Abstract
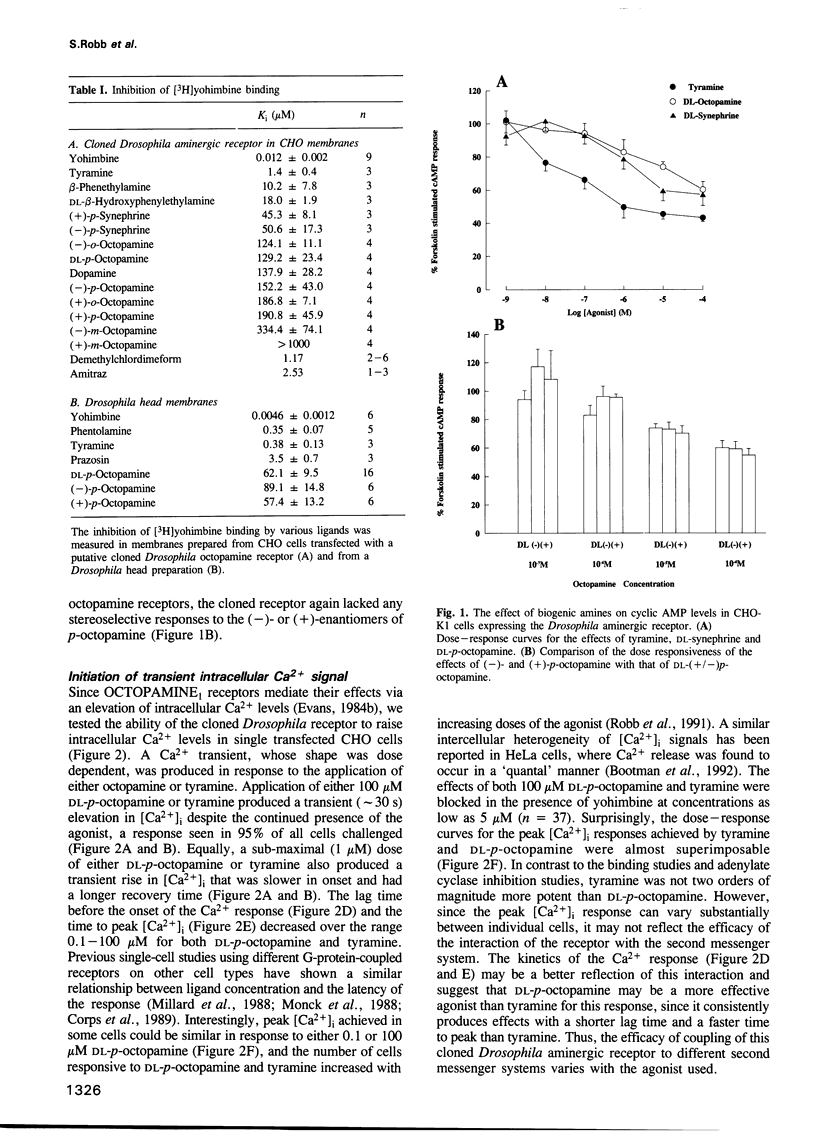
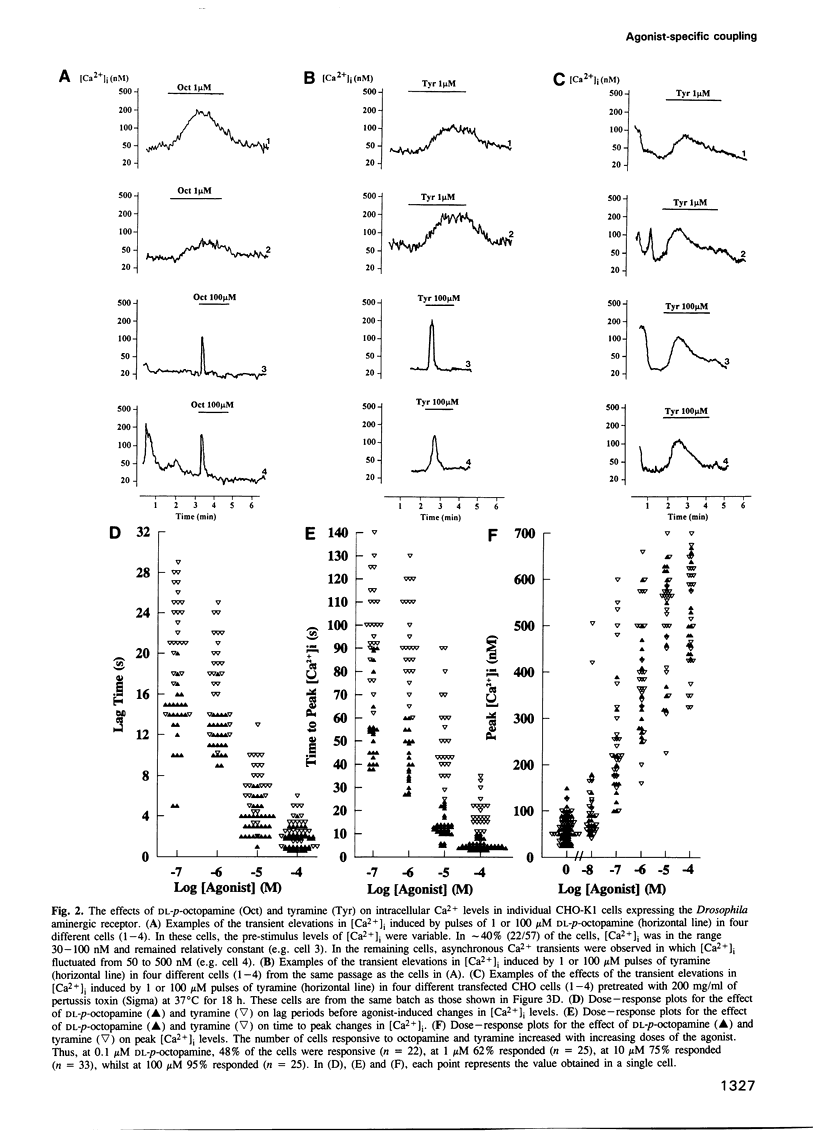
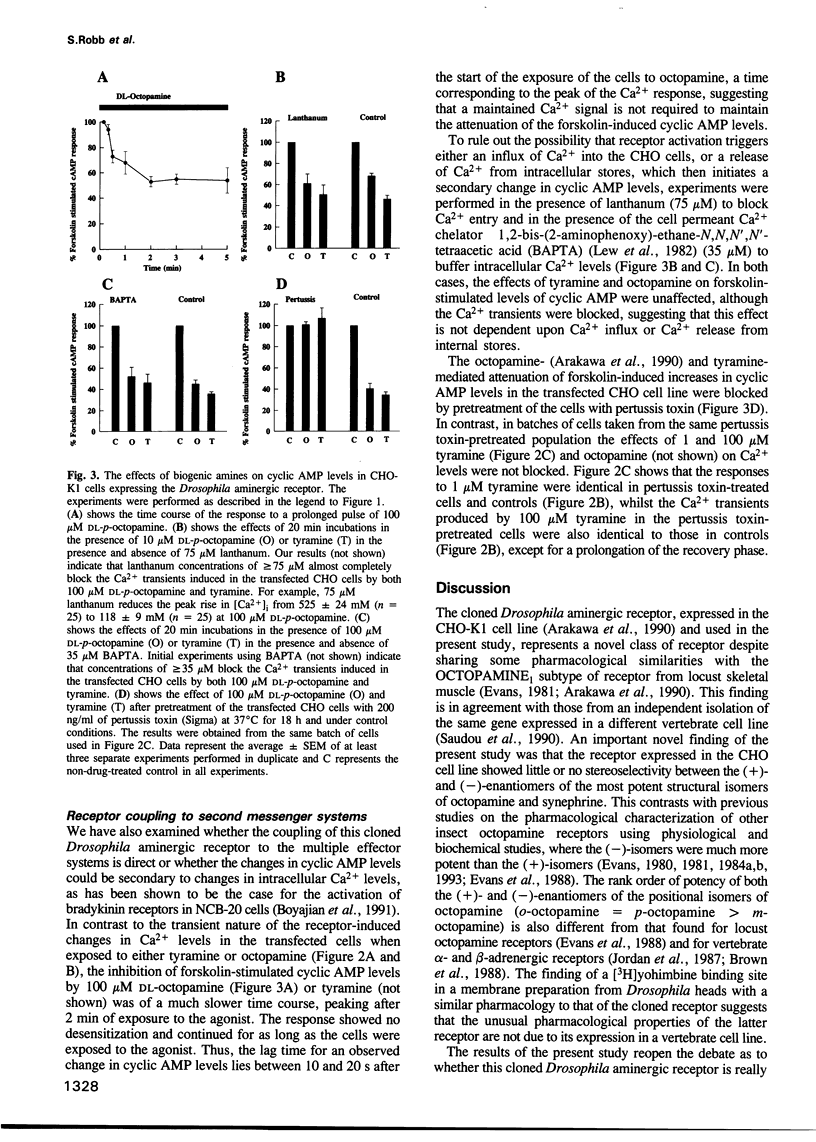
A cloned seven transmembrane-spanning Drosophila octopamine/tyramine receptor, permanently expressed in a Chinese hamster ovary cell line, both inhibits adenylate cyclase activity and leads to the elevation of intracellular Ca2+ levels by separate G-protein-coupled pathways. Agonists of this receptor (octopamine and tyramine), differing by only a single hydroxyl group in their side chain, may be capable of differentially coupling it to different second messenger systems. Thus, a single receptor may have a different pharmacological profile depending on which second messenger system is used to assay its efficacy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Samra A. B., Jüppner H., Force T., Freeman M. W., Kong X. F., Schipani E., Urena P., Richards J., Bonventre J. V., Potts J. T., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa S., Gocayne J. D., McCombie W. R., Urquhart D. A., Hall L. M., Fraser C. M., Venter J. C. Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron. 1990 Mar;4(3):343–354. doi: 10.1016/0896-6273(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Berridge M. J., Taylor C. W. All-or-nothing Ca2+ mobilization from the intracellular stores of single histamine-stimulated HeLa cells. J Physiol. 1992 May;450:163–178. doi: 10.1113/jphysiol.1992.sp019121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyajian C. L., Garritsen A., Cooper D. M. Bradykinin stimulates Ca2+ mobilization in NCB-20 cells leading to direct inhibition of adenylylcyclase. A novel mechanism for inhibition of cAMP production. J Biol Chem. 1991 Mar 15;266(8):4995–5003. [PubMed] [Google Scholar]
- Brown C. M., McGrath J. C., Midgley J. M., Muir A. G., O'Brien J. W., Thonoor C. M., Williams C. M., Wilson V. G. Activities of octopamine and synephrine stereoisomers on alpha-adrenoceptors. Br J Pharmacol. 1988 Feb;93(2):417–429. doi: 10.1111/j.1476-5381.1988.tb11449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund D. B. Subtypes of alpha 1- and alpha 2-adrenergic receptors. FASEB J. 1992 Feb 1;6(3):832–839. doi: 10.1096/fasebj.6.3.1346768. [DOI] [PubMed] [Google Scholar]
- Chabre O., Conklin B. R., Lin H. Y., Lodish H. F., Wilson E., Ives H. E., Catanzariti L., Hemmings B. A., Bourne H. R. A recombinant calcitonin receptor independently stimulates 3',5'-cyclic adenosine monophosphate and Ca2+/inositol phosphate signaling pathways. Mol Endocrinol. 1992 Apr;6(4):551–556. doi: 10.1210/mend.6.4.1316547. [DOI] [PubMed] [Google Scholar]
- Chakraborty M., Chatterjee D., Kellokumpu S., Rasmussen H., Baron R. Cell cycle-dependent coupling of the calcitonin receptor to different G proteins. Science. 1991 Mar 1;251(4997):1078–1082. doi: 10.1126/science.1847755. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Jackson T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Simultaneous measurements of cytosolic calcium and secretion in single bovine adrenal chromaffin cells by fluorescent imaging of fura-2 in cocultured cells. J Cell Biol. 1989 Sep;109(3):1219–1227. doi: 10.1083/jcb.109.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Spatial localization of agonist-induced Ca2+ entry in bovine adrenal chromaffin cells. Different patterns induced by histamine and angiotensin II, and relationship to catecholamine release. J Cell Sci. 1993 Aug;105(Pt 4):913–921. doi: 10.1242/jcs.105.4.913. [DOI] [PubMed] [Google Scholar]
- Corps A. N., Cheek T. R., Moreton R. B., Berridge M. J., Brown K. D. Single-cell analysis of the mitogen-induced calcium responses of normal and protein kinase C-depleted Swiss 3T3 cells. Cell Regul. 1989 Nov;1(1):75–86. doi: 10.1091/mbc.1.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S., Kobilka B. K., Daniel K. W., Nolan R. D., Lapetina E. Y., Caron M. G., Lefkowitz R. J., Regan J. W. Multiple second messenger pathways of alpha-adrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem. 1990 Jan 5;265(1):63–69. [PubMed] [Google Scholar]
- Crawford K. W., Frey E. A., Cote T. E. Angiotensin II receptor recognized by DuP753 regulates two distinct guanine nucleotide-binding protein signaling pathways. Mol Pharmacol. 1992 Jan;41(1):154–162. [PubMed] [Google Scholar]
- Evans P. D. A modulatory octopaminergic neurone increases cyclic nucleotide levels in locust skeletal muscle. J Physiol. 1984 Mar;348:307–324. doi: 10.1113/jphysiol.1984.sp015112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., Gee J. D. Action of formamidine pesticides on octopamine receptors. Nature. 1980 Sep 4;287(5777):60–62. doi: 10.1038/287060a0. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Molecular studies on insect octopamine receptors. EXS. 1993;63:286–296. doi: 10.1007/978-3-0348-7265-2_16. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Multiple receptor types for octopamine in the locust. J Physiol. 1981 Sep;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., Robb S. Octopamine receptor subtypes and their modes of action. Neurochem Res. 1993 Aug;18(8):869–874. doi: 10.1007/BF00998270. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Studies on the mode of action of octopamine, 5-hydroxytryptamine and proctolin on a myogenic rhythm in the locust. J Exp Biol. 1984 May;110:231–251. doi: 10.1242/jeb.110.1.231. [DOI] [PubMed] [Google Scholar]
- Evans P. D., Thonoor C. M., Midgley J. M. Activities of octopamine and synephrine stereoisomers on octopaminergic receptor subtypes in locust skeletal muscle. J Pharm Pharmacol. 1988 Dec;40(12):855–861. doi: 10.1111/j.2042-7158.1988.tb06288.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hosey M. M. Diversity of structure, signaling and regulation within the family of muscarinic cholinergic receptors. FASEB J. 1992 Feb 1;6(3):845–852. [PubMed] [Google Scholar]
- Jackson F. R., Wilson S. D., Strichartz G. R., Hall L. M. Two types of mutants affecting voltage-sensitive sodium channels in Drosophila melanogaster. Nature. 1984 Mar 8;308(5955):189–191. doi: 10.1038/308189a0. [DOI] [PubMed] [Google Scholar]
- Jordan R., Midgley J. M., Thonoor C. M., Williams C. M. Beta-adrenergic activities of octopamine and synephrine stereoisomers on guinea-pig atria and trachea. J Pharm Pharmacol. 1987 Sep;39(9):752–754. doi: 10.1111/j.2042-7158.1987.tb06986.x. [DOI] [PubMed] [Google Scholar]
- Lai J., Waite S. L., Bloom J. W., Yamamura H. I., Roeske W. R. The m2 muscarinic acetylcholine receptors are coupled to multiple signaling pathways via pertussis toxin-sensitive guanine nucleotide regulatory proteins. J Pharmacol Exp Ther. 1991 Sep;258(3):938–944. [PubMed] [Google Scholar]
- Lew V. L., Tsien R. Y., Miner C., Bookchin R. M. Physiological [Ca2+]i level and pump-leak turnover in intact red cells measured using an incorporated Ca chelator. Nature. 1982 Jul 29;298(5873):478–481. doi: 10.1038/298478a0. [DOI] [PubMed] [Google Scholar]
- Millard P. J., Gross D., Webb W. W., Fewtrell C. Imaging asynchronous changes in intracellular Ca2+ in individual stimulated tumor mast cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1854–1858. doi: 10.1073/pnas.85.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Mechanisms of multifunctional signalling by G protein-linked receptors. Trends Pharmacol Sci. 1993 Jun;14(6):239–244. doi: 10.1016/0165-6147(93)90019-g. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi M., Ohashi Y., Shichijo S., Christian C., Sudduth-Klinger J., Harrowe G., Payan D. G. Multiple intracellular signaling pathways of the neuropeptide substance P receptor. J Neurosci Res. 1992 Jul;32(3):437–443. doi: 10.1002/jnr.490320315. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Reynolds E. E., Thomas A. P., Williamson J. R. Novel kinetics of single cell Ca2+ transients in stimulated hepatocytes and A10 cells measured using fura-2 and fluorescent videomicroscopy. J Biol Chem. 1988 Apr 5;263(10):4569–4575. [PubMed] [Google Scholar]
- Nakajima Y., Tsuchida K., Negishi M., Ito S., Nakanishi S. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J Biol Chem. 1992 Feb 5;267(4):2437–2442. [PubMed] [Google Scholar]
- Saudou F., Amlaiky N., Plassat J. L., Borrelli E., Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990 Nov;9(11):3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993 Sep 9;365(6442):170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Taylor C. W. The role of G proteins in transmembrane signalling. Biochem J. 1990 Nov 15;272(1):1–13. doi: 10.1042/bj2720001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. B. Comment: single receptors, dual second messengers. Mol Endocrinol. 1992 Apr;6(4):501–501. doi: 10.1210/mend.6.4.1316545. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Harootunian A. T. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990 Feb-Mar;11(2-3):93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Wolsing D. H., Rosenbaum J. S. The mechanism for the rapid desensitization in bradykinin-stimulated inositol monophosphate production in NG108-15 cells involves interaction of a single receptor with multiple signaling pathways. J Pharmacol Exp Ther. 1993 Jul;266(1):253–261. [PubMed] [Google Scholar]



