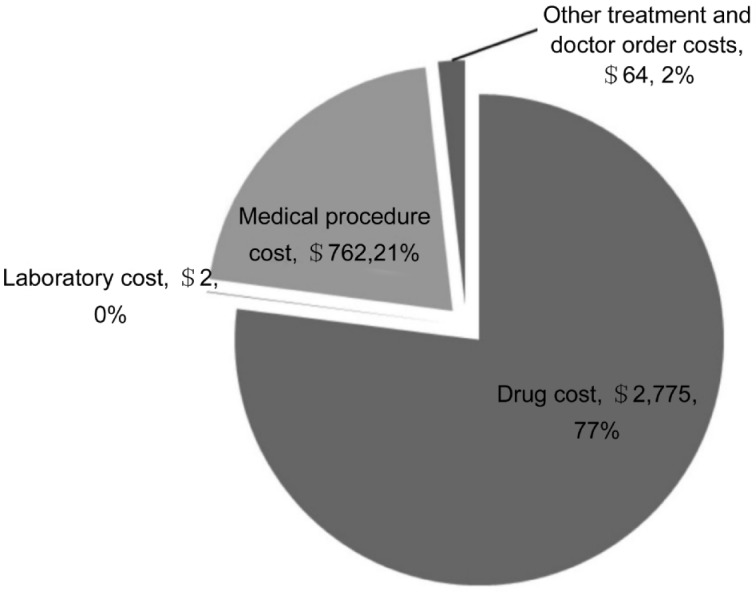
When categorized by the BCVA groups, the average annual costs in the BCVA group of ≥20/50, <20/50-20/160, >20/160-20/400, <20/400 were $3 484, $3 235, $2 489, and $4 909, respectively (Table 3). No statistically-significant differences of the average costs among the BCVA groups were detected (P=0.98). Drug costs were a major driver of direct medical costs, accounting for 77 % of the direct medical costs (Figure 1).
Table 3. Annual direct medical and non-medical costs by level of visual impairment in Thailand.
| Costs of wet AMD | Snellen best corrected visual acuity |
P | |||
| ≥20/50 | <20/50-20/160 | >20/160-20/400 | <20/400 | ||
| Direct medical costs sub-study ($) | 12 (3 484±3 886) | 18 (3 235± 3061) | 15 (2 489±2 368) | 19 (4 909±6 814) | 10.98 |
| 2Direct non-medical costs sub-study ($) | 17 (2 304±5 460) | 15 (2 750±8 091) | 16 (3 989±4 847) | 18 (2 873±7 901) | 10.74 |
AMD: Age-related macular degeneration; SD: Standard deviation. 1Tested by Kruskal-Wallis test; 2One patient did not have information about VA.
n (x±s)
Figure 1. Annual direct medical cost by types of cost.
Direct non-medical costs: The average number of outpatient visits was 8.5±6.1 visits per patient per year. About 71.6% [95% confident interval (CI); 65.9%-86.3%] of patients had at least one relative accompanying them while visiting the outpatient department. Among the patients needing caregivers, the caregivers spent 9.2±9.5h per outpatient visit on average. Only two of the patients [3% (95%CI; 0.0%-7.01%)] were hospitalized during the period of six months prior to the interviews. One patient was hospitalized once and another was hospitalized twice. About one-tenth [9.0% (95%CI; 2.2%-15.9%)] of patients reported that they had to renovate their house or buy accessories because of the low vision associated with wet AMD. Almost a quarter [25.4% (95%CI; 15.0%-35.8%)] of them reported that they required supportive care provided by their family members (so-called “unpaid caregiver services”), which had no associated direct costs. Among those that used unpaid caregiver services, the average number of hours spent for care giving per day was 5.1±4.8h. The average direct non-medical cost was $2 927±6 560. The median of annual direct non-medical cost with inter-quartile range was $207 (74-1 894) (Table 2).
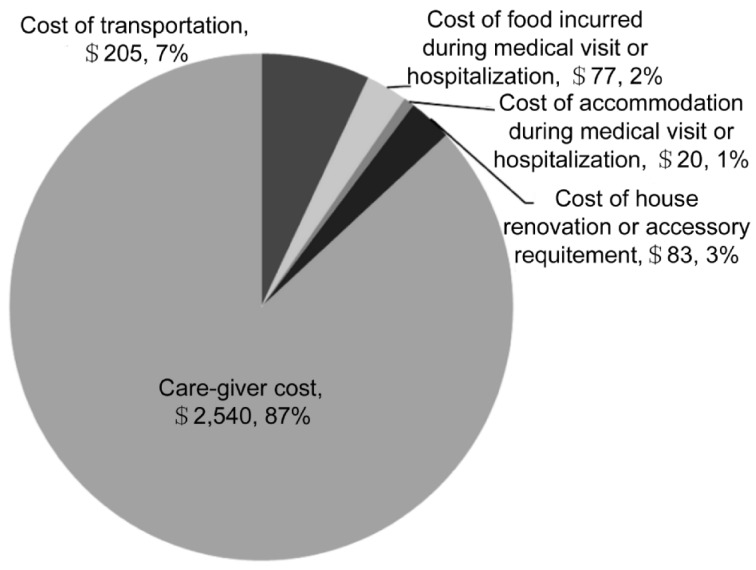
When categorized by the BCVA groups, the average annual costs in the VA group of ≥20/50, <20/50-20/160, >20/160-20/400, <20/400 were $2 304, $2 750, $3 989, and $2 873, respectively (Table 3). There were no statistically-significant differences in the average costs among the BCVA groups (P=0.74). The care-giver cost was the largest component of direct non-medical costs (87%) (Figure 2).
Figure 2. Annual direct non-medical cost by types of cost.
DISCUSSION
Our study is the first that reports the direct medical and non-medical costs of patients with wet AMD in Asia. The findings highlight the importance of the economic burden associated with wet AMD. This information is very crucial for practitioners and policymakers in efficiently planning and allocating scarce resources to achieve optimal eye care at the population level.
Our findings revealed that the composition of total costs in Thailand was different from that in previously-published literature. Drug costs accounted for the largest proportion (42.5%) of total costs associated with wet AMD, while Bonastre et al[16] reported that a major cost driver was the eye examination cost (28.3%). Other studies reported that the caregiver cost was the largest cost incurred in wet AMD care[21],[23]. The difference in the proportion of expenditure could be explained by the different pattern of medical care in the two studies. The proportion of patients receiving medical treatment in Bonastre et al[16] was 31.4% which was substantially lower than that in our study. Another explanation is the difference of healthcare systems. Several anecdotal reports indicated that drugs are a major driver cost in the Thai healthcare system[24],[25]. It was shown that a large proportion of the budget for healthcare management was allocated for drug expenditure. In addition, our study found that only one-fourth of the patients required care from caregivers. The lower rate of caregivers used in Thailand is substantially different from that reported in Lotery et al[23] and Schmier et al[21].
Our study found that there were no significant differences in direct medical and non-medical costs among the 4 BCVA groups. The study of Bonastre et al[16] was the only study that reported a significant difference in direct non-medical costs between patients with wet AMD with BCVA <20/64 and BCVA>20/64, whereas no significant difference in terms of direct medical costs was found between them. Javitt et al[20] investigated the direct medical care costs among patients with three varying BCVA levels (moderate: BCVA <20/40, severe <20/200, and blindness <20/400). They found that at the higher severity level of BCVA, higher average direct medical costs were observed. Schmier et al[21] also evaluated caregiver time costs among patients with either wet or dry AMD and found a trend of elevated costs only with increased loss of vision. Our results are likely to be different from other studies because of the small sample size issue. More studies that investigate the differences in wet AMD-associated costs across the BCVA groups are warranted, especially in Asia Pacific countries.
In our study, an important assumption is that our included patients, aged 60 or older, had no productivity, and this led us to not measure indirect costs. This assumption is suitable for the Thai context because in that system, citizens are retired after the age of 60 years. Therefore, it is difficult for patients with visual problems from AMD in Thailand to be productive after retirement.
The question of whether our research findings can represent national estimates is also debatable. Our study estimated the resource utilizations and healthcare costs from tertiary and university-based hospitals. Moreover, in our fist sub-study, the percentage of male in our study (59.4%) differs from the previous Thai study[26]. It indicated that the percentage of male in patients with AMD was 41.4%. It might be argued that the results are not generalizable to other clinical settings, e.g. provincial and private hospitals. However, most patients with wet AMD seek care from large hospitals, similar to the hospitals in which we conducted this study. The selected settings were the hospitals from which the largest proportion of AMD patients in Thailand seek care. For this reason, we believe that our results are somewhat representative of the overall AMD cost estimates of Thailand.
A couple of limitations exist and deserve to be discussed regarding this study. First, recall bias may have occurred during the interviews regarding the direct non-medical cost data because the patients or the caregivers were asked about all non-medical resource utilizations associated with wet AMD occurring in the last 6 months. Second, even though we attempted to estimate only the costs related to wet AMD, it was very difficult to genuinely distinguish between the costs of wet AMD and the costs of other age-related disorders.
Placed in a larger perspective, our study demonstrated that the total cost associated with wet AMD was substantial. The total annual wet AMD associated costs were $6 528, which were slightly higher than the Gross Domestic Product per capita ($4 972) of Thailand in the year 2011[27]. Policymakers should seriously consider allocating resources and managing them properly and effectively to improve the vision of patients that suffer from this high-cost disease.
In conclusion, our study indicated that wet AMD is associated with a substantial amount of economic burden, especially regarding drug and caregiver costs. This study has highlighted the important issues for practitioners and policy makers, potentially leading to the optimal prevention and management of wet AMD.
Acknowledgments
The authors would like to thank a number of clinical research nurses and physicians involved in the data collection at all sites.
Foundation: Supported by Novartis (Thailand) Ltd.; Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (grant No. PHD/0356/2550 to PD).
Conflicts of Interest: Dilokthornsakul P, None; Chaiyakunapruk N, None; Ruamviboonsuk P, None; Ratanasukon M, None; Ausayakhun S, None; Tungsomeroengwong A, None; Pokawattana N, None; Chanatittarat C was an employee of Novartis (Thailand) Ltd at the time of conducting this study.
REFERENCES
- 1.Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya'ale D, Négrel AD, Resnikoff S. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11(2):67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- 2.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 3.Yuzawa M, Tamakoshi A, Kawamura T, Ohno Y, Uyama M, Honda T. Report on the nationwide epidemiological survey of exudative age-related macular degeneration in Japan. Int Ophthalmol. 1997;21(1):1–3. doi: 10.1023/a:1005845521424. [DOI] [PubMed] [Google Scholar]
- 4.Chen SJ, Cheng CY, Peng KL, Li AF, Hsu WM, Liu JH, Chou P. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2008;49(7):3126–3133. doi: 10.1167/iovs.08-1803. [DOI] [PubMed] [Google Scholar]
- 5.Gupta SK, Murthy GV, Morrison N, Price GM, Dherani M, John N, Fletcher AE, Chakravarthy U. Prevalence of early and late age-related macular degeneration in a rural population in northern India: the INDEYE feasibility study. Invest Ophthalmol Vis Sci. 2007;48(3):1007–1011. doi: 10.1167/iovs.06-0712. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki R, Wang JJ, Aung T, Tan DT, Mitchell P, Sandar M, Saw SM, Wong TY, Singapore Malay Eye Study Group Prevalence of age-related macular degeneration in a Malay population: the Singapore Malay Eye Study. Ophthalmology. 2008;115(10):1735–1741. doi: 10.1016/j.ophtha.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Klein BE, Knudtson MD, Wong TY, Cotch MF, Liu K, Burke G, Saad MF, Jacobs DR., Jr Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113(3):373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Xu L, Jonas JB, Yang H, Ma Y, Li J. Prevalence of age-related maculopathy in the adult population in China: the Beijing eye study. Am J Ophthalmol. 2006;142(5):788–793. doi: 10.1016/j.ajo.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki R, Yasuda M, Song SJ, Chen SJ, Jonas JB, Wang JJ, Mitchell P, Wong TY. The prevalence of age-related macular degeneration in Asians; a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi: 10.1016/j.ophtha.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Jenchitr W, Ruamviboonsuk P, Sanmee A, Pokawattana N. Prevalence of age-related macular degeneration in Thailand. Ophthalmic Epidemiol. 2011;18(1):48–52. doi: 10.3109/09286586.2010.545502. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson AJ, Sparrow JM, Duke AM, Thompson JR, Gibson JM, Rosenthal AR. Prevalence of age-related maculopathy at two points in time in an elderly British population. Eye (Lond) 1997;11(Pt 3):301–314. doi: 10.1038/eye.1997.66. [DOI] [PubMed] [Google Scholar]
- 12.Macular Photocoagulation Study Group Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991;109(9):1220–1231. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 13.Treatment of age-related macular degeneration with photodynamic therapy study group Photodynamic therapy of sub-foveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials: TAP report 1. Arch Ophthalmol. 1999;117(10):1329–1345. [PubMed] [Google Scholar]
- 14.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 15.United Nation Population Division World Population Prospects: the 2008 revision population database. Panel 2: Detailed Data. http://esa.un.org/unpp/index.asp?panel=2 Accessed May 25, 2010.
- 16.Bonastre J, Le Pen C, Soubrane G, Quentel G. The burden of age-related macular degeneration: Results of a cohort study in two French referral centres. Pharmacoeconomics. 2003;21(3):181–190. doi: 10.2165/00019053-200321030-00003. [DOI] [PubMed] [Google Scholar]
- 17.Bank of Thailand Consumer price index by group. 2013. http://www.indexpr.moc.go.th/price_present/tableIndexCpi_bot.asp. Accessed 7 April, 2013.
- 18.Bank of Thailand Daily foreign exchange rates. 2013. p. 2013. http://www.bot.or.th/english/statistics/financialmarkets/exchangerate/_layouts/Application/ExchangeRate/ExchangeRate.aspx. Accessed 7 April, 2013.
- 19.Bandello F, Augustin A, Sahel JA, Benhaddi H, Negrini C, Hieke K, Berdeaux GH. Association between visual acuity and medical and non-medical costs in patients with wet age-related macular degeneration in France, Germany and Italy. Drugs Aging. 2008;25(3):255–268. doi: 10.2165/00002512-200825030-00007. [DOI] [PubMed] [Google Scholar]
- 20.Javitt JC, Zhiyuan Z, Willke RJ. Association between visual loss and higher medical care costs in meidcare beneficiaries. Ophthalmology. 2007;114(2):238–245. doi: 10.1016/j.ophtha.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 21.Schmier JK, Halpern MT, Covert D, Delgado J, Sharma S. Impact of visual impairment on use of caregiving by individuals with age-related macular degeneration. Retina. 2006;26(9):1056–1062. doi: 10.1097/01.iae.0000254890.48272.5a. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of labor. Thailand Notification of the wage on the minimum wage (No.3) and explanation. 2009. http://www.mol.go.th/sites/default/files/prakadkajang_jan0110_0.pdf. Accessed 15 December, 2010.
- 23.Lotery A, Xu X, Zlatava G, Loftus J. Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: results form the UK cohort of a five-country cross-sectional study. Br J Ophthalmol. 2007;91(10):1303–1307. doi: 10.1136/bjo.2007.116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faramnuayphol P, Ekachampaka P, Taverat R, Wattanamano W. Thailand health profile 2005-2007. In: S W, editor. Health services system in Thailand. Bangkok: Printing Press, The War Veterans Organization of Thailand; 2007. [Google Scholar]
- 25.Santoso B, Shein K, Suryawati S. Yogyakarta: World Health Organization; 1997. Financing drugs in South-East Asia. Health Economics and Drugs DAP Series. [Google Scholar]
- 26.Jenchitr R, Ruamviboonsuk P, Sanmee A, Pokawatana N. Prevalence of age-related macular degenertaion in Thailand. Ophthalmic Epidemiol. 2011;18(1):48–52. doi: 10.3109/09286586.2010.545502. [DOI] [PubMed] [Google Scholar]
- 27.The World Bank GDP per capita (current US$) 2013. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 7 April, 2013.