Abstract
This study was done to determine the effectiveness of therapeutic hypothermia (TH) after out-of-hospital cardiac arrest (OHCA) among a large cohort of adults in the Cardiac Arrest Registry to Enhance Survival (CARES), with an emphasis on subgroups with a nonshockable first documented rhythm. This was an IRB approved retrospective cohort study. All adult index events at participating sites from November 2010 to December 2013 were study eligible. All patient data elements were provided. Summary statistics were calculated for all patients with and without TH. For multivariate adjustment, a multilevel (i.e., hierarchical), mixed-effects logistic regression (MLR) model was used with hospitals treated as random effects. Propensity score matching (PSM) on both shockable and nonshockable patients was done as a sensitivity analysis. After predefined exclusions, our final sample size was 6369 records for analysis: shockable=2992 (47.0%); asystole=1657 (26.0%); pulseless electrical activity=1249 (19.6%); other unspecified nonshockable=471 (7.4%). Unadjusted differences in neurological status at hospital discharge with and without TH were similar (p=0.295). After multivariate adjustment, TH had either no association with good neurological status at hospital discharge or that TH was actually associated with worse neurological outcome, particularly in patients with a nonshockable first documented rhythm (i.e., for NS patients, MLR odds ratio for TH=1.444; 95% CI [1.039, 2.006] p=0.029, and OR=1.017, p=0.927 via PSM). Highlighting our limitations, we conclude that when TH is indiscriminately provided to a large population of OHCA survivors with a nonshockable first documented rhythm, evidence for its effectiveness is diminished. We suggest more uniform and rigid guidelines for application.
Introduction
Among out-of-hospital cardiac arrest (OHCA) survivors, prolonged cerebral ischemia with subsequent reperfusion injury is a significant cause of postresuscitation mortality and long-term neurological disability (Edgren et al., 1994). Since 2005, targeted temperature management or therapeutic hypothermia (TH), defined as whole body cooling to 32–34°C for 12–24 hours, has been recommended for comatose adults recovering from ventricular fibrillation (VF) OHCA in an attempt to attenuate reperfusion injury, preserve neurological function, and improve quality survival (ECC Committee and Subcommittees and Task Forces of the American Heart Association, 2005). Over the last decade, TH has emerged as a standard treatment in the care of comatose adults recovering from VF OHCA and is now often extrapolated beyond its proven indications to patients who were specifically excluded from the randomized controlled trials—most notably, patients with a nonshockable first documented rhythm (asystole or pulseless electrical activity [PEA]) (Kim et al., 2012).
The consequence of TH after OHCA due to a nonshockable rhythm is still uncertain (Walters et al., 2011), and as of 2010, it retains a Class IIb (LOE B) recommendation (Peberdy et al., 2010). Recently, a large systematic review and meta-analysis of clinical studies, including 10 with neurological outcomes reported, suggested minimal if any benefit of TH for patients with a nonshockable first documented rhythm (Kim et al., 2012). Since then, six additional case series with somewhat conflicting, but similar results have been published (Dumas et al., 2011; Testori et al., 2011; Kory et al., 2012; Lundbye et al., 2012; Soga et al., 2012; Storm et al., 2012).
This was a retrospective cohort study, done to compare resuscitation outcomes after OHCA resuscitation among a large cohort of adults in the Cardiac Arrest Registry to Enhance Survival (CARES) (https://mycares.net) dataset to determine the effectiveness of TH compared with standard treatment (No-TH) in OHCA survivors, with an emphasis on subgroups of those with a nonshockable first documented rhythm. Our central hypothesis, based on our literature review and preliminary data analysis, was that TH provision after OHCA compared with No-TH would be associated with a modest, but favorable neurological status at hospital discharge and that the effect would vary by rhythm classification (shockable vs. asystole vs. PEA).
Materials and Methods
The study was reviewed and approved by the Institutional Review Board (183049-6) through expedited review.
Design, setting, and population
Initiated in 2004, CARES is a collaborative effort of the Centers for Disease Control and Prevention and the Emory University Department of Emergency Medicine with private funding occurring in 2012 (McNally et al., 2009). Using the Utstein style of reporting on OHCA (Cummins et al., 1991; Jacobs et al., 2004), CARES was developed to serve as a central repository of data and a quality improvement tool on OHCA for Emergency Medical Service (EMS) systems throughout the United States. The registry focuses on a relatively small number of key prehospital variables that can be objectively measured, are clearly defined, and are known to affect patient outcomes (McNally et al., 2009). The program is capable of identifying and tracking all consecutive cases of cardiac arrest in a defined geographic area through the continuum of care from EMS activation to hospital discharge. There are currently more than 400 EMS agencies and 900 hospitals participating across the United States with a catchment of over 50 million people (Appendix 1). The CARES design, development, and data elements included have been published previously and described in great detail (McNally et al., 2009).
This was a secondary analysis of prospectively collected data submitted to CARES from November 1, 2010, which is when TH became a mandatory reporting field, to December 31, 2012. All adult (≥18 years of age) index arrests of presumed cardiac etiology with attempted resuscitation at participating sites were screened for study eligibility. Sustained return of spontaneous circulation (ROSC) with survival to hospital admission was a prerequisite for study inclusion. The cohort was defined by cooling in the field that was continued after hospital admission or cooling initiated after hospital arrival and continued during hospitalization. All records with missing TH status or outcome classification were excluded. Furthermore, we excluded patients whose arrest occurred in a long-term care facility as a surrogate for likely poor baseline health status potentially negatively biasing the hypothermia group toward poor outcomes. Finally, we excluded patients whose arrest occurred after arrival of EMS as a surrogate for likely short arrest duration to reduce bias toward good outcomes in the control (No-TH) group. All remaining records were categorized as shockable (i.e., VF, pulseless ventricular tachycardia or other unspecified shockable rhythm), asystole, PEA, or other unspecified nonshockable rhythm for analysis based on the patients' first documented rhythm. All data elements in the registry relevant to the research question were considered for modeling.
Data collection and processing
Prehospital providers initiate a CARES event based on criteria set forth by the project coordinator and the data dictionary. A limited set of prehospital data is gathered on each occurrence (Appendix 2). A contact person at each EMS agency (CARES liaison) ensures adherence to the criteria and provides routine communication with the project coordinator for issues, concerns, and questions. The data are submitted in one of the three ways: direct entry into the website database, exportation of electronic data from field software programs, or from an existing database. Hospitals receive notification to provide outcome data through an e-mail, which is automatically generated by the database. The five basic hospital level questions include emergency department outcome, TH treatment, hospital outcome, disposition location, and neurological status at the time of hospital discharge. The neurological status is categorized using the Cerebral Performance Category (CPC) scale as follows: 1=good cerebral performance; 2=moderate disability; 3=severe cerebral disability; 4=coma or vegetative state; and 5=death. It is a CARES policy that only appropriately trained medical providers, such as a nurse or physician, makes the hospital discharge CPC scale determination, and each CARES site is provided with training materials to educate staff with regard to CPC scoring. In cases of inadequate documentation, the physician responsible for the patient discharge may be contacted for clarification.
The CARES staff reviews all records each month to identify any anomalies and immediately address all concerns. Data fidelity is assured through multilevel review, including software logic checks, and a data analyst examines each submitted report. If elements are missing from a report, an audit file is sent to the responsible site for clarification. Once the essential elements pass quality checks, individual identifiers are stripped from the record and the case is permanently entered into the registry database. For this study, the deidentified data, stored on a secure web server (Sansio Corporation, Minneapolis, MS), were exported to an excel file format (Microsoft Corporation, Redmond, WA) and electronically transferred to the principal investigator for analysis.
Statistical analysis
Summary statistics of medians and interquartile ranges, means and standard deviations, and proportions with 95% confidence intervals, when appropriate, were calculated for all eligible patients with and without TH. The outcome of interest was a CPC score indicating good cerebral performance or moderate disability (CPC=1 or 2) versus a lower CPC score (3, 4, or 5) indicating severe cerebral disability, coma/vegetative state, or death, respectively. As per convention, we made the worse outcome the event, so odds ratios greater than 1 in the multivariate models indicate an association with a worse outcome.
For multivariate adjustment, a multilevel (i.e., hierarchical), mixed-effects logistic regression (MLR) model was used with hospitals treated as random effects (Rabe-Hesketh and Skrondal, 2008). This model allows for the possibility of serial correlation in outcomes among patients treated within the same hospital. With this model structure, we created several models. We created regression models using all eligible patients and only included all mandatory fields deemed clinically relevant: age, gender, race, arrest location, witnessed arrest, TH given in the hospital (the main predictor of interest), TH given in the field, and the first documented rhythm category. Additional optional variables relating to chronic comorbidities, drugs used during resuscitation, and use of an impedance threshold device (ITD) were added as covariates in subsequent models. We performed no imputation and since these additional covariates were optional (and therefore missing from some patient records), the sample size in these regression models decreased. In the regression models, we also tested if an interaction effect was present with TH and the first documented rhythm category.
As an additional sensitivity analysis, we performed a propensity score matching analysis, where the propensity (probability) of having TH was modeled as the outcome using first the mandatory variables and then on all variables (comorbidities, drugs, ITD, prehospital time). Separate propensity scores were created for shockable and nonshockable patients (i.e., patients were directly matched on the rhythm category first) as a preliminary analysis showed that propensity matching on the entire dataset could not adequately match TH and No-TH patients by the first documented rhythm category. After matching the patients using a 5:1 greedy match algorithm, diagnostics were performed to ensure that the covariates (i.e., age, gender) were balanced between groups. Inferences done on the matched sample took the paired nature of the data into account.
Results
Population
There were 89,278 OHCA records available in the CARES database through December 31, 2012 representing a mixed dataset of prehospital and in-hospital cooling. After our predefined exclusions (Fig. 1), we were left with a final sample size for this study of 6369 records (shockable=2992 [47.0%]; asystole=1657 [26.0%]; PEA=1249 [19.6%]; other unspecified nonshockable=471 [7.4%]) for analysis.
FIG. 1.
Exclusion criteria: There were 89,278 out-of-hospital cardiac arrest records available in the Cardiac Arrest Registry to Enhance Survival (CARES) database through December 31, 2012. After our predefined exclusions, we were left with a final sample size for this study of 6369 records for analysis.
Stratified by TH status
Overall, more than half of the patients in this cohort were assigned to the TH group (3452 [54.2%] vs. 2917 [45.8%] in the No-TH group). Table 1A presents the key mandatory variables by treatment status for all included patients. The TH group was slightly younger, but TH usage did not differ by gender or race/ethnicity. The TH group had a higher proportion of witnessed cardiac arrests. The initiation of TH in the field was strongly associated with TH provision after hospital admission. As expected, patients with a shockable first documented rhythm were more likely to receive TH than those initially found with a nonshockable rhythm. There were no differences between groups in the proportion of patients with a CPC score of 1 or 2, a CPC score of 3 or 4, and a CPC score of 5 (death), p=0.295.
Table 1A.
Raw Differences Between Therapeutic Hypothermia Provided and No-Therapeutic Hypothermia Provided for All Patients (N=6369)
| Variable | TH provided n=3452 (54.2%) | No-TH provided n=2917 (45.8%) | p-Value |
|---|---|---|---|
| Age (median, IQR) | 62 (52, 72) | 64 (54, 77) | <0.001 |
| Age categories | <0.001 | ||
| 18–49 | 685 (19.84) | 497 (17.04) | |
| 50–59 | 824 (23.87) | 627 (21.49) | |
| 60–69 | 935 (27.09) | 688 (23.59) | |
| 70–79 | 607 (17.58) | 565 (19.37) | |
| 80–89 | 358 (10.37) | 441 (15.12) | |
| 90+ | 43 (1.25) | 99 (3.39) | |
| % Female | 1204 (34.88) | 1073 (36.78) | 0.114 |
| Race | 0.06 | ||
| White | 1574 (45.60) | 1253 (42.96) | |
| Black | 540 (15.64) | 446 (15.29) | |
| Hispanic | 231 (6.69) | 192 (6.58) | |
| Asian/other | 88 (2.55) | 66 (2.26) | |
| Unknown race | 1019 (29.52) | 960 (32.91) | |
| Location of the event | 0.069 | ||
| Home | 2491 (72.16) | 2109 (72.30) | |
| Street | 288 (8.34) | 205 (7.03) | |
| Recreation location | 108 (3.13) | 117 (4.01) | |
| Public area/commercial area/jail/other | 565 (16.37) | 486 (16.66) | |
| Event known to be witnessed | 2252 (65.24) | 1810 (62.05) | 0.008 |
| Resuscitation initiated by (no CPR: n=13) | 0.348 | ||
| Layman initiated CPR | 1448 (41.95) | 1237 (42.41) | |
| EMS initiated CPR | 955 (27.67) | 839 (28.76) | |
| First responder initiated CPR | 1043 (30.21) | 835 (28.63) | |
| AED used during resuscitation | 191 (5.53) | 196 (6.72) | 0.048 |
| Hypothermia initiated in the field (missing imputed to be none) | 1688 (48.32) | 628 (21.53) | <0.001 |
| First documented rhythm | <0.001 | ||
| Shockable (includes VFib, V Tach, and unknown shockable) | 1851 (53.62) | 1141 (39.12) | |
| Asystole | 794 (23.00) | 863 (29.59) | |
| Idioventricular/PEA | 592 (17.15) | 657 (22.52) | |
| Unknown unshockable rhythm | 215 (6.23) | 256 (8.78) | |
| Hospital outcome | 0.295 | ||
| CPC 1/2 | 1175 (34.04) | 983 (33.70) | |
| CPC 3/4 | 208 (6.03) | 151 (5.18) | |
| CPC 5 | 2069 (59.94) | 1783 (61.12) |
TH, therapeutic hypothermia; PEA, pulseless electrical activity; EMS, Emergency Medical Service; CPC, Cerebral Performance Category; CPR, cardiopulmonary resuscitation.
With respect to those variables that were optionally reported, patients with cancer, renal disease, and chronic heart disease comorbidities were less likely to receive TH treatment. (Table 1B) Regarding drugs given during resuscitation, there were significant differences between groups in the delivery of epinephrine, amiodarone, atropine, vasopressin, and lidocaine—with all but vasopressin being more prevalent in the TH group. Notably, patients with the longer times from ambulance dispatch to arrival to the ED were more likely to receive TH as well.
Table 1B.
Raw Differences Between Groups for the Subset with Optional Fields Completed
| Variable | TH provided n=2255 (55.1%) | No-TH provided n=1835 (44.9%) | p-Value |
|---|---|---|---|
| Comorbid conditions | |||
| Diabetes | 431 (19.11) | 347 (18.91) | 0.869 |
| Cancer | 105 (4.66) | 131 (7.14) | 0.001 |
| Hypertension | 602 (26.70) | 537 (29.26) | 0.068 |
| Renal disease | 103 (4.57) | 115 (6.27) | 0.016 |
| Heart disease | 655 (29.05) | 589 (32.10) | 0.035 |
| Respiratory disease | 273 (12.11) | 227 (12.37) | 0.798 |
| Hyperlipidemia | 111 (4.92) | 100 (5.45) | 0.448 |
| Stroke | 71 (3.15) | 741 (3.87) | 0.211 |
| Variable | TH provided n=2543 (55.5%) | No-TH provided n=2040 (44.5%) | p-Value |
|---|---|---|---|
| Resuscitation drugs given | |||
| Epinephrine | 1998 (78.57) | 1439 (70.54) | <0.001 |
| Atropine | 626 (24.62) | 450 (22.06) | 0.042 |
| Bicarbonate | 501 (19.70) | 448 (21.96) | 0.061 |
| Amiodarone | 583 (22.93) | 295 (14.46) | <0.001 |
| Vasopressin | 402 (15.81) | 383 (18.77) | 0.008 |
| Dextrose | 114 (4.48) | 95 (4.66) | 0.779 |
| Lidocaine | 309 (12.15) | 192 (9.41) | 0.003 |
| Variable | TH provided n=2540 (55.6%) | No-TH provided n=2031 (44.4%) | p-Value |
|---|---|---|---|
| Impedence threshold device used | 673 (26.5) | 431 (21.2) | <0.001 |
| Variable | TH provided n=2749 (50.7%) | No-TH provided n=2040 (49.3%) | p-Value |
|---|---|---|---|
| Time from ambulance dispatch to ED arrival (minutes); groups represent quintiles of equal size | <0.001 | ||
| 17:10 to 28:26 | 483 (17.57) | 602 (22.48) | |
| 28:28 to 34:59 | 505 (18.37) | 514 (19.19) | |
| 35:00 to 41:17 | 583 (21.21) | 567 (21.17) | |
| 41:18 to 50:40 | 585 (21.28) | 502 (18.75) | |
| 50:41 or Later | 593 (21.57) | 493 (18.41) |
Unadjusted patient CPC status at the time of hospital discharge is presented in Figure 2. Overall, patients with a shockable first documented rhythm had less in-hospital mortality and more favorable neurological outcomes compared with those who were found to be in a nonshockable rhythm on EMS arrival. Across the rhythm categories, those with TH had either similar proportions of patients with good or moderate disability CPC status as those with No-TH or had statistically significantly fewer patients with this outcome.
FIG. 2.
Unadjusted patient Cerebral Performance Category results at the time of hospital discharge.
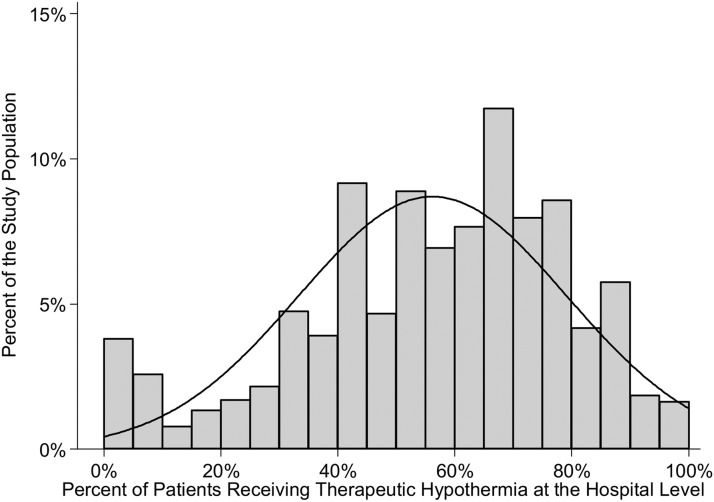
At the hospital level, the proportion of patients treated with TH varied widely across institutions as indicated by the histogram in Figure 3. This analysis excluded any hospital that contributed fewer than 10 eligible patients so as to not distort the hospital-level TH rate. Whereas the average proportion of patients treated with TH in the cohort exceeded 50%, the range was quite broad with some hospitals rarely using TH, while others provide it to the vast majority of their patients. Moreover, at the hospital level, the correlation between the TH rate and percent of patients with nonshockable rhythm was modest, r=0.30, p<0.001.
FIG. 3.
Histogram of the percentage of patients treated with therapeutic hypothermia (TH) across institutions—the percentage of patients receiving TH varied considerably by hospital. Some hospitals rarely provided TH and others almost always. Overall, about half the patients received TH, but at the hospital level, the rate of TH was not consistently 50% and it varied from very low (<10%) to very high (>90%).
Multivariate results are presented in Table 2 and the multivariate sensitivity analyses are given in Table 3. The multilevel (i.e., hierarchical), MLR model in Table 2 shows that TH for all patients, after adjusting only for the mandatory fields, was significantly associated with a worse neurological outcome (CPC score of 3, 4, or 5—severe cerebral disability, coma, or death). The model includes an interaction term with the first documented rhythm category and for the unknown nonshockable rhythm category; the interaction was significant (interaction term odds ratio=2.18). This means that the main effect odds ratio for TH of 1.35 is not constant, but is higher (by a factor of 2.177) when the rhythm category is unknown nonshockable (i.e., these patients do worse than expected). A likelihood ratio test for this model compared with a logistic regression model that did not take hospital-level effects into account was significant (p<0.001) indicating that the hospital for which the patient was treated was significantly associated with outcome.
Table 2.
Multivariate Results with an Interaction Term (N=6369)
| Variable | Odds ratio | 95% confidence intervals | p>|z| |
|---|---|---|---|
| Therapeutic hypothermia | 1.353 | (1.134–1.616) | 0.001 |
| Age categories | |||
| 18–49 | 1 | ||
| 50–59 | 1.228 | (1.014–1.488) | 0.036 |
| 60–69 | 1.774 | (1.468–2.144) | <0.001 |
| 70–79 | 2.723 | (2.199–3.371) | <0.001 |
| 80–89 | 4.14 | (3.194–5.367) | <0.001 |
| 90+ | 5.536 | (3.103–9.879) | <0.001 |
| Female gender | 1.404 | (1.219–1.616) | <0.001 |
| Race | |||
| White | 1 | ||
| Black | 1.291 | (1.049–1.590) | 0.016 |
| Hispanic | 1.53 | (1.110–2.110) | 0.009 |
| Asian/other | 0.966 | (0.618–1.508) | 0.879 |
| Unknown race | 1.069 | (0.903–1.267) | 0.437 |
| Location of the event | |||
| Home | 1 | ||
| Street | 0.655 | (0.522–0.823) | <0.001 |
| Recreation location | 0.288 | (0.201–0.411) | <0.001 |
| Public area/commercial area/jail/other | 0.524 | (0.443–0.620) | <0.001 |
| Event known to be witnessed (yes=1) | 0.678 | (0.587–0.784) | <0.001 |
| Resuscitation initiated by | |||
| EMS initiated CPR | 1 | ||
| Layman initiated CPR | 0.75 | (0.635–0.886) | 0.001 |
| First responder initiated CPR | 0.9 | (0.749–1.081) | 0.26 |
| Hypothermia initiated in the field (yes=1) | 1.194 | (0.019–1.400) | 0.028 |
| First documented rhythm | |||
| Shockable (includes VFib, V Tach, and unknown shockable) | 1 | ||
| Asystole | 13.219 | (9.763–17.899) | <0.001 |
| Idioventricular/PEA | 4.398 | (3.421–5.655) | <0.001 |
| Unknown unshockable rhythm | 1.757 | (1.290–2.393) | <0.001 |
| TH×rhythm category | |||
| TH×shockable | 1 | ||
| TH×asystole | 0.942 | (0.615–1.444) | 0.784 |
| TH×PEA | 1.163 | (0.810–1.672) | 0.413 |
| TH×unknown unshockable | 2.177 | (1.360–3.485) | 0.001 |
Table 3.
Multivariate Sensitivity Analyses
| Group | Odds ratio for TH; higher odds ratio=worse outcome | 95% confidence intervals | p-Value | Sample size | |
|---|---|---|---|---|---|
| Using all variables | |||||
| Greedy matched | Shockable only | 0.921 | (0.683, 1.244) | 0.593 | 363 matched pairs |
| No matching, but all available variables in a multilevel mixed-effects logistic regression | Shockable only | 1.11 | (0.849, 1.451) | 0.447 | 1726 patients |
| Greedy matched | Nonshockable only | 1.017 | (0.710, 1.457) | 0.927 | 425 matched pairs |
| No matching, but all available variables in a multilevel mixed-effects logistic regression | Nonshockable only | 1.444 | (1.039, 2.006) | 0.029 | 1830 patients |
| Using all patients on mandatory variables | |||||
| Multilevel mixed-effects logistic regression | All patients | 1.353 | (1.134–1.616) | 0.001 | 6369 (note an interaction term is present) |
| Greedy matched | Shockable only | 1.313 | (1.056, 1.630) | 0.014 | 681 matched pairs |
| Greedy matched | Nonshockable only | 1.746 | (1.293, 2.358) | <0.001 | 741 matched pairs |
Table 2 presents the results for all patients. Table 3 presents the multivariate results applied to the subsets of shockable and nonshockable patients. In this study, we see that when we use all variables (i.e., the variables in Table 2 plus comorbidities, drugs, and time from ambulance dispatch to ED arrival as shown in Table 1B), TH appears nonsignificant in all shockable patients and in the propensity matched sample of shockable patients. The propensity matched sample of nonshockable patients using all variables also found TH to have no significant relationship with outcome. In contrast, the propensity-matched samples that only adjusted for the mandatory variables (in the last two rows of Table 3) showed that TH was associated with significantly worse outcomes for both shockable and nonshockable patients. Thus, the propensity-matched results mirrored the corresponding regression model results that used the same variables.
Discussion
Comparative Effectiveness Research (CER) examines the real-world outcomes of two or more treatment options for a given medical condition—as opposed to efficacy, which is the ideal therapeutic effect under rigidly controlled conditions as in a randomized controlled clinical trial. CER research, such as ours, always has a selection bias (i.e., the treatment choice is not randomly assigned), which must be attenuated with multivariate methods that adjust for confounding variables. CER research also tends to have more data collection errors or missing data due to the real-world setting and often, important confounders are simply not recorded or available for analysis. In this study, done to determine the effectiveness of TH compared with No-TH in OHCA survivors with an emphasis on subgroups of those with a nonshockable first documented rhythm, we found that after multivariate adjustment, TH had either no association with neurological status at hospital discharge or that TH was actually associated with worse neurological outcomes, particularly in patients with a nonshockable first documented rhythm.
These results differ from a recent systematic review and meta-analysis, which once again reaffirmed that induced mild TH improves survival and neurological outcomes after cardiac arrest resuscitation (Arrich et al., 2012). However, systematic review and meta-analysis included only randomized and quasirandomized controlled trials and so is more an assessment of TH efficacy. In addition, the total number of patients in the five included studies was small (253 treatment patients, 226 controls) with over 70% of the TH patients and more than 75% of the controls originating from the two landmark randomized control trials that are now over 10 years old. Our study is an examination of the real-world outcomes between patients treated with TH and those not treated, and included over 6000 patients and so is more a reflection of current day treatment effectiveness subject to the limitations intrinsic to any CER study.
Our study is unique in that, it is the first longitudinally collected and validated single source data analysis of TH treatment large enough to stratify outcomes by first documented rhythm subgroups. Further, the data included key predictive resuscitation variables for inclusion in our modeling to better explain the raw group differences. Because this study is not a random trial and the TH was not randomly assigned (i.e., it appeared that more acutely ill patients were given this treatment), our reported multivariate results are more definitive than the raw results. However, since the CARES dataset has important clinical factors to be optionally reported, we are faced with two types of models. The first are models with the largest sample size possible (i.e., only mandatory variables), while the second adjusts for all variables and examines only subsets of patients by rhythm category with this reported data. The former show us how TH does in a more broadly defined population of patients. The latter have the advantage of adjusting for more confounders and with propensity matching; we are getting samples on No-TH patients most like their TH counterparts. Whereas these latter models are less generalizable to all OHCA patients, they are also less biased to unmeasured confounding and the sample sizes are still large and much larger than previous studies in the literature.
With our propensity-matched models using all variables, we saw that TH had no association with outcome for either shockable or nonshockable patients. When we did not adjust for all variables, either using the multilevel logistic regression model on all patients or stratifying by rhythm with propensity matching, we saw that TH was associated with a worse outcome. Since our result differed when more covariates were added to the models, we feel that the models that did not adjust for comorbidities, drugs, and prehospital time are biased. Nevertheless, in the model in Table 2, we did observe a significant interaction effect indicating that TH was associated with a worse outcome than expected for patients with unknown nonshockable rhythms. This interaction odds ratio was similar in magnitude (OR=1.975), but borderline nonsignificant (p=0.055) when we examined these factors in a model that adjusted for all variables, but we note that interactions require a much larger sample size to be adequately powered (usually four times the sample size as main effects). Thus, our regression results show that, with shockable as the referent, patients with a nonshockable first documented rhythm as a whole have much worse outcomes when provided TH, and the effect is not constant across the nonshockable rhythm subcategories.
The utilization of TH varied widely across participating hospitals, and its use was not strongly correlated with the percent of patients at each with shockable rhythms. Our results show that getting TH is strongly dependent on the admitting hospital type, which suggests that endorsed treatment guidelines for the care of resuscitated OHCA patients are not consistently followed nationwide.
We stress that our results should not be interpreted as evidence that TH is not beneficial, but merely that when applied indiscriminately across a large heterogeneous cohort of OHCA survivors, as in routine clinical practice, its contribution to patient outcomes is diluted and evidence of a measurable effect is lost. Our findings are important because as the effectiveness of the treatment diminishes, the number needed to treat (estimated to be six per life saved) increases substantially (Holzer et al., 2005), and the cost-effectiveness of the therapy (initially calculated as $47,168 per quality-adjusted life year) may be greater than expected (Merchant et al., 2009).
Study limitations
The use of registry data has inherent limitations. The most important of which, for appropriately interpreting our study results, is various sources of bias (i.e., selection and misclassification bias). There was no independent verification of the appropriateness of point-of-care inclusion and exclusion decisions or classification accuracy.
As with all retrospective analyses, unmeasured confounders can generate unexpected results. We must stress that CARES is structured to collect binary information on the initiation of TH (i.e., cooled? yes or no) and does not take into account many of the confounding variables related to implementation and time to initiation. As such, no details are available on pre-existing advanced directive or withdrawal of care once initiated; pretreatment neurological status; hemodynamic stability or management during treatment; or the timing, quality, or duration of treatment (i.e., time to target temperature or cooling/rewarming procedures).
In addition, the time from arrest to first documented rhythm is challenging to capture and is not a component of the registry. It is possible that group differences in neurological outcome could be due, in part, to the duration of ischemia before attempted resuscitation. Furthermore, we had no data on quality of resuscitation efforts or time to ROSC. In addition, the initial ECG rhythm classification was not independently confirmed. However, there is no reason to believe that a systematic bias exists in the way rhythm categories were recorded. Finally, the CARES database is limited in the number of comorbidities it tracks and so earlier conditions not collected, such as morbid obesity and their confounding effect on mortality or CPC scores, are unknown.
In terms of outcome, we acknowledge that patients' long-term (30-day or 90-day) neurological status is unknown since CPC scores are only recorded at the time of hospital discharge. Therefore, any neurological improvement occurring beyond this time point (Raina et al., 2008) would not be captured. However, the CPC at hospital discharge has recently been shown to be a useful surrogate measure of long-term quality survival after cardiac arrest resuscitation (Phelps et al., 2013).
Future studies should begin by addressing these limitations, and we encourage a multicenter randomized clinical trial to specifically examine the effect of TH on patients after successful cardiac arrest resuscitation with a nonshockable first documented rhythm.
Conclusion
Highlighting our limitations, we conclude that when TH is indiscriminately provided to a large population of OHCA survivors with a nonshockable first documented rhythm, evidence for its effectiveness is diminished. We emphasize that TH may still be effective, particularly for certain patient subsets, but perhaps, more uniform and rigid guidelines for application are needed to assure more appropriate application, and a randomized controlled clinical trial on nonshockable patients appears warranted.
Appendix
APPENDIX 1:
Site Map
APPENDIX 2:
Data Collection Form
Acknowledgments
The Cardiac Arrest Registry to Enhance Survival (CARES) is funded by grant support from the American Red Cross, the Medtronic Foundation Heart Rescue Project, the American Heart Association, and the Zoll Corporation. The project was supported by the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the National Institutes of Health, through grant number UL1 RR025752. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The data analysis was funded by a project grant from the Laerdal Foundation for Acute Care Medicine.
Disclosure Statement
All authors declare that no competing financial interests exist.
References
- Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2012;9:CD004128. [DOI] [PubMed] [Google Scholar]
- Cummins RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation 1991;84:960–975 [DOI] [PubMed] [Google Scholar]
- Dumas F, Grimaldi D, Zuber B, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients?: insights from a large registry. Circulation 2011;123:877–886 [DOI] [PubMed] [Google Scholar]
- ECC Committee, Subcommittees and Task Forces of the American Heart Association 2005American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005;112:IV1–203 [DOI] [PubMed] [Google Scholar]
- Edgren E, Hedstrand U, Kelsey S, Sutton-Tyrrell K, Safar P. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet 1994;343:1055–1059 [DOI] [PubMed] [Google Scholar]
- Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med 2005;33:414–418 [DOI] [PubMed] [Google Scholar]
- Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation 2004;110:3385–3397 [DOI] [PubMed] [Google Scholar]
- Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non-shockable initial rhythms? A systematic review and meta-analysis of randomized and non-randomized studies. Resuscitation 2012;83:188–196 [DOI] [PubMed] [Google Scholar]
- Kory P, Fukunaga M, Mathew JP, et al. Outcomes of mild therapeutic hypothermia after in-hospital cardiac arrest. Neurocrit Care 2012;16:406–412 [DOI] [PubMed] [Google Scholar]
- Lundbye JB, Rai M, Ramu B, et al Therapeutic hypothermia is associated with improved neurologic outcome and survival in cardiac arrest survivors of non-shockable rhythms. Resuscitation 2012;83:202–207 [DOI] [PubMed] [Google Scholar]
- McNally B, Stokes A, Crouch A, Kellermann AL. CARES: cardiac arrest registry to enhance survival. Ann Emerg Med 2009;54:674–683.e2. [DOI] [PubMed] [Google Scholar]
- Merchant RM, Becker LB, Abella BS, Asch DA, Groeneveld PW. Cost-effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes 2009;2:421–428 [DOI] [PubMed] [Google Scholar]
- Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122:S768–S786 [DOI] [PubMed] [Google Scholar]
- Phelps R, Dumas F, Maynard C, Silver J, Rea T. Cerebral performance category and long-term prognosis following out-of-hospital cardiac arrest. Crit Care Med 2013;41:1252–1257 [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A.Multilevel and Longitudinal Modeling Using Stata. 2nd ed. College Station, TX: Stata Press, 2008 [Google Scholar]
- Raina KD, Callaway C, Rittenberger JC, Holm MB. Neurological and functional status following cardiac arrest: method and tool utility. Resuscitation 2008;79:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga T, Nagao K, Sawano H, et al. Neurological benefit of therapeutic hypothermia following return of spontaneous circulation for out-of-hospital non-shockable cardiac arrest. Circ J 2012;76:2579–2585 [DOI] [PubMed] [Google Scholar]
- Storm C, Nee J, Roser M, Jorres A, Hasper D. Mild hypothermia treatment in patients resuscitated from non-shockable cardiac arrest. Emerg Med J 2012;29:100–103 [DOI] [PubMed] [Google Scholar]
- Testori C, Sterz F, Behringer W, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation 2011;82:1162–1167 [DOI] [PubMed] [Google Scholar]
- Walters JH, Morley PT, Nolan JP. The role of hypothermia in post-cardiac arrest patients with return of spontaneous circulation: a systematic review. Resuscitation 2011:508–516 [DOI] [PubMed] [Google Scholar]
- Williams GR, Jr., Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958;148:462–468 [DOI] [PMC free article] [PubMed] [Google Scholar]