Abstract
Recently, clinical application of autogenous tooth bone-graft materials has been reported. Autogenous tooth bone graft has been used in implant surgery. Familial tooth bone graft is a more advanced procedure than autogenous teeth bone graft in that extracted teeth can be used for bone graft materials of implant and teeth donation between siblings is possible. We used autogenous tooth and familial tooth bone-graft materials for ridge augmentation and sinus bone graft and obtained satisfactory results. The cases are presented herein.
Keywords: Familial tooth, Alveolar ridge augmentation, Sinus floor augmentation
I. Introduction
Ridge augmentation refers to the procedure that was performed for cases with insufficient vertical or horizontal volume of bone to augment the height or width of the alveolar ridge by executing particulated or block bone graft. Vertical and horizontal augmentation can be performed simultaneously or separately. Ridge augmentation is a type of onlay graft, and thus, the possibility of bone resorption after grafting and dehiscence of the upper soft tissues is high. The procedure involves one wall of the bony defect, and the healing of grafted tissues is dependent on the blood supply from the recipient bone tissues. Therefore, autogenous bone graft is strongly recommended for alveolar ridge augmentation1.
The maxillary sinus is wrapped well within its walls, and maxillary sinus bone graft shows healing similar to the wound created by tooth extraction. Therefore, use of any biocompatible bone graft material will result in good healing. Nevertheless, for cases whose residual bone volume is definitely insufficient and cases with poor blood supply, or to shorten the healing period, autogenous bone graft is recommended2,3. Nonetheless, due to several problems associated with the harvest of autogenous bone, recently, the possibility that autogenous tooth bone graft may be a substitution for autogenous bone graft has been suggested4. Autogenous tooth bone grafts always require that the tooth of the patient be extracted, and thus, its use is limited in many cases. Therefore, familial tooth bone grafts that treat the extracted teeth of immediate family members as bone-graft materials have been introduced. Such cases are presented together with relevant results from the literature.
II. Cases Report
1. Case 1
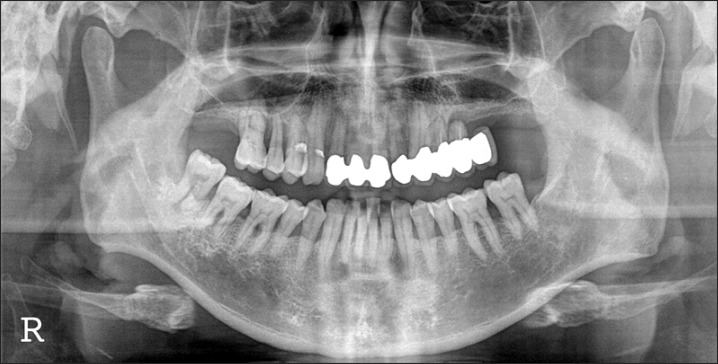
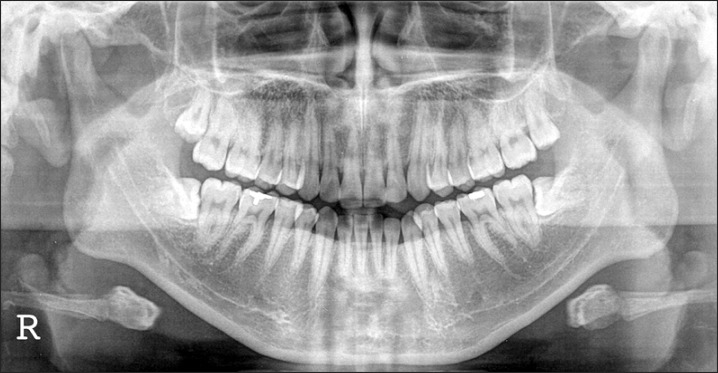
A 45-year-old male patient was transferred from another dental clinic for maxillary anterior horizontal ridge augmentation. The dentist planned to insert a 6-unit fixed prosthesis after implant placement in the #13 and #23 areas. The condition of the #13 and #23 areas involved an alveolar ridge of insufficient width.(Fig. 1) The decision was made to extract the lower right 3rd molar (#48) of the patient and to treat this tissue as autogenous tooth bone graft material. The dentist planned to extract two impacted 3rd molars from the patient's daughter, prepare them as blocks and powder, and use them as graft materials.(Figs. 2, 3) On April 10th, 2010, the lower right 3rd molar of the patient was extracted and prepared as powder-type graft material. On the same day, the impacted 3rd molars of the daughter were extracted, prepared as block and powder graft materials, and stored. On April 26th, 2010, in the maxillary anterior edentulous area (#13-#23), a flap was elevated by crestal incision. Block graft materials were grafted to the labial side of the maxillary canine area (#13 and #23 labial area), and additionally, powder graft materials were grafted to the vicinity with tissue adhesive (Greenplast; Green Cross Corp., Yongin, Korea).(Figs. 4-6) The area was covered with an absorbable collagen membrane (Ossix Plus; OraPharma Inc., Louis Drive Warminster, PA, USA), and the wound was sutured. Good bone healing without special complications was observed, and the patient was returned to the referred dental clinic for implant placement.(Fig. 7) Eight months after the surgery, implantation was performed at a local clinic. The condition of horizontal ridge augmentation was good, and thus 4 implants were placed in the #13, #12, #22, and #23 areas. After 1 week, a temporary prosthesis was installed.(Figs. 8, 9) The final prosthesis was carried out in local clinic successfully, further follow up was not performed.
Fig. 1.
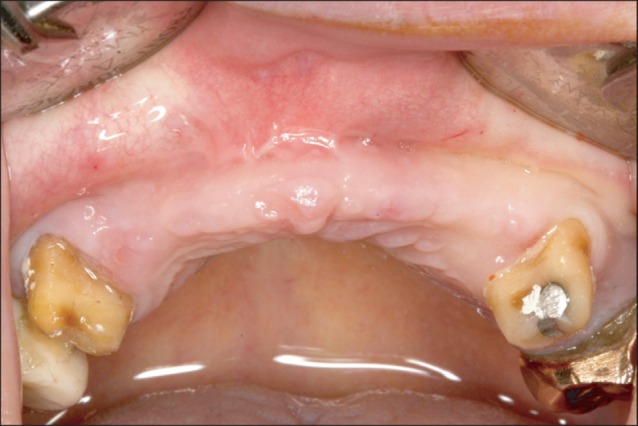
A photograph of the oral cavity prior to surgery.
Fig. 2.
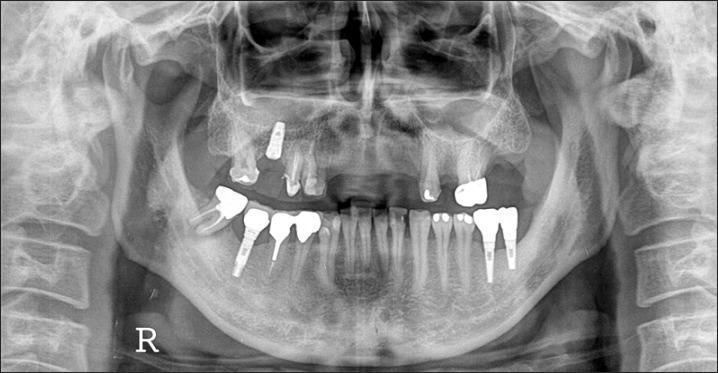
A panorama radiograph at the initial diagnosis. The dentist at another clinic was expected to place implants in the #13 and #23 areas. The patient was referred to us for horizontal augmentation. The #48 area was planned for extraction and used as bone graft material.
Fig. 3.
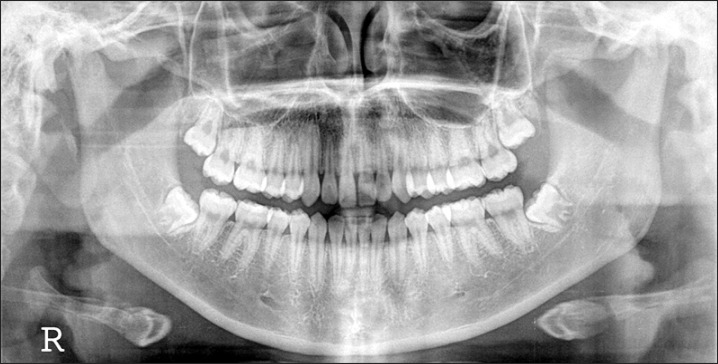
A panorama radiograph of a 15-year-old female patient who was the daughter of the patient. The decision was made to extract the #18 and #48 impacted molars and prepare them as block and powder bone-graft materials.
Fig. 4.
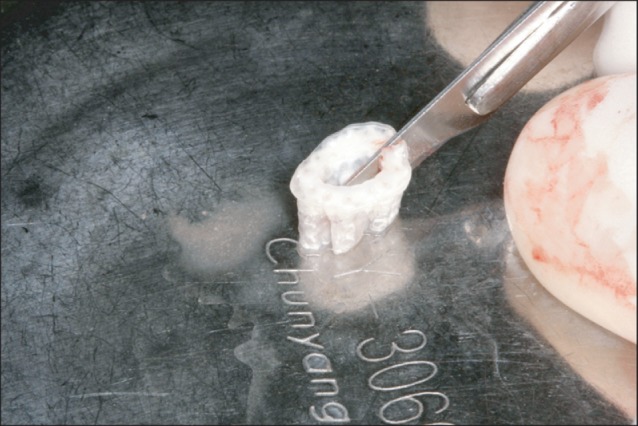
The appearance of trimmed familial tooth bone blocks. Block-type graft materials were hydrated for 30 minutes in normal saline, divided to two blocks with a #15 blade.
Fig. 6.
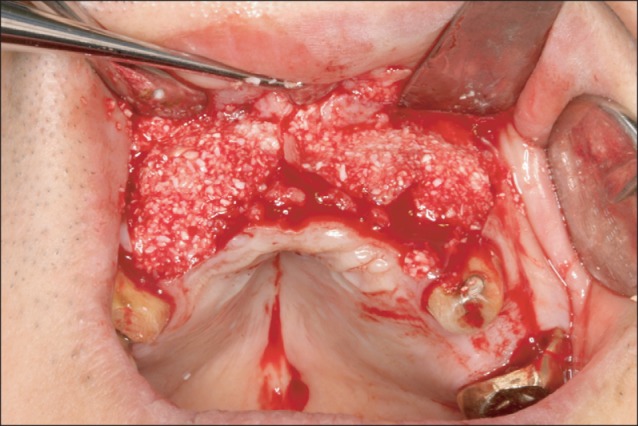
The appearance after grafting powder-type bone-graft materials in the vicinity of the block.
Fig. 7.
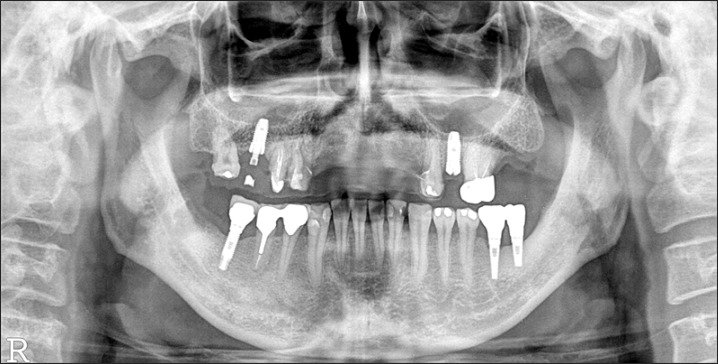
A panorama radiograph taken 5 months after bone graft. During the healing period, implants were placed in the maxillary left 2nd premolar area in a local clinic.
Fig. 8.
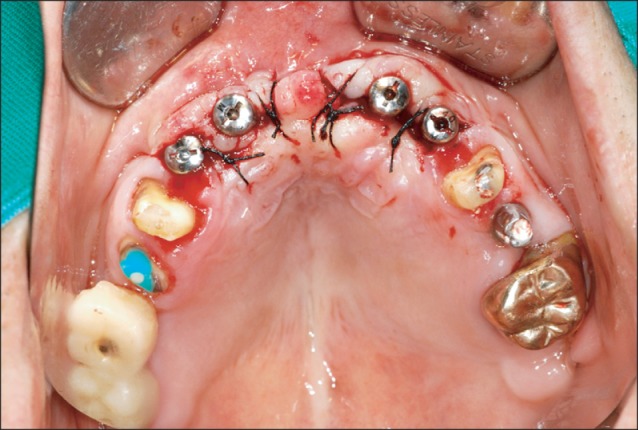
The appearance after implant placement. Eight months after bone graft, in a local clinic, 4 implants were placed in a non-submerged type. The early fixation was excellent, and thus, after 1 week, a temporary prosthesis was installed (Anyang More Dental Clinic, Byoengdoek Yu and Gyuhyong Lee's case).
Fig. 9.
A panoramic radiograph taken 3 months after the installation of a temporary prosthesis (Anyang More Dental Clinic, Byoengdoek Yu and Gyuhyong Lee's case).
2. Case 2
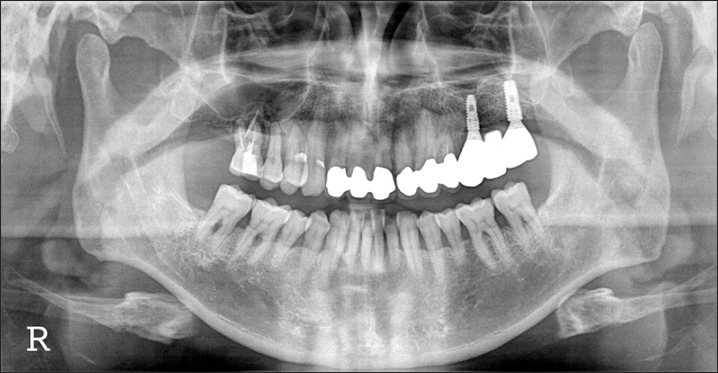
A 49-year-old male patient visited our clinic for pain in the maxillary left 2nd premolar (#25) and the treatment of implants in the 1st and 2nd molar areas (#26-#27). After diagnosis of an abscess in the #25 root apex, tooth extraction was decided upon. The patient was scheduled to undergo extraction of the mandibular right impacted 3rd molar (#48 impacted molar). This tooth and an impacted tooth from his son were prepared as graft materials, and maxillary bone graft was performed in the #26-#27 areas. Delayed implant placement was planned.(Figs. 10, 11) On April 22nd, 2010, the #48 was extracted, and the left impacted 3rd molar of the patient's 22-year-old son was extracted. These teeth were prepared as powder bone-graft materials. On May 7th, 2010, the prosthesis between the #24-#25 area was cut, and the #25 was extracted. A flap was elevated by performing crestal incision on the #25-#27 area, and maxillary sinus bone graft was performed by lateral approach. Autogenous and familial tooth bone graft in powder form were used as bone-graft materials. On August 3rd, 2010, implants (Osstem TS III SA; Osstem, Seoul, Korea, #25: 4 diameter/11.5 length, #27: 5 diameter/11.5 length) were placed in the #25 and #27 area, in a non-submerged type. The primary stability value measured by the Osstell mentor (Integration Diagnostics AB, Savedalen, Sweden) was shown to be #25: 67 implant stability quotient (ISQ) and #27: 71 ISQ, respectively.(Fig. 12) Prior to implant placement, a bony specimen was harvested from the #27 area using a 2.0 mm diameter trephine bur. A healing abutment was connected, and the wound was sutured. On December 30, 2010, a final prosthesis was installed (Prosthetic treatment was performed by Dr. Yangjin Yi).(Fig. 13)
Fig. 10.
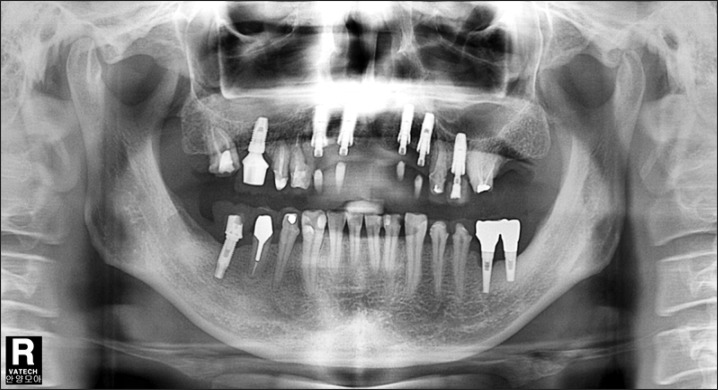
A panorama radiograph at the time of initial diagnosis. The abscess in the #25 root apex and periodontitis in the #15-#16 were in severe condition. The decision was made to first treat the left maxillary molar area. We planned to extract the #48 and treat with autogenous tooth bone-graft materials.
Fig. 11.
A panorama radiograph of the 22-year-old son. The #28 and #38 teeth were extracted and prepared as powder bone-graft materials.
Fig. 12.
A panorama radiograph after implant placement. Implants were placed 3 months after bone graft.
Fig. 13.
A panorama radiograph 24 months after final prosthetic delivery.
The bone tissue specimens collected after 3 months were examined under light microscope. It was confirmed that in the sinus bone graft area, new bone had formed actively in the vicinity of graft materials. New bones formed along the border of the graft material were woven bones, and some bones had already formed short trabecular patterns. In some areas, they formed loose anastomosis with other trabecular new bones formed along the border of other graft materials. The more developed new trabecular bones were connected to the residual alveolar bone in a pattern that was more densely anastomosed.(Figs. 14, 15)
Fig. 14.
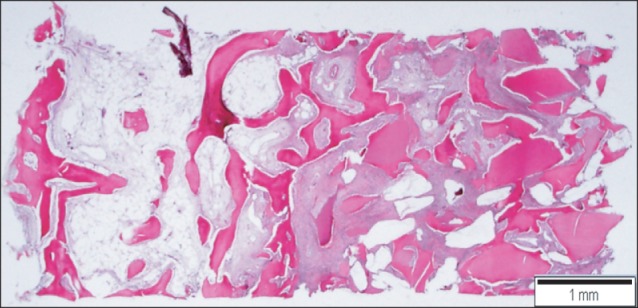
Scanning view of the implantation site. New bone formation is demonstrated around the implant materials. Left: residual alveolar bone site, right: sinus bone graft area. H&E staining, scale bar=1 mm.
Fig. 15.
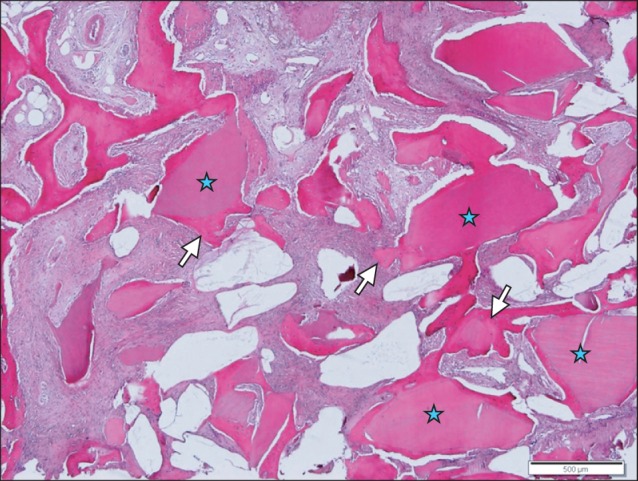
New bone forming anastomosing trabeculae (arrows) is identified around the implant materials (asterisks). H&E staining, scale bar=500 µm.
III. Discussion
It has been reported that in allogenic tooth graft between parents and children and between siblings, specific immune reactions are delayed or reduced if tissue-located histocompatibility antigens (H-antigens) are compatible. Kim et al.5 have reported that if the teeth of 9 to 11-year-old children whose orthodontic therapy was planned were transplanted to the defect area of their parents or siblings, good results can be anticipated.
Through a treatment process similar to that used for autogenous tooth bone-graft materials, the teeth extracted from immediate family members were preserved, treated, and stored. Tooth material was prepared as bone graft material; this could be donated, distributed, and grafted to the family for therapeutic purposes. Here, the term "family" refers to brothers, sisters, parents or children. The extraction and the tooth to be donated were teeth that could not be saved by dental treatments, the deciduous teeth, and the wisdom teeth. When a tooth is extracted from a family member and donated or received, the dental clinic obtains the individual's consent for donation as well as consent for transplantation. The consent is signed by the patients themselves or guardians, and a written agreement for treatment of the extracted tooth is signed. Donors are submitted to basic tests for venereal diseases and acquired immunodeficient virus if requested by the donor or the recipient. The recipient should prepare a document that proves the family relationship and sign agreement to transplantation6.
Isograft refers to the graft between genetically identical individuals (e.g., monozygotic twins). It has been reported that monozygotic twins have identical major histocompatibility complexes, and thus graft rejection reactions never occur. Therefore, this type of graft is favored in the context of organ transplant. Isograft is generally considered to be an autologous graft7. The genetic combination involved in tooth bone graft among immediate family members is not 100% identical; nonetheless, it shows a similar trend and the risk for immune rejection reactions is almost removed by the treatments (i.e., decalcification and freeze-drying). Allogenic bone-graft materials treated with decalcification processes do not require tissue compatibility tests such as ABO typing. Because familial tooth bone-graft materials are also treated with the decalcification process, special tissue compatibility tests are not required7.
Barrett and Reade8 performed tooth isograft in murine renal subcapsular sites and histologically evaluated the results. It has been reported that in teeth in which the root was not completely formed, the formation of alveolar bone and periodontal ligaments was observed. However, in the teeth with complete roots, formation of neither alveolar bone nor periodontal ligaments was observed. In this study, after the isograft of incomplete teeth, the alveolar bones were formed. This process may be mediated by osteoconduction and indirect osteoinduction. In addition, in 1982, experimental studies were conducted on the isograft of immature molars in the intraosseous as well as extraosseous areas in mice. Bone formation was observed not only in the tibial area but also in the kidney area9. Steidler and Reade10 examined the effect of the extracorporeal time involved in tooth isograft in rodents. It was observed that if the extracorporeal time was longer than 30 minutes, pulp degeneration, necrosis of the cementum, resorption of the roots, and osseointegration occurred. In this study, the new bone formation was observed in the vicinity of tooth isograft in the tibia. It could be speculated to be the result of osteoinduction caused by the tooth.
Barrett and Reade11,12 have reported the following results of animal experiments that were performed for the immunological examination of tooth isograft. The 3rd molar tooth of mature mice was grafted to the tibial shaft medulla and the renal subcapsular kidney site. Histological tests were performed after 6 months. It was observed that in both sites, early pulp degeneration was detected, and the continuous regeneration process associated with the formation of dentin was observed. Tibial shaft isografts were surrounded by bones, and continuous resorption initiated by the coronal dentin was observed. The teeth grafted to the subcapsular kidney site were not surrounded by bones, and coronal resorption findings were not shown. In other words, with regard to the tooth isograft transplanted in the intraosseous area, diverse levels of root resorption and osseointegration were observed. Nevertheless, the reformation of periodontal ligaments was not observed.
We used familial tooth bone-graft materials for alveolar ridge augmentation and maxillary bone graft and obtained good clinical outcomes. The impacted third molars of the patients themselves were extracted and prepared as autogenous tooth bone-graft materials. Nonetheless, the volume was not sufficient, and thus, the bone-graft materials prepared from the impacted third molar of their children were used in parallel. Other bone-graft materials were not used. Four months after alveolar ridge augmentation and 3 months after maxillary bone graft, implants were placed secondarily. Bone healing was good, and the early stability of implants could be obtained readily. The results of histological tests of the specimens collected from case 2 showed excellent new bone formation and bone remodeling phenomena. In this study, only 2 cases were presented, and a mixture of autogenous tooth bone materials and family tooth bone materials was used. The problem of Case 1 was that further follow up was not performed in our hospital after completion of final prosthesis in local clinic. The grafted bone in sinus and peri-implant bone were maintained well in Case 2. Thus, it is difficult to perform an accurate clinical evaluation of familial tooth bone graft. Prospective clinical and histomorphometric studies involving more cases are required.
Fig. 5.
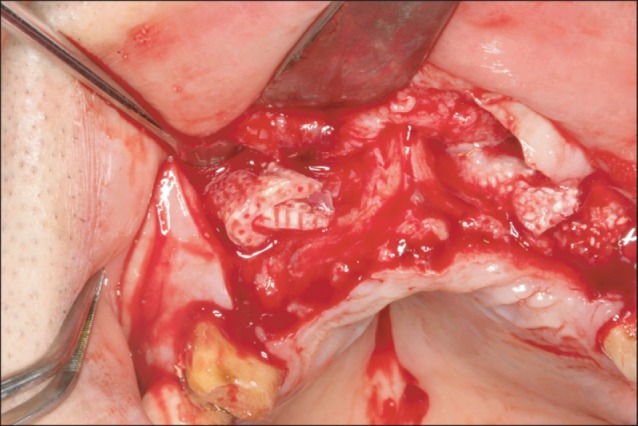
Insertion of the block to the labial side of the maxillary canine area. It was not specially fixed with screws.
Acknowledgements
We wish to thank Dr. Byoengdoek Yu and Gyuhyong Lee from Anyang More Dental Clinic for their work on the prosthodontic procedure.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kim YK, Yi YJ, Yun PY, Yeo IS, Lee HJ, Kim SG, et al. All about implants in Q&A (I) Yongin: Korea Quintessence; 2010. pp. 304–331. [Google Scholar]
- 2.Kim SG, Lee BG. Bone graft and implant. Vol. 2-2. Clinical application of a variety of bone graft. Seoul: Narae Publishing; 2007. pp. 240–258. [Google Scholar]
- 3.Del Fabbro M, Testori T, Francetti L, Weinstein R. Systematic review of survival rates for implants placed in the grafted maxillary sinus. Int J Periodontics Restorative Dent. 2004;24:565–577. [PubMed] [Google Scholar]
- 4.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Kim YM, Kim MR. Present and future of autogenout tooth graft. Dent Success. 2010;30:790–800. [Google Scholar]
- 6.Korea Auto-Tooth and Bone Bank Committee. Familial tooth bank standards. 2010. [Google Scholar]
- 7.Pearson KA, Brubaker SA, Anderson ML. Standards for tissue banking. 12th ed. McLean: American Association of Tissue Banks (AATB); 2008. pp. 13–108. [Google Scholar]
- 8.Barrett AP, Reade PC. A histological investigations of isografts of immature mouse molars to an intrabony and extrabony site. Arch Oral Biol. 1982;27:59–63. doi: 10.1016/0003-9969(82)90177-7. [DOI] [PubMed] [Google Scholar]
- 9.Barrett AP, Reade PC. A histological investigation of isografts of mature mouse molars to an intrabony and an extrabony site. Transplantation. 1981;31:353–357. doi: 10.1097/00007890-198105010-00010. [DOI] [PubMed] [Google Scholar]
- 10.Steidler NE, Reade PC. An histological study of the effects of extra-corporeal time on murine dental isografts. Arch Oral Biol. 1979;24:165–169. doi: 10.1016/0003-9969(79)90065-7. [DOI] [PubMed] [Google Scholar]
- 11.Barrett AP, Reade PC. Changes in periodontal fibre organization in mature bone/tooth isografts in mice. J Oral Pathol. 1981;10:276–283. doi: 10.1111/j.1600-0714.1981.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 12.Barrett AP, Reade PC. The relationship between degree of development of tooth isografts and the subsequent formation of bone and periodontal ligament. J Periodontal Res. 1981;16:456–465. doi: 10.1111/j.1600-0765.1981.tb00996.x. [DOI] [PubMed] [Google Scholar]