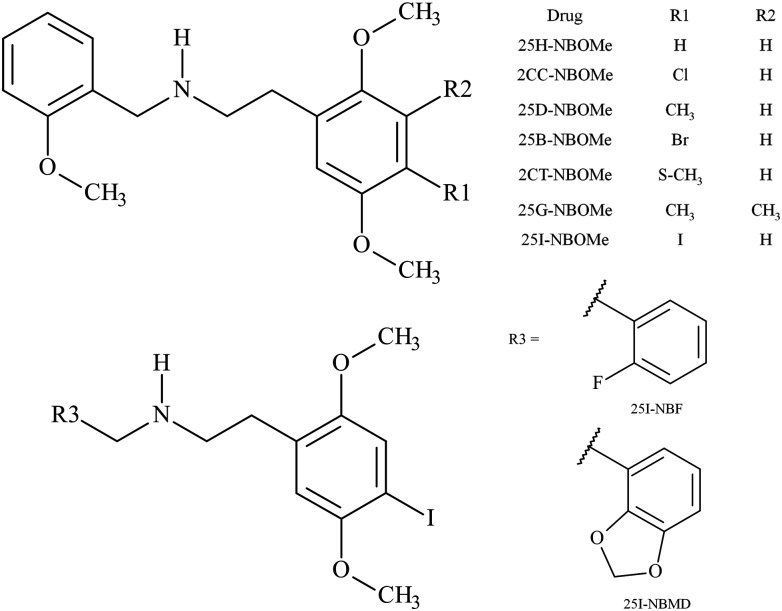
Abstract
We present a high-performance liquid chromatography triple quadrupole mass spectrometry (HPLC–MS-MS) method for the identification and quantification of nine serotonin 5-HT2A receptor agonist hallucinogenic substances from a new class of N-methoxybenzyl derivatives of methoxyphenylethylamine (NBOMe) designer drugs in human urine: 25H-NBOMe, 2CC-NBOMe, 25I-NBF, 25D-NBOMe, 25B-NBOMe, 2CT-NBOMe, 25I-NBMD, 25G-NBOMe and 25I-NBOMe. This assay was developed for the Virginia Commonwealth University Clinical and Forensic Toxicology laboratory to screen emergency department specimens in response to an outbreak of N-benzyl-phenethylamine derivative abuse and overdose cases in Virginia. The NBOMe derivatives were rapidly extracted from the urine specimens by use of FASt™ solid-phase extraction columns. Assay performance was determined as recommended for validation by the Scientific Working Group for Forensic Toxicology (SWGTOX) for linearity, lower limit of quantification, lower limit of detection, accuracy/bias, precision, dilution integrity, carryover, selectivity, absolute recovery, ion suppression and stability. Linearity was verified to be from 1 to 100 ng/mL for each of the nine analytes. The bias determined for the NBOMe derivatives was 86–116% with a <14% coefficient of variation over the linear range of the assay. Four different NBOMe derivatives were detected using the presented method in patient urine specimens.
Introduction
It is generally accepted that the characteristic subjective and behavioral effects of hallucinogens in both humans and animals are mediated by activation of the 5-HT2A receptor (1). Additionally, the 5-HT2A receptor has been closely linked to complex behaviors including working memory and cognitive processes. It has also been implicated in the pathophysiology of affective disorders, such as depression and schizophrenia. As a result, the therapeutic effects of atypical antipsychotics are due to antagonism at 5-HT2A receptors (2). All serotonergic hallucinogens, including the indole derivative lysergic acid diethylamide (LSD), act as 5-HT2A agonists. Phenylalkylamine hallucinogens are selective for both 5-HT2A and 5-HT2C sites (3). Members of the phenylalkylamine hallucinogen class can be further subdivided into two subclasses: the phenethylamines referred to as ‘2C’ compounds including mescaline, 2,5-dimethoxy-4-bromophenethylamine (2CB) and 2,5-dimethoxy-4-iodophenethylamine (2C-I), and the phenylisopropylamines, such as 2,5-dimethoxy-4-iodoamphetamine (DOI) and 2,5-dimethoxy-4-methylamphetamine (DOM). The terminology ‘2C’ is an abbreviation invented by Alexander Shulgin signifying only two carbons, an ethyl linkage, between the phenyl and amine groups in the perceptional distorting and/or hallucinogenic phenylethylamine derivatives he synthetized (4–6). In 1974, he synthesized 4-bromo-2,5-dimethoxyphenethylamine (2C-B) as a replacement for 4-methylenedioxymethamphetamine (MDMA) in psychotherapy after MDMA was scheduled in the USA (7).
In 2012, Zuba and Sekuła (8) reported analytical properties of three potent serotonin 5-HT2A receptor agonist hallucinogenic substances from a new class of N-methoxybenzyl derivatives of methoxyphenylethylamine (NBOMe) derivatives identified in blotter papers seized from the drug market in Poland: 2-(2,5-dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe), 2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25E-NBOMe) and 2-(2,5-dimethoxy-3,4-dimethylphenyl)-N-(2-methoxybenzyl)ethanamine (25G-NBOMe). Recently, N-(2-methoxybenzyl)- 2,5-dimethoxy-4-chlorophenethylamine (2CC-NBOMe) and 2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2,3-methylenedioxyphenyl)methyl]ethanamine (25I-NBMD) have been identified on blotter paper (9, 10). The addition of an N-benzyl substituent to phenethylamine hallucinogens dramatically increases 5-HT2A receptor affinity (11). NBOMe derivatives such as N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (25I-NBOMe) and N-(2-methoxybenzyl)-2,5-dimethoxy-4-bromophenethylamine (25B-NBOMe), which display high affinity and efficacy, have become important ligands in the investigation of the 5-HT2A receptor (12) and in positron emission tomography radiotracers (13).
Currently, 25I-NBOMe sold as a powder or on blotter paper under the names ‘N-Bomb’ and ‘Smiles’ is the most widely abused of the many NBOMe derivatives.
Anecdotal reports indicate that the powder in doses of 50–250 µg may be administered sublingually, by insufflation or applied to the buccal cavity. Blotter paper usually contains higher doses of 500–800 µg, apparently due to low bioavailability of the drug (14). Several published abstracts and a few papers have described signs and symptoms of 25I-NBOMe intoxication (15–19).
These reports contain a total of 22 cases of 25I-NBOMe intoxication with the following demographics: the average age of the patients was 18 years with a range of 14–29 years; 13 of these cases included the sex of the patient, all were male (15, 16, 18) except one (19). The incidence of clinical presentations as a percentage of the total reported cases is as follows: tachycardia (95%), agitation (77%), hypertension (73%) and seizures (45%). A case of serve intoxication due to ingestion of 25B-NBOMe has also been reported (20). Many of these patients exhibited seizures, bizarre behavior and agitation persisting for as long as 3 days. Given the high potency and ease of synthesis of NBOMe derivatives, it is likely that the abuse of these hallucinogens will become more widespread in the future. Five states including Virginia, Louisiana, Arkansas, Florida and Georgia had regulated 25I-NBOMe as a Schedule 1 controlled substance prior to 10 October 2013 when the Drug Enforcement Administration temporarily placed 25I-NBOMe, 25B-NBOMe and 2CC-NBOMe on Schedule 1 of the Controlled Substances Act. None of the other N-benzylphenethylamines are scheduled federally or subject to international controls.
We present a high-performance liquid chromatography triple quadrupole mass spectrometry (HPLC–MS-MS) method for the identification and quantification in human urine of nine NBOMe derivatives including: 2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzylidene)ethanamine (25H-NBOMe), 2CC-NBOMe, N-(2-fluorobenzyl)-2-(4-iodo-2,5-dimethoxyphenyl)ethanamine (25I-NBF), 25D-NBOMe, 25B-NBOMe, 2-(2,5-dimethoxy-4-(methylthio)phenyl)-N-(2-methoxybenzyl)ethanamine (2CT-NBOMe), 25I-NBMD, 25G-NBOMe and 25I-NBOMe (Figure 1). The NBOMe derivatives are rapidly isolated within seconds from urine by use of FASt™ solid-phase extraction columns. The method is novel in that presently there are no published procedures for the analysis of NBOMe derivatives in human urine specimens. The assay was developed to screen emergency department specimens in response to an outbreak of 25-NBOMe derivative abuse and overdose cases in Virginia during early 2012 (16, 17, 19, 20).
Figure 1.
Structure of the NBOMe derivatives.
Methods
Reagents
The primary reference materials for 25H-NBOMe, 2CC-NBOMe, 25I-NBF, 25D-NBOMe, 25B-NBOMe, 2CT-NBOMe, 25I-NBMD, 25G-NBOMe, 25I-NBOMe and the internal standard (ISTD) 4-iodo-2,5-dimethoxy-N-[(2-methoxyphenyl)methyl]-benzeneethanamine-d3 (25I-NBOMe-d3) were purchased from Cayman Chemical Company (Ann Arbor, MI, USA) as hydrochloride salts. Ammonium acetate, formic acid, methanol and deionized (DI) water were purchased from Fisher Scientific (Hanover Park, IL, USA). All reagents were ACS grade or better. Medical grade nitrogen was purchased from National Welders Supply Company (Richmond, VA, USA), the Clean Screen FASt™ extraction columns were purchased from UCT (Bristol, PA, USA) and the Liquichek™ Urine Toxicology Control, Level C3, was purchased from Bio-Rad (Hercules, CA, USA). In-house drug-free urine was obtained from laboratory personnel who did not use tobacco products nor take prescription medications, over-the-counter or illicit drugs.
Sample preparation
Twenty-five microliters of ISTD containing 200 ng/mL (5 ng total) of 25I-NBOMe-d3 were added to 0.5 mL aliquots of calibrators, quality control (QC) specimens or patient samples followed by the addition of 0.5 mL of methanol. Samples were mixed using a vortex mixer for 30 s. Three hundred microliters of the calibrator, QC or patient sample were transferred to Clean Screen FASt™ extraction columns and rapidly aspirated into 250 µL of auto-sampler vials with nitrogen under 80 psi in an UCT Positive Pressure Manifold. Vials were then capped and placed on the HPLC–MS-MS for analysis.
Instrumental analysis
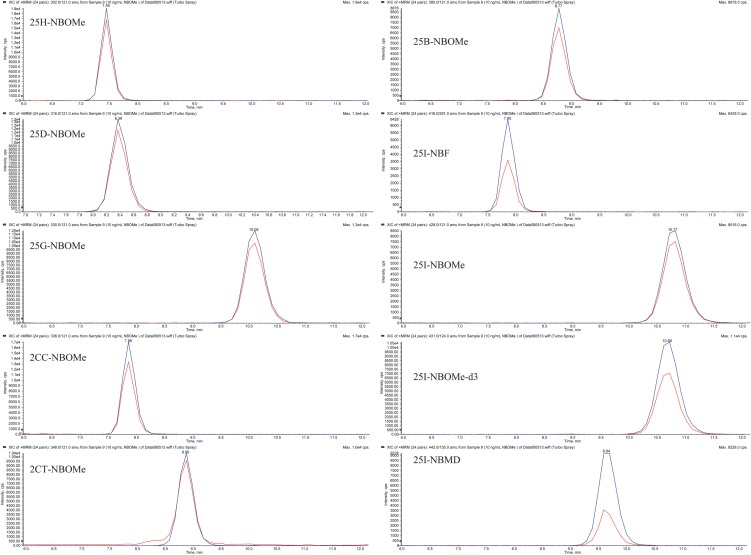
The HPLC–MS-MS analysis was performed on an Applied Biosystems 3200 Q trap with a turbo V source for Turbolon Spray attached to a Shimadzu SCL HPLC system controlled by the Analyst 1.4.2 software. Chromatographic separation was performed on a Restek Allure Biphenyl 5 µ 100 × 3.2 mm column (Bellefonte, PA, USA; Figure 2). The mobile phase consisted of A: DI water with 10 mM ammonium acetate and 0.1% formic acid and B: methanol. The following gradient was used: 0.00–1.0 min starting at 50% B with a linear gradient to 80% B, then using linear gradient ending at 10.0 min to 70% B and finally returning at 10.1 min to 50% B. The source temperature was set at 650°C and had a curtain gas flow rate of 30 mL/min. The ionspray voltage was 5,000 V, with the ion source gases one and two at flow rates of 30 mL/min. The acquisition mode used was multiple reaction monitoring. The retention times (min), declustering potential (V), transition ions (m/z) and corresponding collection energies (eV) for all the compounds can be found in Table I. The total run time for the analytical method was 13 min.
Figure 2.
The chromatographic separation of nine NBOMe derivatives and 25I-NBOMe-d3, the ISTD.
Table I.
The HPLC–MS-MS acquisition parameters
| Designer drug | RT (min) | DP (V) | Trans ions (m/z) | CE (eV) |
|---|---|---|---|---|
| 25H-NBOMe | 7.45 | 45 | 302 > 121 | 26 |
| 302 > 91 | 55 | |||
| 2CC-NBOMe | 7.86 | 40 | 336 > 121 | 25 |
| 336 > 91 | 58 | |||
| 25I-NBF | 7.86 | 60 | 416 > 291 | 26 |
| 416 > 109 | 65 | |||
| 25D-NBOMe | 8.36 | 45 | 316 > 121 | 26 |
| 316 > 91 | 60 | |||
| 25B-NBOMe | 8.77 | 45 | 380 > 121 | 27 |
| 380 > 91 | 65 | |||
| 2CT-NBOMe | 8.86 | 45 | 348 > 121 | 28 |
| 348 > 91 | 60 | |||
| 25I-NBMD | 9.64 | 60 | 442 > 135 | 36 |
| 442 > 77 | 90 | |||
| 25G-NBOMe | 10.08 | 42 | 330 > 121 | 27 |
| 330 > 91 | 60 | |||
| 25I-NBOMe-d3 | 10.68 | 50 | 431 > 124 | 30 |
| 431 > 92 | 75 | |||
| 25I-NBOMe | 10.77 | 55 | 428 > 121 | 30 |
| 428 > 91 | 70 |
Method validation
The evaluation of the urine assay was conducted over 7 separate days. Sample batches were analyzed as recommended for biomedical assay validation (21, 22) for linearity, lower limit of quantification (LOQ), lower limit of detection (LOD), accuracy/bias, precision, dilution integrity, carryover, selectivity, absolute recovery, ion suppression and stability. Stock standard stability was also accessed. Validation sample batches contained calibrators in duplicate, drug-free control (negative control) with ISTD added, a double negative control containing neither NBOMe derivatives nor ISTD and aliquots of the QC urine specimens were analyzed in triplicate on 6 days and for the inter-day precision study in replicates of six on a single day yielding a total of 24 data points for each of the following QC urine specimen containing the NBOMe derivatives: limit of quantification QC (LOQC), target concentration of 1.0 ng/mL; low control (LQC), target concentration of 3.0 ng/mL; medium control (MQC), target concentration of 30 ng/mL; high control (HQC), target concentration of 75 ng/mL; dilution control (DQC), target concentration of 500 ng/mL and a limit of detection control (LODQC), target concentration of 0.10 ng/mL. All QC samples were stored at −20°C until testing.
Linearity, limit of quantification and limit of detection
Linearity was verified from seven point calibration curves prepared in duplicate with concentrations of 1, 2, 5, 10, 25, 50 and 100 ng/mL in ‘in-house’ certified drug-free urine. A linear regression of the ratio of the peak area counts of NBOMe derivatives and 25I-NBOMe-d3 the ISTD versus concentration was used to construct the calibration curves. The linear regression correlation coefficients (r2) for all the NBOMe calibration curves in the seven batches yielded the least fit mean r2 of 0.997 ± 0.004. The LOQ and LOD were administratively set at 1 and 0.1 ng/mL, respectively. LOQC samples were used to verify that the LOQ was within ±20% of the target value and had a response at least five times greater than the signal-to-noise ratio of drug-free urine. The LODQC samples verified that the signal-to-noise ratio was three times the noise level of the background signal from the lot of drug-free urine samples.
Accuracy/bias and precision
Accuracy/bias and precision were determined from the prepared QC urine samples. The largest calculated within-run % coefficient of variation (CV) for each concentration over the seven validation runs was used to assess within-run precision. Between-run precision was calculated for each concentration over the seven validation runs by using the combined QC values (n = 6) from the single day and the daily QC values (n = 3) over 6 days for a total of 24 replicates at each concentration (Table II). The accuracy/bias was determined for all nine NBOMe derivatives using the QC values (n = 6) from a single day. These QC were determined to have a range of 86–116% and did not exceed a 14% CV for all QC samples (Table III).
Table II.
Assay precision
| Precision | Mean concentration (% CV) | Within-run (n = 3) | |||
|---|---|---|---|---|---|
| Designer drug | LOQ (1.0 ng/mL) | Low QC (3.0 ng/mL) | Mid QC (30 ng/mL) | High QC (75 ng/mL) | Dil QC (500 ng/mL) |
| 25H-NBOMe | 1.0 (8) | 3.3 (7) | 29 (4) | 74 (3) | 490 (4) |
| 2CC-NBOMe | 0.9 (8) | 3.3 (4) | 31 (3) | 73 (4) | 490 (4) |
| 25I-NBF | 0.9 (14) | 3.4 (7) | 30 (3) | 71(4) | 490 (2) |
| 25D-NBOMe | 1.0 (8) | 3.1 (8) | 30 (3) | 74 (2) | 490 (4) |
| 25B-NBOMe | 1.0 (12) | 3.2 (5) | 31 (4) | 75 (3) | 480 (2) |
| 2CT-NBOMe | 1.1 (7) | 3.4 (3) | 33 (4) | 70 (4) | 500 (4) |
| 25I-NBMD | 1.0 (6) | 3.0 (5) | 29 (2) | 75 (3) | 470 (2) |
| 25G-NBOMe | 1.0 (7) | 3.2 (3) | 29 (3) | 74 (2) | 480 (3) |
| 25I-NBOMe | 1.0 (11) | 3.1 (4) | 31 (3) | 76 (3) | 460 (2) |
| Between-run 7 days (n = 24) |
|||||
| 25H-NBOMe | 1.0 (6) | 3.1 (7) | 29 (7) | 71 (5) | 480 (5) |
| 2CC-NBOMe | 1.0 (8) | 3.1 (5) | 30 (5) | 71 (4) | 490 (5) |
| 25I-NBF | 1.0 (9) | 3.2 (5) | 30 (4) | 73 (3) | 490 (5) |
| 25D-NBOMe | 1.0 (6) | 3.0 (6) | 28 (5) | 71 (5) | 470 (4) |
| 25B-NBOMe | 1.1 (6) | 3.2 (4) | 30 (4) | 73 (4) | 490 (4) |
| 2CT-NBOMe | 1.1 (9) | 3.2 (5) | 30 (7) | 70 (10) | 480 (4) |
| 25I-NBMD | 1.0 (9) | 3.1 (5) | 29 (3) | 73 (3) | 480 (4) |
| 25G-NBOMe | 1.0 (8) | 3.0 (5) | 29 (6) | 71 (4) | 470 (5) |
| 25I-NBOMe | 1.1 (9) | 3.1 (3) | 30 (4) | 73 (4) | 480 (4) |
Table III.
Accuracy/bias (n = 6)
| Designer drug | Control | Mean concentration ± SD (ng/mL) | Accuracy/bias (%) |
|---|---|---|---|
| 25H-NBOMe | LOQ (1.0 ng/mL) | 1.0 ± 0.1 | 103 |
| Low QC (3.0 ng/mL) | 2.8 ± 0.2 | 94 | |
| Mid QC (30 ng/mL) | 26 ± 1 | 86 | |
| High QC (75 ng/mL) | 67 ± 3 | 89 | |
| Dil QC (500 ng/mL) | 460 ± 11 | 92 | |
| 2CC-NBOMe | LOQ (1.0 ng/mL) | 1.1 ± 0.1 | 106 |
| Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 104 | |
| Mid QC (30 ng/mL) | 28 ± 1 | 92 | |
| High QC (75 ng/mL) | 70 ± 3 | 93 | |
| Dil QC (500 ng/mL) | 488 ± 16 | 98 | |
| 25I-NFB | LOQ (1.0 ng/mL) | 1.1 ± 0.1 | 106 |
| Low QC (3.0 ng/mL) | 3.2 ± 0.1 | 106 | |
| Mid QC (30 ng/mL) | 29 ± 1 | 97 | |
| High QC (75 ng/mL) | 73 ± 3 | 97 | |
| Dil QC (500 ng/mL) | 497 ± 16 | 99 | |
| 25D-NBOMe | LOQ (1.0 ng/mL) | 1.0 ± 0.1 | 102 |
| Low QC (3.0 ng/mL) | 2.9 ± 0.2 | 97 | |
| Mid QC (30 ng/mL) | 26 ± 1 | 88 | |
| High QC (75 ng/mL) | 67 ± 3 | 90 | |
| Dil QC (500 ng/mL) | 466 ± 12 | 93 | |
| 25B-NBOMe | LOQ (1.0 ng/mL) | 1.1 ± 0.1 | 111 |
| Low QC (3.0 ng/mL) | 3.2 ± 0.1 | 107 | |
| Mid QC (30 ng/mL) | 28 ± 1 | 94 | |
| High QC (75 ng/mL) | 71 ± 2 | 95 | |
| Dil QC (500 ng/mL) | 490 ± 15 | 98 | |
| 2CT-NBOMe | LOQ (1.0 ng/mL) | 1.1 ± 0.1 | 112 |
| Low QC (3.0 ng/mL) | 3.2 ± 0.1 | 106 | |
| Mid QC (30 ng/mL) | 28 ± 1 | 94 | |
| High QC (75 ng/mL) | 72 ± 3 | 96 | |
| Dil QC (500 ng/mL) | 495 ± 15 | 99 | |
| 25I-NBMD | LOQ (1.0 ng/mL) | 0.9 ± 0.1 | 89 |
| Low QC (3.0 ng/mL) | 2.9 ± 0.1 | 98 | |
| Mid QC (30 ng/mL) | 29 ± 1 | 95 | |
| High QC (75 ng/mL) | 73 ± 3 | 98 | |
| Dil QC (500 ng/mL) | 494 ± 17 | 99 | |
| 25G-NBOMe | LOQ (1.0 ng/mL) | 1.1 ± 0.1 | 105 |
| Low QC (3.0 ng/mL) | 2.9 ± 0.1 | 96 | |
| Mid QC (30 ng/mL) | 27 ± 1 | 89 | |
| High QC (75 ng/mL) | 69 ± 3 | 92 | |
| Dil QC (500 ng/mL) | 473 ± 17 | 95 | |
| 25I-NBOMe | LOQ (1.0 ng/mL) | 1.2 ± 0.0 | 116 |
| Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 103 | |
| Mid QC (30 ng/mL) | 28 ± 1 | 95 | |
| High QC (75 ng/mL) | 73 ± 3 | 97 | |
| Dil QC (500 ng/mL) | 494 ± 18 | 99 |
Dilution integrity
Dilution integrity was determined from the prepared DQC urine sample. This QC sample was diluted 1 : 10 with drug-free urine before analysis. The largest calculated within-run % CV for each NBOMe over the seven validation runs was used to assess within-run precision. The between-run precision was calculated for each NBOMe derivative over the seven validation runs by using the combined data from all replicates. Both between- and within-run precisions have an accuracy/bias that was determined for all nine NBOMe derivatives to have a range of 92–100% and did not exceed a 5% CV (Table II).
Carryover
Sample carryover was evaluated in each of the seven validation batches using two different procedures. First, immediately following the injection of the 100-ng/mL NBOMe calibrator, a negative control was injected. No carryover was detected in the negative control for any of the NBOMe derivatives. Secondly, an injection of the HQC (75 ng/mL) sample was immediately followed by the injection of the LQC (3 ng/mL) sample. This procedure was routinely applied each time the 100-ng/mL calibrator, HQC (75 ng/mL) and LQC (3 ng/mL) samples were analyzed. The lack of carryover was confirmed as none of the NBOMe derivatives were detected in the negative control and the LQC samples did not demonstrate a significant quantified bias of >20%.
Selectivity
The selectivity of the assay was determined using 10 different lots of NBOMe-free urine. These lots of urines included specimens that were hemolyzed, icteric and lipemic. Each individual lot was analyzed with and without ISTD. No peaks were detected that co-eluted with the targeted NBOMe derivatives or with the ISTD. This ensured that endogenous urine components did not interfere with the assay. Possible inter-subject matrix effects were determined by preparing 10 lots of urines from different sources and fortifying them with 3 ng/mL of each of the NBOMe derivatives. These urine specimens were then analyzed in triplicate. The results deviated by less than ±20% of the prepared concentration in each of the 10 urines for all the NBOMe derivatives except with 25H-NBOMe, 2CC-NBOMe and 25D-NBOMe. The NBOMe derivatives which yielded results that deviated >20% of the prepared concentration of 3 ng/mL were: 25H-NBOMe, 5 of the 10 lots yielded values ranging from 73 to 54% of the expected concentration; 2CC-NBOMe, 3 of the 10 lots yielded 77, 72 and 56% of the expected concentration and 25D-NBOMe, 4 of the 10 lots yielded values ranging from 74 to 66% of the expected concentration. Individual urine matrix effects were shown to potentially decrease the reported concentrations of 25H-NBOMe, 2CC-NBOMe and 25D-NBOMe in specimens that are hemolyzed, icteric or lipemic. No interferences were observed from compounds in the following commercially available control, Liquichek™ Urine Toxicology Control, Level C3, containing the drugs listed in Table IV.
Table IV.
Selectivity: compounds not yielding an interference with the assay
| Liquichek™ urine toxicology control | |
|---|---|
| Drug of abuse | Concentration (ng/mL) |
| d-Amphetamine | 625 |
| d-Methamphetamine | 625 |
| MDMA | 312 |
| 3,4-Methylenedioxyamphetamine (MDA) | 312 |
| 3,4-Methylenedioxy-N-ethylamphetamine (MDEA) | 312 |
| Amobarbital | 250 |
| Butalbital | 250 |
| Pentobarbital | 250 |
| Phenobarbitital | 250 |
| Secobarbital | 250 |
| α-Hydroxyalprazolam | 375 |
| Nordiazepam | 375 |
| Oxazepam | a |
| 11-nor-9-carboxy-delta-9-tetrahydrocannabinol | 18.5 |
| Benzoylecgonine | 185 |
| Ethanol (mg/dL) | 70 |
| LSD | 0.65 |
| Methadone | a |
| EDDP (methadone metabolite) | 375 |
| Methaqualone | 375 |
| 6-Monoacetylmorphine | a |
| Codeine | 2500 |
| Morphine-3-β-d-Glucuronide | 2500 |
| Phencyclidine (PCP) | 31 |
| Propoxyphene | 375 |
| Norpropoxyphene | 375 |
aAnalyte present, no claims made regarding performance or stability.
Absolute recovery and ion suppression
The absolute percent recovery and percent ion suppression of the assay for the NBOMe derivatives was determined at three different concentrations spanning the linear range of the assay at concentrations of 3, 30 and 150 ng/mL (n = 6). The absolute percent recovery was determined by first preparing matrix samples. These samples were prepared at concentrations 1, 3 or 75 ng/mL of the NBOMe derivatives with 10 ng/mL of the ISTD added. First, drug-free urine samples were eluted though the FASt™ columns. Then, the NBOMe derivatives and ISTD were added at the appropriate concentrations. These samples were then brought to their original volume with methanol : water (1∶1). The addition of the drugs to the extracted urines was used to mitigate any matrix effects in the recovery studies. The absolute recovery of the assay was determined by comparing the absolute area of the extracted aliquots of 1, 3 or 75 ng/mL of NBOMe derivatives with 10 ng/mL of ISTD brought to their original volume with methanol : water (1 : 1) solution to the absolute peak area of matrix samples. The ion suppression of the assay was determined by comparing the absolute area of the matrix samples with that of unextracted 1, 3 or 75 ng/mL of NBOMe derivatives with 10 ng/mL of ISTD samples prepared in methanol : water (1 : 1) solutions. The absolute percent recovery of the assay for the nine NBOMe derivatives at 3 ng/mL ranged from 83 to 90%, at 30 ng/mL from 76 to 83% and at 75 ng/mL from 68 to 84% (Table V). Overall, absolute recovery over the linear range of the assay varied from a low of 68% exhibited by 2CT-NBOMe at a concentration of 75 ng/mL, to 90% 25B-NBOMe at a concentration of 3 ng/mL. The absolute recovery of ISTD 25I-NBOMe-d3 was 82 ± 7% (n = 18). The percent ion suppression of the assay for the nine NBOMe derivatives at 3 ng/mL ranged from 20 to 14%, at 30 ng/mL from 14 to 8% and at 75 ng/mL from 10 to 1% (Table V). Overall, percent ion suppression over the linear range of the assay varied from a low of 1% exhibited by 25I-NBOMe at a concentration of 75 ng/mL, to a high of 20% exhibited by 25I-NBF at a concentration of 3 ng/mL. The percent ion suppression of ISTD 25I-NBOMe was 9 ± 7% (n = 18).
Table V.
Recovery and ion suppression
| Designer drug | % Mean ± SD (n = 6) |
|||||
|---|---|---|---|---|---|---|
| 3 ng/mL |
30 ng/mL |
75 ng/mL |
||||
| % Recovery | Ion suppression | % Recovery | Ion suppression | % Recovery | Ion suppression | |
| 25H-NBOMe | 85 ± 4 | 18 ± 10 | 82 ± 6 | 14 ± 8 | 84 ± 8 | 10 ± 8 |
| 2CC-NBOMe | 83 ± 9 | 16 ± 7 | 81 ± 5 | 13 ± 9 | 81 ± 7 | 8 ± 8 |
| 25I-NBF | 90 ± 8 | 20 ± 5 | 81 ± 7 | 11 ± 7 | 79 ± 7 | 5 ± 9 |
| 25D-NBOMe | 85 ± 8 | 14 ± 10 | 80 ± 4 | 10 ± 8 | 83 ± 8 | 8 ± 9 |
| 25B-NBOMe | 90 ± 5 | 17 ± 7 | 83 ± 4 | 8 ± 8 | 79 ± 7 | 3 ± 8 |
| 2CT-NBOMe | 85 ± 7 | 16 ± 7 | 76 ± 6 | 10 ± 8 | 68 ± 7 | 10 ± 9 |
| 25I-NBMD | 85 ± 7 | 15 ± 5 | 80 ± 5 | 8 ± 7 | 78 ± 8 | 2 ± 9 |
| 25G-NBOMe | 85 ± 7 | 14 ± 8 | 80 ± 3 | 9 ± 8 | 83 ± 8 | 5 ± 10 |
| 25I-NBOMe | 89 ± 6 | 17 ± 4 | 82 ± 5 | 8 ± 9 | 79 ± 7 | 1 ± 8 |
Stability
Stability of the NBOMe derivatives in urine was determined under several specific conditions and time intervals. The experiments were performed using three of the NBOMe control specimens: LQC (3 ng/mL), MQC (30 ng/mL) and HQC (75 ng/mL). All studies included six replicate analyses of each QC specimen. Since urine specimens are often stored frozen and thawed for re-analysis, the stability of NBOMe derivatives in urine was determined after three freeze–thaw cycles. The QC specimens were stored at −20°C, then twice removed and allowed to thaw. Once thawed, they were re-frozen for 24 h. The specimens were removed a third time, allowed to thaw and then analyzed. The freeze–thaw QC samples were extracted and quantified against freshly prepared calibrators. The ‘bench-top’ stability of the NBOMe derivatives in urine at room temperature was assessed to evaluate the possible effects of specimen transportation and processing in the laboratory by having the QC specimens sit at room temperature for 72 h. The 1-month QC evaluated the long-term stability of NBOMe in urine. These samples were stored at −20°C for 1 month, then thawed, extracted and quantified against freshly prepared calibrators. Batch analysis was performed with an auto-sampler connected to the HPLC–MS-MS. The ‘postpreparative’ stability of the NBOMe derivatives was evaluated by having extracts sit in the HPLC–MS-MS's auto-sampler. A batch of the extracted LQC, MQC and HQC were quantified against a freshly prepared calibration. The extracted controls were then allowed to sit in the auto-sampler for 72 h at 5°C after which they were re-injected and quantified from the initial calibration. The results of the initial analysis were compared with those of the re-injected samples. NBOMe derivatives were considered stable under the conditions of the freeze–thaw, bench-top and 1-month stability studies if the concentrations of the QC samples were within ±20% of the target concentration samples. In the postpreparative study, NBOMe derivatives were considered stable if the concentrations of the re-injected QC samples were within ±20% of their concentration determined by their initial injection. Under the tested conditions, NBOMe derivatives were stable in frozen or room temperature urine, as well as, in urine extracts in the auto-sampler (Table VI). The sole exception was 2CT-NBOMe which had a −32 and −43% deviation from the target concentrations of the MQC and HQC specimens stored at room temperature for 72 h, respectively.
Table VI.
Stability
| Designer drug | Control | Mean concentration ± SD (ng/mL) | Accuracy/bias (%) |
|---|---|---|---|
| Freeze/thaw (n = 6) | |||
| 25H-NBOMe | Low QC (3.0 ng/mL) | 2.9 ± 0.1 | 97 |
| Mid QC (30 ng/mL) | 28 ± 1 | 93 | |
| High QC (75 ng/mL) | 71 ± 3 | 95 | |
| 2CC-NBOMe | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 102 |
| Mid QC (30 ng/mL) | 29 ± 1 | 96 | |
| High QC (75 ng/mL) | 72 ± 4 | 97 | |
| 25I-NFB | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 103 |
| Mid QC (30 ng/mL) | 30 ± 1 | 100 | |
| High QC (75 ng/mL) | 75 ± 2 | 100 | |
| 25D-NBOMe | Low QC (3.0 ng/mL) | 2.8 ± 0.1 | 93 |
| Mid QC (30 ng/mL) | 27 ± 1 | 91 | |
| High QC (75 ng/mL) | 69 ± 5 | 92 | |
| 25B-NBOMe | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 103 |
| Mid QC (30 ng/mL) | 30 ± 1 | 99 | |
| High QC (75 ng/mL) | 74 ± 3 | 99 | |
| 2CT-NBOMe | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 102 |
| Mid QC (30 ng/mL) | 30 ± 1 | 100 | |
| High QC (75 ng/mL) | 74 ± 3 | 98 | |
| 25I-NBMD | Low QC (3.0 ng/mL) | 2.8 ± 0.1 | 95 |
| Mid QC (30 ng/mL) | 29 ± 1 | 97 | |
| High QC (75 ng/mL) | 73 ± 1 | 97 | |
| 25G-NBOMe | Low QC (3.0 ng/mL) | 2.9 ± 0.1 | 96 |
| Mid QC (30 ng/mL) | 27 ± 1 | 93 | |
| High QC (75 ng/mL) | 72 ± 5 | 95 | |
| 25I-NBOMe | Low QC (3.0 ng/mL) | 2.9 ± 0.1 | 98 |
| Mid QC (30 ng/mL) | 29 ± 1 | 95 | |
| High QC (75 ng/mL) | 73 ± 2 | 97 | |
| Bench top 72 h (n = 6) | |||
| 25H-NBOMe | Low QC (3.0 ng/mL) | 3.0 ± 0.3 | 99 |
| Mid QC (30 ng/mL) | 27 ± 1 | 92 | |
| High QC (75 ng/mL) | 68 ± 2 | 91 | |
| 2CC-NBOMe | Low QC (3.0 ng/mL) | 3.0 ± 0.3 | 101 |
| Mid QC (30 ng/mL) | 28 ± 0 | 93 | |
| High QC (75 ng/mL) | 69 ± 2 | 92 | |
| 25I-NFB | Low QC (3.0 ng/mL) | 3.1 ± 0.2 | 102 |
| Mid QC (30 ng/mL) | 28 ± 1 | 95 | |
| High QC (75 ng/mL) | 73 ± 3 | 97 | |
| 25D-NBOMe | Low QC (3.0 ng/mL) | 3.0 ± 0.2 | 100 |
| Mid QC (30 ng/mL) | 26 ± 0 | 86 | |
| High QC (75 ng/mL) | 65 ± 2 | 87 | |
| 25B-NBOMe | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 103 |
| Mid QC (30 ng/mL) | 29 ± 1 | 95 | |
| High QC (75 ng/mL) | 74 ± 2 | 98 | |
| 2CT-NBOMe | Low QC (3.0 ng/mL) | 2.6 ± 0.2 | 86 |
| Mid QC (30 ng/mL) | 20 ± 1 | 68 | |
| High QC (75 ng/mL) | 43 ± 1 | 57 | |
| 25I-NBMD | Low QC (3.0 ng/mL) | 2.9 ± 0.1 | 96 |
| Mid QC (30 ng/mL) | 29 ± 1 | 91 | |
| High QC (75 ng/mL) | 71 ± 1 | 95 | |
| 25G-NBOMe | Low QC (3.0 ng/mL) | 2.9 ± 0.3 | 98 |
| Mid QC (30 ng/mL) | 26 ± 0 | 87 | |
| High QC (75 ng/mL) | 67 ± 2 | 90 | |
| 25I-NBOMe | Low QC (3.0 ng/mL) | 2.8 ± 0.2 | 94 |
| Mid QC (30 ng/mL) | 27 ± 1 | 90 | |
| High QC (75 ng/mL) | 71 ± 2 | 94 | |
| 72 h Post-prep (n = 3) | |||
| 25H-NBOMe | Low QC (3.0 ng/mL) | 3.5 ± 0.2 | 118 |
| Mid QC (30 ng/mL) | 35 ± 1 | 117 | |
| High QC (75 ng/mL) | 85 ± 4 | 113 | |
| 2CC-NBOMe | Low QC (3.0 ng/mL) | 3.6 ± 0.0 | 119 |
| Mid QC (30 ng/mL) | 33 ± 2 | 109 | |
| High QC (75 ng/mL) | 80 ± 3 | 107 | |
| 25I-NFB | Low QC (3.0 ng/mL) | 3.1 ± 0.2 | 103 |
| Mid QC (30 ng/mL) | 29 ± 1 | 95 | |
| High QC (75 ng/mL) | 70 ± 2 | 93 | |
| 25D-NBOMe | Low QC (3.0 ng/mL) | 3.5 ± 0.1 | 117 |
| Mid QC (30 ng/mL) | 34 ± 2 | 114 | |
| High QC (75 ng/mL) | 85 ± 3 | 113 | |
| 25B-NBOMe | Low QC (3.0 ng/mL) | 3.3 ± 0.3 | 110 |
| Mid QC (30 ng/mL) | 30 ± 1 | 101 | |
| High QC (75 ng/mL) | 74 ± 2 | 98 | |
| 2CT-NBOMe | Low QC (3.0 ng/mL) | 3.2 ± 0.3 | 108 |
| Mid QC (30 ng/mL) | 29 ± 3 | 97 | |
| High QC (75 ng/mL) | 71 ± 1 | 95 | |
| 25I-NBMD | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 104 |
| Mid QC (30 ng/mL) | 30 ± 1 | 100 | |
| High QC (75 ng/mL) | 77 ± 2 | 102 | |
| 25G-NBOMe | Low QC (3.0 ng/mL) | 3.4 ± 0.1 | 112 |
| Mid QC (30 ng/mL) | 32 ± 0 | 107 | |
| High QC (75 ng/mL) | 81 ± 2 | 107 | |
| 25I-NBOMe | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 104 |
| Mid QC (30 ng/mL) | 30 ± 1 | 100 | |
| High QC (75 ng/mL) | 77 ± 2 | 102 | |
| One month (n = 6) | |||
| 25H-NBOMe | Low QC (3.0 ng/mL) | 3.1 ± 0.0 | 103 |
| Mid QC (30 ng/mL) | 31 ± 1 | 104 | |
| High QC (75 ng/mL) | 69 ± 2 | 92 | |
| 2CC-NBOMe | Low QC (3.0 ng/mL) | 3.1 ± 0.0 | 104 |
| Mid QC (30 ng/mL) | 31 ± 0 | 105 | |
| High QC (75 ng/mL) | 69 ± 1 | 92 | |
| 25I-NFB | Low QC (3.0 ng/mL) | 3.2 ± 0.1 | 106 |
| Mid QC (30 ng/mL) | 32 ± 1 | 107 | |
| High QC (75 ng/mL) | 71 ± 3 | 95 | |
| 25D-NBOMe | Low QC (3.0 ng/mL) | 3.0 ± 0.1 | 99 |
| Mid QC (30 ng/mL) | 30 ± 0 | 99 | |
| High QC (75 ng/mL) | 68 ± 1 | 94 | |
| 25B-NBOMe | Low QC (3.0 ng/mL) | 3.3 ± 0.1 | 108 |
| Mid QC (30 ng/mL) | 31 ± 1 | 104 | |
| High QC (75 ng/mL) | 70 ± 1 | 93 | |
| 2CT-NBOMe | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 102 |
| Mid QC (30 ng/mL) | 30 ± 1 | 99 | |
| High QC (75 ng/mL) | 61 ± 2 | 82 | |
| 25I-NBMD | Low QC (3.0 ng/mL) | 3.1 ± 0.1 | 104 |
| Mid QC (30 ng/mL) | 30 ± 0 | 101 | |
| High QC (75 ng/mL) | 69 ± 2 | 92 | |
| 25G-NBOMe | Low QC (3.0 ng/mL) | 3.2 ± 0.1 | 105 |
| Mid QC (30 ng/mL) | 31 ± 0 | 104 | |
| High QC (75 ng/mL) | 70 ± 2 | 94 | |
| 25I-NBOMe | Low QC (3.0 ng/mL) | 3.2 ± 0.1 | 108 |
| Mid QC (30 ng/mL) | 31 ± 0 | 103 | |
| High QC (75 ng/mL) | 71 ± 2 | 94 | |
Stock standard stability
The stability of stock solutions of the NBOMe derivatives and the ISTD, 25I-NBOMe-d3, was evaluated by allowing aliquots of the stock standards to sit at room temperature for 6 h. These aliquots and freshly prepared stock standards were analyzed by the presented HPLC–MS-MS method. The absolute areas of aliquots of NBOMe derivatives kept at room temperature for 6 h were compared with the freshly prepared stock standards. The absolute percent difference of the three stock standards used in the preparation of the assay for the nine NBOMe derivatives and the ISTD, 25I-NBOMe-d3, was within −6 and 5% of the expected values.
Application of the method
Applying the presented method, we detected the presence of NBOMe derivatives in four specimens from emergency room patients. These patients presented with signs and symptoms of drug intoxication including: tachycardia, hypertension, severe agitation and seizures. The specimens were kept at −20°C until analysis. Four different NBOMe derivatives were detected; however, 25I-NBOMe, consistent with its apparent popularity, was detected in three of the specimens. One specimen was determined to contain only 25B-NBOMe at 1.7 ng/mL while another contained only 25I-NBOMe at <1.0 ng/mL, and this specimen quantitated to 0.1 ng/mL equal to the LOD. The specimen containing 25B-NBOMe had previously been found to contain 1.9 ng/mL by a different HPLC–MS-MS method (20). That assay used liquid/liquid extraction, a different analytical column and different instrumental parameters. The re-analysis of the same urine specimen by the presented method demonstrated a good correlation between these analytical methods. The other urines contained 25I-NBOMe with smaller concentrations of other NBOMe derivatives; one contained 2.3 ng/mL of 25I-NBOMe and <1.0 mg/mL of 2CC-NBOMe; and the other 1.2 ng/mL of 25I-NBOMe and <1.0 ng/mL of 25H-NBOMe.
Results and discussion
The presented HPLC–MS-MS method demonstrated acceptable reliability and reproducibility for the detection and quantification of nine NBOMe derivatives in urine specimens using a rapid solid-phase extraction method. Accuracy as well as intra- and inter-day precision of the assay were determined not to exceed CVs of ±10% over the dynamic range of the assay. This performance is consistent with industry standards for urine drug testing. It certainly demonstrates the robustness of the assay. Additionally, the assay was free of significant interference from matrix effects and free from significant analyte carryover. Three of the NBOMe derivatives, such as 25H-NBOMe, 2CC-NBOMe and 25D-NBOMe, demonstrated a potential for individual specimen matrix effect when hemolyzed, icteric and lipemic urine specimens are analyzed. These individual matrix effects can affect quantification of the urine concentrations. The use of deuterated ISTDs for these compounds should help reduce this effect if or when they become available. The NBOMe derivatives were shown to be stable under conditions of specimen handling in the laboratory with the exception of 2CT-NBOMe, which was found to be unstable at room temperatures after 72 h but stable at −20°C for 1 month. Therefore, specimens suspected to contain 2CT-NBOMe should be kept frozen.
While the presented method includes the detection and quantification of nine NBOMe derivatives, previously published HPLC–MS-MS methods have only addressed the analysis of one or two NBOMe derivatives. Validated serum and whole blood methods for the determination of 2CC-NBOMe, 25B-NBOMe and 25I-NBOMe have been used to report urine concentrations. Unlike the presented assay, these methods did not use a deuterated ISTD and involved a time-consuming liquid/liquid or traditional solid-phase extraction (17, 20, 23, 24). A urine HPLC–MS-MS method with limited validation data for the determination of only 25I-NBOMe and 25H-NBOMe was included as part of a case report (19). This method used a simple dilution of the urine prior to injection and reported urine concentrations of 7.5 ng/mL of 25I-NBOMe and 0.9 ng/mL of 25H-NBOMe. The presented method used a similar dilution method, but added the extraction through the use of the Clean Screen FASt™ extraction columns to quickly and efficiently reduce the amount of unwanted matrix and particulates in the final sample to lessen the chance of matrix produced ion suppression and analytical column clogging.
The presented assay allows for the determination of six of the seven NBOMe derivatives reported present on blotter paper or in human specimens: 25H-NBOMe, 2CC-NBOMe, 25D-NBOMe, 25B-NBOMe, 25I-NBMD and 25I-NBOMe. No primary reference material is presently available for 25E-NBOMe, which has been detected on blotter paper (8). 25E-NBOMe could be easily added to the method when a primary reference material becomes available. At present, two NBOMe derivatives determined by the presented method, 2CT-NBOMe and 25I-NBF, have not been reported to be available on the designer drug market. They were included in the method because primary reference materials were available. As these drugs are partial agonists of the 5-HT2A receptor (11), they were included in the presented method as an attempt to stay ahead of the ever evolving designer drug market.
Conclusion
A HPLC–MS-MS method for the determination of nine NBOMe derivatives in urine was developed. It used a simple FASt™ column extraction procedure prior to chromatographic analysis, and it is particularly suited for analysis of urine samples from emergency room patients. Furthermore, the assay may be easily adapted for the analysis of NBOMe derivatives in research and forensic urine specimens.
Funding
This project was supported by the National Institutes of Health (NIH) Center grant P30DA033934.
Conflict of interest
None declared.
References
- 1.Geyer M.A., Vollenweider F.X. Serotonin research: contributions to understanding psychoses. Trends in Pharmacological Sciences. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Abbas A., Roth B.L. Pimavanserin tartrate: a 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders. Expert Opinion on Pharmacotherapy. 2008;9:3251–3259. doi: 10.1517/14656560802532707. [DOI] [PubMed] [Google Scholar]
- 3.Nichols D.E. Hallucinogens. Pharmacology and Therapeutics. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Shulgin A.T., Carter M.F. Centrally active phenethylamines. Psychopharmacol Commun. 1975;1:93–98. [PubMed] [Google Scholar]
- 5.Shulgin A., Shulgin A. Berkeley, CA: Transform Press; 1991. PIHKAL: A Chemical Love Story. [Google Scholar]
- 6.Dean B.V., Stellpflug S.J., Burnett S.M., Engebretsen K.M. 2C or not 2C: phenethylamine designer drug review. Journal of Medical Toxicology. 2013;9:172. doi: 10.1007/s13181-013-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glennon R.A., Kier L.B., Shulgin A.T. Molecular connectivity analysis of hallucinogenic mescaline analogs. Journal of Pharmaceutical Sciences. 1979;68:906–907. doi: 10.1002/jps.2600680733. [DOI] [PubMed] [Google Scholar]
- 8.Zuba D., Sekuła K. Analytical characterization of three hallucinogenic N-(2-methoxy)benzyl derivatives of the 2C-series of phenethylamine drugs. Drug Testing and Analysis. 2013;8:634–645. doi: 10.1002/dta.1397. [DOI] [PubMed] [Google Scholar]
- 9.Zuba D., Sekula K., Buczek A. 25C-NBOMe-new potent hallucinogenic substance identified on the drug market. Forensic Science International. 2013;227:7–14. doi: 10.1016/j.forsciint.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Sekuła K., Zuba D. Structural elucidation and identification of a new derivative of phenethylamine using quadrupole time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2013;18:2081–2090. doi: 10.1002/rcm.6667. [DOI] [PubMed] [Google Scholar]
- 11.Braden M.R., Parrish J.C., Naylor J.C., Nichols D.E. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Molecular Pharmacology. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- 12.Nichols D.E., Frescas S.P., Chemel B.R., Rehder K.S., Zhong D., Lewin A.H. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): a high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorganic and Medicinal Chemistry. 2008;16:6116–6123. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettrup A., Holm S., Hansen M., Wasim W., Santini M.A., Palner M., et al. Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [11C]Cimbi-36. Molecular Imaging and Biology. 2013;15:376. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- 14.2013. Erowid. 25IeNBOMe (2C-I-NBOMe) Dose http://www.erowid.org/chemicals/2ci_nbome/2ci_nbome_dose.shtml. (accessed Aug 10, 2013)
- 15.Kelly A., Eisenga B., Riley B., Judge B. Case series of 25I-NBOMe exposures with laboratory confirmation. Clinical Toxicology (Philadelphia) 2012;50:702. [Google Scholar]
- 16.Rose S.R., Cumpston K.L., Stromberg P.E., Wills B.K. Severe poisoning following self-reported use of 25-I, a novel substituted amphetamine. Clinical Toxicology (Philadelphia) 2012;50:707–708. [Google Scholar]
- 17.Rose R.S., Poklis J.L., Poklis A. A case of 25I-NBOMe (25-I) intoxication: a new potent 5HT2a agonist designer drug. Clinical Toxicology (Philadelphia) 2013;51:174–177. doi: 10.3109/15563650.2013.772191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill S.L., Doris T., Gurung S., Katebe S., Lomas A., Dunn M., et al. Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clinical Toxicology (Philadelphia) 2013;51:487–492. doi: 10.3109/15563650.2013.802795. [DOI] [PubMed] [Google Scholar]
- 19.Stellpflug S.J., Kealey S.E., Hegarty C.B., Janis G.C. 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe): clinical case with unique confirmatory testing. Journal of Medical Toxicology. 2013 doi: 10.1007/s13181-013-0314-y. doi:10.1007/s13181-013-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poklis J.L., Nanco C.R., Troendle M.M., Wolf C.E., Poklis A. Determination of 4-bromo-2,5-dimethoxy-N-[(2-methoxyphenyl)methyl]-benzeneethanamine (25B-NBOMe) in serum and urine by high performance liquid chromatography with tandem mass spectrometry in a case of severe intoxication. Drug Testing and Analysis. 2013 doi: 10.1002/dta.1522. doi:10.1002/dta.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2001. [Google Scholar]
- 22.Report from the Scientific Working Group for Forensic Toxicology: Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. Journal of Analytical Toxicology. 2013;37:452–474. doi: 10.1093/jat/bkt054. [DOI] [PubMed] [Google Scholar]
- 23.Poklis J.L., Charles J., Wolf C.E., Poklis A. High performance liquid chromatography tandem mass spectrometry method for the determination of 2CC-NBOMe and 25I-NBOMe in human serum. Biomedical Chromatography. 2013 doi: 10.1002/bmc.2999. doi:10.1002/bmc.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poklis J.L., Devers K.G., Arbefeville E.F., Julia M., Pearson J.M., Houston E., et al. Postmortem detection of 25I-NBOMe [2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2 methoxyphenyl)methyl]ethanamine] in fluids and tissues determined by high performance liquid chromatography with tandem mass spectrometry from a traumatic death. Forensic Science International. 2013. doi:10.1016/j.forsciint.2013.10.015. [DOI] [PMC free article] [PubMed]