Abstract
Significant vascular changes occur subsequent to spinal cord injury (SCI), which contribute to progressive pathophysiology. In the present study, we used female Wistar rats (300–350 g) and a 35-g clip-compression injury at T6 to T7 to characterize the spatial and temporal vascular changes that ensue post-SCI. Before sacrifice, animals were injected with vascular tracing dyes (2% Evans Blue (EB) or fluorescein isothiocyanate/Lycopersicon esculentum agglutinin [FITC-LEA]) to assess blood–spinal cord barrier (BSCB) integrity or vascular architecture, respectively. Spectrophotometry of EB tissue showed maximal BSCB disruption at 24 h postinjury, with significant disruption observed until 5 days postinjury (p<0.01). FITC-LEA-identified functional vasculature was dramatically reduced by 24 h. Similarly, RECA-1 immunohistochemistry showed a significant decrease in the number of vessels at 24 h postinjury, compared to uninjured animals (p<0.01), with slight increases in endogenous revascularization by 10 days postinjury. White versus gray matter (GM) quantification showed that GM vessels are more susceptible to SCI. Finally, we observed an endogenous angiogenic response between 3 and 7 days postinjury: maximal endothelial cell proliferation was observed at day 5. These data indicate that BSCB disruption and endogenous revascularization occur at specific time points after injury, which may be important for developing effective therapeutic interventions for SCI.
Key words: : blood–spinal cord barrier, endogenous angiogenesis, spinal cord injury, vascular injury, vascular permeability
Introduction
Traumatic spinal cord injury (SCI) results in dramatic alterations in spinal cord blood flow and can cause systemic hypotension as a result of interruption of descending sympathetic circuits.1 However, at the cellular level, SCI pathology results in rapid, permanent changes to the structure and function of the microvessels.2–4 This includes loss of microcirculation, disruption to the blood–spinal cord barrier (BSCB), loss of structural organization, endothelial cell (EC) death and vascular remodeling.4,5 These changes have more widespread effects, because vascular damage assists in spreading and enhancing the secondary injury cascades after SCI. Notably, breakdown of the BSCB increases inflammatory response (allowing inflammatory cells to enter the injury site). Moreover, the death of ECs, severed vascular networks, and ischemia result in apoptosis and cell death of other central nervous system (CNS) cells, because they cannot survive without an adequate blood supply.6–9
The major form of vascular regeneration that occurs in injuries and wound healing is angiogenesis (although postnatal vasculogenesis has been shown to occur).10 Angiogenesis, defined as the production of blood vessels from preexisting vessels, is a complex process and critical for the remodeling and survival of tissues after injury.11 Previous SCI research has confirmed that a relationship exists between blood-vessel density and improved functional outcomes; therefore, sparing or regenerating vasculature postinjury would be a desirable outcome.12–15 These findings are further supported by studies that have demonstrated that CNS microvessels provide trophic support and are essential for survival of localized tissue.16,17 Recently, Bearden and Segal showed that regenerating axons have a tendency to grow along blood vessels, suggesting that the vasculature may act as a scaffold and provide guidance for axonal sprouting after injury.18
Angiogenesis does occur after injury; however, unfortunately the endogenous mechanisms cannot provide a sufficient amount of repair. This leaves the injury site in a constant state of hypoxia ischemia, leading to further cell death. It has been proposed that after SCI, angiogenic response occurs within the first week postinjury, with signs of endogenous revasularization disappearing shortly after.3,19 Logically, novel vascular therapies would aim to target early vascular mechanisms (between 3 and 7 days) to maximally improve local vasculature and blood supply, in turn reducing the amount of cell loss and neurological deficits. Moreover, residual vessels or newly generated vessels that are damaged and/or immature present an opportunity for adverse physiological events, notably providing a direct route to the lesion site for circulating inflammatory mediators. Because the BSCB is disrupted, this allows a large influx of inflammatory cells to enter into an otherwise immune-protected CNS.
Previous groups have characterized and investigated many of the cellular events after SCI.7,20–22 Additionally, previous work has been published that describes some of the vascular changes that result from SCI, including reduction in blood flow, disruption of the BSCB, and loss of structural organization.2–5,23–26 However, our study investigates a number of novel aspects, compared with other SCI vascular studies: 1) In a model of clip-compression injury, BSCB permeability has not been examined. 2) BSCB disruption and blood vessels have been examined separately in previous studies. In the current research, we examine both aspects of vascular injury, providing a more detailed assessment of the damage after SCI. 3) In clip-compression injury, vascular studies have not extended past 24 h. Here, we examine the vascular changes to 14 days post-SCI. 4) Clip-compression injury offers an alternative SCI model for research and may exhibit varied vascular profiles, compared to contusion or transection models. 5) Endogenous angiogenic response after SCI remains largely uncharacterized and has not yet been examined in a model of clip-compression injury.
To the best of our knowledge, previous studies have only examined the cellular changes that occur in “mild” SCI; the NYU impactor model has been commonly used7,24,27–29 or transection models.25,26 Other studies that have used the clip-compression model have examined blood flow, hemorrhage, and blood gases; however, none have investigated cellular profiles or angiogenic response. Moreover, the majority of these studies have examined only acute vascular changes (≤24 h), whereas we have examined up to 14 days postinjury. The clip-compression model of SCI presents an alternative SCI model that offers differences, compared to other models, ultimately helping to mimic the diversity and heterogeneity observed in the clinic. The clip-compression model has a number of key advantages over other models of SCI, which may more accurately portray the human condition. Of note, clip-compression injury results in both dorsal and ventral damage of the cord, and the clip-compression model also creates temporary ischemia and impaired blood flow, which contribute to secondary pathology of the injury, and are commonly observed in humans.
This study aimed to examine the vascular changes that result from a moderately severe injury and determine the temporal and spatial profile of these changes. To critically assess the outcomes of any therapeutic intervention, it is imperative that we first understand the vascular changes involved in the clip-compression model of SCI. Although previous research has investigated the vascular changes of mild SCIs, the amount of damage and disruption to microvascular structures is anticipated to be more extensive and will therefore result in different characteristics and outcomes within the injury epicenter and penumbra. This research presents novelty in describing the vascular changes in an alternate, well-utilized model of SCI (the clip-compression model). Additionally, this study employs some modern techniques for assessing vasculature, specifically highlighting the differences in perfused and nonperfused vessels. Importantly, the results of this study provide key insights into the dynamic vascular alterations and endogenous repair that occur after SCI. Taken together, these data may help elucidate ideal time points and spatial areas that could maximize the effectiveness of therapeutics aiming to target spinal vasculature after injury.
Methods
Animal model of spinal cord injury
All animal protocols (979.31 and 891.9) and procedures were approved by the animal care committee at the University Health Network (Toronto, Ontario, Canada).
Animals were subject to a contusive-compressive SCI using a modified aneurysm clip, which has been extensively characterized by our laboratory and previously described.30 Briefly, adult female Wistar rats (250–300 g; Charles River, Montreal, Quebec, Canada) were deeply anesthetized using 4% halothane and sedated for the remainder of the surgery under 2% halothane. Animals received a two-level laminectomy of mid-thoracic vertebral segments T6 and T7. A modified clip calibrated to a closing force of 35g was applied extradurally to the cord for 1 min and then removed. The incision was closed in layers using standard silk sutures, and animals were given a single dose of buprenorphine (0.05 mg/kg). Animals were allowed to recover in their cage under a heat lamp and, subsequently, were housed in a temperature-controlled warm room (26°C) with free access to food and water. Animals were given buprenorphine (0.05 mg/kg) every 12 h for 48 h after surgery, and their bladders were manually voided three times daily. The number of animals used in each experiment is outlined in Table 1.
Table 1.
Number of Animals Used per Experiment
| Experiment | Group | Original animal no. | Final animal no. |
|---|---|---|---|
| Evans Blue | Sham | 6 | 6 |
| (Fig. 1) | 1 h | 10 | 8 |
| 4 h | 10 | 8 | |
| 24 h | 10 | 8 | |
| 3 day | 10 | 10 | |
| 5 day | 10 | 10 | |
| 7 day | 10 | 8 | |
| 10 day | 10 | 10 | |
| 14 day | 10 | 9 | |
| RECA/FITC-LEA | Sham | 5 | 5 |
| (Figs. 2–4; Table 2) | 1 h | 5 | 5 |
| 4 h | 5 | 5 | |
| 24 h | 5 | 5 | |
| 3 day | 5 | 4 | |
| 5 day | 5 | 5 | |
| 7 day | 5 | 5 | |
| 10 day | 5 | 4 | |
| 14 day | 5 | 5 | |
| Ki67/RECA-1 | Sham | 5 | 5 |
| (Fig. 5) | 1 h | 5 | 5 |
| 4 h | 5 | 5 | |
| 24 h | 5 | 5 | |
| 3 day | 5 | 4 | |
| 5 day | 5 | 5 | |
| 7 day | 5 | 5 | |
| 10 day | 5 | 4 | |
| 14 day | 5 | 5 |
n values for individual experiments and the results from each study are outlined and account for the variation in final n values between experiments.
FITC-LEA, fluorescein isothiocyanate/Lycopersicon esculentum agglutinin.
Evans Blue: Blood–spinal cord barrier disruption
Animals were injected with 1 mL of 2% Evans Blue (EB) into the tail vein.31,32 EB was allowed to circulate for 20–30 min, and then animals were transcardially perfused with 180 mL of sterile saline. Perfusion of fluid was administered at a rate of 60 mL/min, which is only slightly lower than physiological flow rates, because we did not want to induce vascular injury during this procedure. One centimeter of the spinal cord surrounding the injury site was extracted, weighed, and snap-frozen in dry ice. Samples were then homogenized in 400 μL of N,N’-dimethylformamide (DMF) and incubated at 50°C for 72 h. Samples were centrifuged at 18,000 rpm for 30 min. The supernatant was collected, aliquoted into a 48-well glass plate, and colorimetric measurements were performed using a PerkinElmer Victor-3 1420 spectrophotometer at the absorption maximum for EB (620 nm; PerkinElmer, Inc., Waltham, MA). Samples were normalized to the original sample weight, and EB concentration was calculated based on a standard curve of EB in DMF (data reported as EB per spinal cord weight: μg/g).
Histochemistry
Histological processing
After deep inhalation anesthetic, animals were transcardially perfused with 60 mL of sterile saline and 180 mL of 4% paraformaldehyde (PFA) in 0.1 M of phosphate-buffered saline (PBS). Perfusion of fluids was administered at a rate of 60 mL/min. Tissues were cryoprotected in 20% sucrose in PBS. A 10-mm (1-cm) length of the spinal cord centered at the injury site was fixed in tissue-embedding medium. The tissue segment was snap-frozen on dry ice and sectioned on a cryostat at a thickness of 14 μm. Serial spinal cord sections at 500-μm intervals were stained with myelin-selective pigment Luxol Fast Blue and the cellular stain, hematoxylin and eosin to identify the injury epicenter. Tissue sections showing the largest cystic cavity and greatest demyelination were taken to represent the injury epicenter. Rostrocaudal spinal cord maps were created by calculating the distance from the epicenter to the corresponding tissue sections.
Immunohistochemistry
Mouse anti-RECA-1 (1:25; Serotec Inc., Raleigh, NC) and rabbit anti-Ki67 (1:1000; Abcam, Toronto, Ontario, Canada) were used to stain ECs and proliferating cells, respectively. Sections were rinsed three times in PBS after primary antibody (Ab) incubation and incubated with either fluorescent Alexa 568, 647, or 488 goat anti-mouse/rabbit secondary Ab (1:400; Invitrogen, Burlington, Ontario, Canada) for 1 h. Sections were rinsed three times with PBS and cover slipped with Mowiol mounting medium containing 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc., Burlingame, CA) to counterstain the nuclei. Images were taken using a Leica MZ FLIII epifluorescence microscope (Leica Microsystems, Richmond Hill, Ontario, Canada).
Quantification of blood vessels
At 1 and 4 h and 1, 3, 5, 7, 10, and 14 days postinjury, animals were sedated with inhalation anesthetic and the right femoral vein was surgically exposed. Fluorescein isothiocyanate/Lycopersicon esculentum agglutinin (FITC-LEA; 0.5 mg), diluted in saline to a final volume of 1 mL, was injected into the femoral vein and allowed to circulate for 20–30 min (FITC-conjugated Lycopersicon esculentum agglutinin; catalog no. L0401; Sigma-Aldrich, Oakville, Ontario, Canada). Animals were transcardially perfused with 4% PFA, and tissues were fixed and processed as described above. Tissue sections at the injury epicenter and 1000, 500, and 250 μm (rostral and caudal) from the epicenter were used to assess the spatial distribution of vascular damage. Tissue sections were stained with RECA-1 (1:25; Serotec) to identify ECs and blood vessels. Vessels that were colabeled with FITC-LEA and RECA-1 were identified as “functional,” because the presence of FITC-LEA indicated that these vessels were connected to the systemic vasculature and exhibited a perfusion state. Vascular quantification was performed on four selected fields (ventral horn, dorsal horn, and left and right lateral columns) in each section under 25× magnification (0.14 mm2) and was conducted in a blinded manner. Nonbiased stereological equipment and software was not available at the time of the data collection; therefore, we used a simple spinal cord trace to consistently sample in the same areas at various distances from the injury epicenter. Uninjured sections at each distance from the “epicenter” were traced (because gray [GM] and white matter [WM] areas are not consistent throughout the spinal cord), and four areas were marked to represent the ventral GM, dorsal GM, and left and right later WM column sampling areas. These traces were used as a template for selecting nonbiased, consistent sampling sites from injured tissue. Quantification only included RECA-1 and FITC-LEA staining that displayed distinct vascular structures, such as cross-sectional vessels displaying a lumen, branched structures, or structures sectioned in a longitudinal plane. Data are presented as separate WM and GM counts, because these regions showed distinct variation in their vascular responses.
Quantification of angiogenesis
Tissue sections—taken from animals sacrificed 1 and 4 h and 1, 3, 5, 7, 10, and 14 days postinjury—were used to quantify angiogenesis after SCI. Angiogenesis was calculated as vessels colabeled with RECA-1 and Ki67 (cellular proliferation). Angiogenic quantification was performed on four selected fields (ventral horn, dorsal horn, and left and right lateral columns) in each section under 25× magnification (0.14 mm2) and was conducted in a blinded manner. Nonbiased stereological equipment and software was not available at the time of the data collection; therefore, we used a simple spinal cord trace to consistently sample in the same areas at various distances from the injury epicenter. Uninjured sections at each distance from the epicenter were traced (because GM and WM areas are not consistent throughout the spinal cord), and four areas were marked to represent the ventral GM, dorsal GM, and left and right later WM column sampling areas (Fig. 2D). These traces were used as a template for selecting nonbiased, consistent sampling sites from injured tissue. The number of angiogenic vessels was calculated at 500 μm and 1, 2 and 3 mm, both rostral and caudal from the epicenter, for each animal. Rostral and caudal values were pooled for each distance.
FIG. 2.
Spatial-temporal disruption of spinal cord vasculature after clip-compression injury. (A–H) Spatiotemporal comparison of RECA-1 quantification and FITC-LEA quantification with reference to sham animals. Tissues were examined at the epicenter and 250, 500, and 1000 μm rostral and caudal to the injury epicenter. Animals were sacrificed at (A) 1 h, (B) 4 h, (C) 24 h, (D) 3 days, (E) 5 days, (F) 7 days, (G) 10 days, and (H) 14 days post-SCI. Significant disruption of the vasculature was observed as early as 1 h postinjury, with the epicenter and adjacent areas being most significantly affected. Vasculature was significantly decreased from sham animal values between 1 h and 10 days postinjury, but, at 14 days, RECA-1 and FITC-LEA values were not significantly different from sham animals. (I) Representative images taken from the dorsal GM at 500 μm rostral to the epicenter at 10 days postinjury. White arrowheads indicate vessels that are labeled with RECA-1, but not FITC-LEA. Lack of double labeling indicates that vascular structures (i.e., endothelial cells) are present; however, the blood vessel did not have an active perfusion state. (J) Schematic of RECA-1/FITC-LEA quantification method. Four areas (dorsal horn, ventral horn, and left and right lateral white matter columns) were preselected in uninjured tissue, and these areas were quantified in injured sections. *p<0.05; **p<0.001. Scale bar, 100 μm. Green, FITC-LEA; red, RECA-1; blue, DAPI. n=4–5 animals per time point (see Table 1). See Table 3 for details on the statistical comparisons shown in panels (A–H). FITC-LEA, fluorescein isothiocyanate/Lycopersicon esculentum agglutinin; SCI, spinal cord injury; DAPI, 4’,6-diamidino-2-phenylindole.
Statistical analysis
Data were analyzed with SigmaPlot statistical software (Systat Software Inc., San Jose, CA). For data that investigated the percentage of cells, the data were subject to an arcsine transformation before statistical analysis to attain a more normal distribution. For comparison of groups sampled at various distances from the injury site, we used two-way analysis of variance (ANOVA) with repeated measures, followed by Holm-Sidak's post-hoc test. For data where only one variable after injury was analyzed (Fig. 1), a one-way ANOVA was performed, followed by Dunn's post-hoc test. In all figures, the mean value±standard error of the mean (SEM) are used to describe the results. Statistical significance was accepted for p values of <0.05.
FIG. 1.
Blood–spinal cord barrier permeability after traumatic spinal cord injury. Evans Blue extravasation between 1 h and 14 days post–spinal cord injury. Evans Blue extravasation after spinal cord injury was quantified by spectrophotometry at 630 nm. Evans Blue concentrations were calculated from a standard curve using concentrations between 0 and 50 ng. Tissue samples were normalized to their wet weight. Values are shown as the mean±standard error of the mean. n values are displayed in the figure legend (n=6–10 animals/group; see Table 1). One-way analysis of variance, Dunn's post-hoc test. *p<0.05, compared to sham.
Results
Blood–spinal cord barrier permeability after spinal cord injury
A key component in understanding the disruption of the vasculature after SCI is to investigate vascular/BSCB permeability. The opening of the BSCB results in negative events that propagate SCI pathology—including inflammation, edema, hemorrhage, and homeostatic dysregulation—however, we may be able to take advantage of BSCB breakdown because it may allow for certain drugs and therapies to enter the spinal cord, which may otherwise be excluded from the highly regulated CNS environment. We hypothesized that elucidating BSCB permeability after SCI may offer important information that may identify the most optimal time window for therapeutic intervention. Here, we have examined the temporal changes to BSCB permeability, from 1 h postinjury to 14 days postinjury. We observe that the BSCB is disrupted very early on (as early as 1 h after injury), maximally disrupted at 24 h post-SCI, and appears to be restored by 14 days (Fig. 1; Table 2). The BSCB shows significant disruption and permeability at 1 h, 4 h, 24 h, 3 days, and 5 days after SCI. At 24 h postinjury, the BSCB is significantly disrupted, compared to sham animals, as well as all other sampled time points, indicating that the BSCB is maximally disrupted at 24 h after clip-compression SCI. Although 24 h is the peak of BSCB permeability, we suggest that there is a relatively large time window to exploit on the disruption of the BSCB. In a model of clip-compression injury, therapies administered between 1 h and 5 days (and, most notably, at 24 h postinjury), may have an added advantage in reaching their CNS targets.
Table 2.
Spatial and Temporal Data from FITC-LEA and RECA-1 Analysis
| Time point | FITC-LEA+vessel counts | RECA-1+vessel counts | FITC vs. RECA counts (%) | % FITC/RECA in gray matter | % FITC/RECA in white matter |
|---|---|---|---|---|---|
| Sham animals | 265±8.8 | 269±11.4 | 98.7 | 98.5 | 99.6 |
| 1 hour postinjury | |||||
| 1000 μm caudal | 179±19.3 | 195±16.2 | 91.9 | 92.8 | 89.0 |
| 500 μm caudal | 141±14.8 | 202±34.7 | 69.8 | 68.3 | 76.1 |
| 250 μm caudal | 131±2.8 | 217±32.3 | 60.5 | 60.2 | 61.8 |
| Epicenter | 63±14.4 | 138±19.8 | 45.8 | 46.7 | 42.5 |
| 250 μm rostral | 94±12.9 | 183±34.5 | 51.2 | 44.2 | 74.6 |
| 500 μm rostral | 114±29.9 | 190±15.3 | 60.0 | 58.3 | 65.4 |
| 1000 μm rostral | 165±15.3 | 198±8.1 | 83.4 | 83.0 | 85.3 |
| 4 hours postinjury | |||||
| 1000 μm caudal | 125±20.9 | 150±28.9 | 83.6 | 78.9 | 94.5 |
| 500 μm caudal | 83±15.5 | 125±7.0 | 66.7 | 59.4 | 87.6 |
| 250 μm caudal | 68±6.2 | 122±18.2 | 55.2 | 46.9 | 80.8 |
| Epicenter | 27±8.1 | 81±5.7 | 33.8 | 27.0 | 49.0 |
| 250 μm rostral | 87±21.6 | 130±33.3 | 66.8 | 57.5 | 89.5 |
| 500 μm rostral | 93±22.7 | 114±26.7 | 81.7 | 80.7 | 83.7 |
| 1000 μm rostral | 110±22.4 | 141±24.9 | 78.3 | 73.5 | 89.1 |
| 24 hours postinjury | |||||
| 1000 μm caudal | 144±13.4 | 208±12.4 | 68.7 | 68.1 | 70.9 |
| 500 μm caudal | 129±16.3 | 191±21.0 | 67.3 | 68.6 | 64.1 |
| 250 μm caudal | 112±7.3 | 188±13.3 | 59.2 | 54.0 | 74.9 |
| Epicenter | 57±5.9 | 135±26.9 | 41.9 | 23.4 | 78.5 |
| 250 μm rostral | 138±13.2 | 223±33.0 | 61.8 | 55.2 | 80.8 |
| 500 μm rostral | 149±21.5 | 197±26.3 | 75.5 | 72.5 | 85.6 |
| 1000 μm rostral | 200±36.9 | 225±30.6 | 88.9 | 88.9 | 88.8 |
| 3 days postinjury | |||||
| 1000 μm caudal | 191±22.4 | 218±10.2 | 87.2 | 85.5 | 92.2 |
| 500 μm caudal | 194±44.0 | 215±44.2 | 89.8 | 87.4 | 97.0 |
| 250 μm caudal | 131±15.4 | 160±31.5 | 81.9 | 78.9 | 89.0 |
| Epicenter | 112±15.3 | 143±15.5 | 78.4 | 73.3 | 88.8 |
| 250 μm rostral | 106±14.8 | 139±21.8 | 76.7 | 70.6 | 91.0 |
| 500 μm rostral | 121±25.9 | 141±23.4 | 85.8 | 81.1 | 95.2 |
| 1000 μm rostral | 185±20.4 | 211±21.0 | 87.8 | 85.9 | 94.6 |
| 5 days postinjury | |||||
| 1000 μm caudal | 163±21.2 | 234±24.7 | 69.7 | 68.6 | 74.0 |
| 500 μm caudal | 117±25.4 | 183±27.3 | 63.8 | 60.6 | 72.8 |
| 250 μm caudal | 106±9.8 | 195±26.8 | 54.4 | 48.5 | 77.3 |
| Epicenter | 72±16.0 | 124±16.3 | 58.1 | 53.0 | 68.5 |
| 250 μm rostral | 93±19.1 | 158±18.5 | 58.8 | 49.0 | 86.3 |
| 500 μm rostral | 121±28.9 | 189±18.7 | 63.8 | 58.9 | 76.3 |
| 1000 μm rostral | 174±28.2 | 232±42.7 | 75.0 | 68.0 | 94.5 |
| 7 days postinjury | |||||
| 1000 μm caudal | 228±12.8 | 250±12.7 | 91.2 | 90.9 | 92.2 |
| 500 μm caudal | 213±14.4 | 243±15.2 | 87.4 | 86.0 | 90.9 |
| 250 μm caudal | 153±4.8 | 207±8.7 | 74.0 | 66.7 | 93.0 |
| Epicenter | 83±9.0 | 158±20.8 | 52.7 | 35.2 | 85.5 |
| 250 μm rostral | 81±15.1 | 124±8.7 | 65.0 | 51.7 | 90.6 |
| 500 μm rostral | 137±16.7 | 169±13.3 | 81.0 | 77.2 | 91.6 |
| 1000 μm rostral | 241±30.1 | 251±31.5 | 95.8 | 94.8 | 99.2 |
| 10 days postinjury | |||||
| 1000 μm caudal | 191±8.7 | 209±11.3 | 91.4 | 90.7 | 94.4 |
| 500 μm caudal | 132±24.7 | 141±21.3 | 93.8 | 93.2 | 95.5 |
| 250 μm caudal | 86±14.1 | 112±6.6 | 77.4 | 71.8 | 88.5 |
| Epicenter | 49±1.8 | 76±4.2 | 64.7 | 49.2 | 89.7 |
| 250 μm rostral | 141±26.9 | 153±26.9 | 92.1 | 90.1 | 97.6 |
| 500 μm rostral | 187±27.2 | 203±26.9 | 92.0 | 91.1 | 96.4 |
| 1000 μm rostral | 231±20.6 | 236±23.1 | 97.2 | 96.4 | 100.0 |
| 14 days postinjury | |||||
| 1000 μm caudal | 248±8.1 | 269±10.8 | 92.0 | 91.3 | 94.6 |
| 500 μm caudal | 207±21.3 | 227±23.1 | 91.1 | 90.1 | 94.8 |
| 250 μm caudal | 213±15.5 | 230±16.6 | 92.5 | 91.8 | 94.5 |
| Epicenter | 148±20.8 | 171±31.5 | 86.5 | 84.6 | 93.0 |
| 250 μm rostral | 196±13.0 | 217±11.6 | 90.5 | 89.1 | 94.1 |
| 500 μm rostral | 216±13.6 | 231±13.7 | 93.5 | 94.8 | 90.2 |
| 1000 μm rostral | 252±19.6 | 263±19.6 | 95.8 | 95.9 | 95.4 |
Values are provided from the data presented in Figures 1–3. FITC-LEA and RECA-1 counts within the spinal cord (columns 1 and 2, respectively) are shown, and ratios of FITC-LEA/RECA-1 staining observed overall (column 3), in the gray matter (column 4), and in the white matter (column 5) are displayed. Values are provided for each time point between 1 h and 14 days post–spinal cord injury, and the spatial quantification within each time point is also shown (between 1000 μm rostral and 1000 μm caudal to the injury site). Data are presented as either the mean±standard error of the mean (columns 1 and 2) or as percentages (columns 3–5).
FITC-LEA, fluorescein isothiocyanate/Lycopersicon esculentum agglutinin.
Spatial-temporal disruption of the vasculature
Our next aim was to delineate the distribution of vascular damage after clip-compression injury, compared to uninjured tissue. Not surprisingly, we detected a significant disruption to the localized vasculature (Figs. 1 and 2; Tables 2 and 3). The epicenter of the injury is most significantly disturbed at all time points, with the adjacent sections exhibiting less damage. Interestingly, the vascular changes appear to stay relatively confined, spanning approximately 2 mm rostrocaudal from the injury epicenter (Fig. 2; Table 2). Two-way ANOVA showed that tissues at 1 h after injury and extending to 10 days postinjury display significant vascular decreases, compared to sham animals (Fig. 2A–G; p<0.05 and p<0.001). At 14 days postinjury, RECA-1 counts are not significantly different from sham values. Adjacent sections to the epicenter appear to show vascular recovery (particularly more distal areas), in comparison with the lesion epicenter, which remains notably altered after injury at all time points investigated (1 h to 14 days). Overall, the penumbra of vascular damage remains relatively small (spanning approximately 1 mm by 14 days), suggesting that vascular therapies may only need to target a localized area to be effective.
Table 3.
Statistical Comparisons of RECA-1 and FITC-LEA Data
| Sham vs. RECA-1 | Significance | Sham vs. FITC-LEA | Significance | RECA-1 vs. FITC-LEA | Significance | |
|---|---|---|---|---|---|---|
| Figure | ||||||
| A | Sham vs. 1 h | <0.001 | Sham vs. 1 h | <0.001 | 1 h | <0.001 |
| B | Sham vs. 4 h | <0.001 | Sham vs. 4 h | <0.001 | 4 h | <0.001 |
| C | Sham vs. 24 h | <0.001 | Sham vs. 24 h | <0.001 | 24 h | <0.001 |
| D | Sham vs. 3 day | <0.001 | Sham vs. 3 day | <0.001 | 3 day | <0.050 |
| E | Sham vs. 5 day | <0.001 | Sham vs. 5 day | <0.001 | 5 day | <0.001 |
| F | Sham vs. 7 day | <0.001 | Sham vs. 7 day | <0.001 | 7 day | 0.066 |
| G | Sham vs. 10 day | <0.001 | Sham vs. 10 day | <0.001 | 10 day | 0.404 |
| H | Sham vs. 14 day | 0.095 | Sham vs. 14 day | 0.006 | 14 day | 0.103 |
The results of the statistical tests performed within and between RECA-1 time points and FITC-LEA time points are provided. This table is provided as a reference to the data depicted in Figure 2.
FITC-LEA, fluorescein isothiocyanate/Lycopersicon esculentum agglutinin.
To assess the functionality of the vasculature after injury, we compared the number of vessels marked by in vivo tracer (FITC-LEA) and ex vivo histological RECA-1 staining (Fig. 2; Table 2). We observed that RECA-1 counts were higher than FITC-LEA-positive vessels, indicating that although vascular components may be preserved post-SCI, perfusion of vessels is diminished. This trend was observed at all time points; however, the difference was greatest in tissues examined between 1 h and 5 days post-SCI (Fig. 2A–E). Vessels that are RECA-1 positive, but not FITC-LEA postive, may be physically blocked (by blood clots), disconnected from the blood supply (as a result of mechanical trauma), or new vessels that have not yet been connected to the vascular network (immature or angiogenic vessels). The latter case is supported by the trend that, over time, both RECA-1 and FITC-LEA values increased; however, FITC-LEA values were always less than RECA-1 values (Fig. 2A–H). This suggests that an endogenous vascular response occurs, and that vessels undergo growth and remodeling before they become functional.
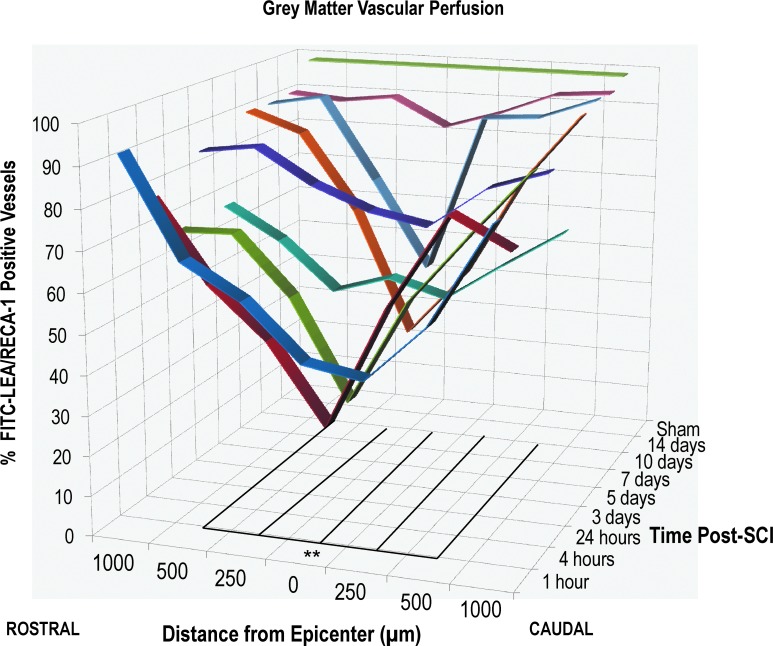
Because the vasculature of the GM and WM varies drastically in an uninjured spinal cord, it was of interest to investigate the two regions separately to determine whether one area was more vulnerable to vascular disruption after SCI. The results indicate that both GM and WM vasculature is disrupted after SCI; however, we observed that GM vessels (Fig. 3; Table 2) were more susceptible to interruptions in blood flow after SCI, compared to WM vessels (Fig. 4; Table 2). Moreover, we observed that the WM vasculature endogenously recovered to over 90% of an uninjured cord by 5 days postinjury, and that restoration of vascular flow occurred much more quickly (over 70% of vessels had a perfusion state by 24 h post-SCI). In the WM, points between 500 μm rostral and 500 μm caudal were significantly disrupted at 1 h after injury, and only the epicenter was significantly disrupted at 4 h post-SCI (Fig. 4; p<0.001). In the GM, the vasculature recovered much slower and did not recover to the same extent. Between 500 μm rostral and 500 μm caudal, a significant disruption was observed from 1 h and extended to 10 days post-SCI (Fig. 3; p<0.001). By 10 days post-SCI, less than 50% of vessels were perfused; however, by 14 days, 84% of vessels showed a perfusion state. Here, we report on the percentage of FITC-LEA-labeled vessels, relative to the number RECA-1-positive vessels, indicating a ratio of perfused (or functional) of the vessels.
FIG. 3.
Vascular disruption of the gray matter after traumatic SCI. Spatiotemporal quantification of vessel perfusion in the gray matter. Data shown are the percentage of FITC-LEA vessels, compared to RECA-1-positive vessels, at various distances from the epicenter (0–1000 μm rostral and caudal), as well as various time points after injury (1 h to 14 days post-SCI). A drastic reduction in vascular perfusion was observed as early as 1 h after injury, and a partial restoration was observed by 14 days post-SCI. The epicenter and areas directly adjacent to the epicenter show the most disruption (between 500 μm rostral and 500 μm caudal), whereas more-distal sections appear less affected. n=4–5 animals per time point (see Table 1). **p<0.001. Lines indicate the distance from the epicenter that show significant disruption, compared to sham animals. Lines extend along the z-axis to show the time window in which the disruption persists. FITC-LEA, fluorescein isothiocyanate/Lycopersicon esculentum agglutinin; SCI, spinal cord injury.
FIG. 4.
Vascular disruption of the white matter after traumatic SCI. Spatiotemporal quantification of vessel perfusion in the white matter. Data shown are the percentage of FITC-LEA vessels, compared to RECA-1-positive vessels, at various distances from the epicenter (0–1000 μm rostral and caudal), as well as various time points after injury (1 h to 14 days post-SCI). A drastic reduction in vascular perfusion was observed as early as 1 h after injury; however, the vasculature appears 95% restored by 24 h post-SCI. The epicenter and areas directly adjacent to the epicenter show the most disruption, whereas more-distal sections appear less affected. Compared to the gray matter vasculature, vascular disruption in the white matter was confined to a narrower rostrocaudal distribution as well as a significantly shorter time window. n=4–5 animals per time point (see Table 1). **p<0.001. Lines indicate the distance from the epicenter that show significant disruption, compared to sham animals. Lines extend along the z-axis to show the time window in which the disruption persists. FITC-LEA, fluorescein isothiocyanate/Lycopersicon esculentum agglutinin; SCI, spinal cord injury.
Endogenous angiogenesis occurs after spinal cord injury
Very few studies have examined the endogenous angiogenic response after SCI.3,5,33 Moreover, none of them have investigated such a response using a model of clip-compression injury, or extensively examined the spatiotemporal angiogenic response, as described in this research. In our studies, we observed a dynamic angiogenic response initiated after a moderately severe clip-compression injury. We quantified proliferating ECs (RECA-1/Ki67) to assess endogenous angiogenesis. Results showed angiogenesis occurring as early as 3 days after injury and ending by 10 days, with maximal angiogenesis occurring at 5 days post-SCI. Significant angiogenesis was observed at both 3- and 7-day time points, compared to sham animals, at all distances from the lesion (between 2000 μm rostral and 2000 μm caudal; p<0.001; Fig. 5). Additionally, tissue at 5 days post-SCI showed significant angiogenesis, compared with 3 and 7 days, spanning all distances from the injury (p<0.001; Fig. 5). At the peak of angiogenesis (5 days post-SCI), we noted that 1 mm distal to the epicenter showed the most proliferating ECs, with approximately 15% of vessels marked as Ki67 positive.
FIG. 5.
Endogenous angiogenic response after traumatic thoracic SCI. (A) Schematic representation of angiogenesis quantification. (B) Representative Ki67/RECA-1 double-label imaging (RECA-1, green; Ki67, red). Image taken from tissue at 5 days post-SCI within the ventral gray matter and 1000 μm caudal from injury. (C) Spatial and temporal quantification of the angiogenic response after SCI. Uninjured (sham) spinal cord tissue showed relatively low basal levels of endothelial cell proliferation (1%), compared to days 3, 5, and 7, after injury (6–15%) (**). Maximal proliferation was observed at 5 days post-SCI, with angiogenesis occurring most notably around 1000 μm distal to the injury site. Angiogenesis at 5 days was significantly increased, compared to days 3 and 7, at all distances (*). The epicenter did exhibit some vascular proliferation; however, as the result of a diminished number of vessels present, the ratio of regenerating endothelial cells was notably less than more-distal areas. Scale bar, 100 μm. *p<0.001; **p<0.001. SCI, spinal cord injury.
Discussion
In the present study, we aimed to identify some of the major vascular alterations that occur after SCI. We examined the temporal and spatial loss of vasculature, as well as the perfusion of the vasculature and vascular permeability. We observed that the BSCB is significantly disrupted early on, but appears to partially recover by 14 days post-SCI. Additionally, we noted that significant vascular loss and function occurs, and GM vessels in particular are more affected and less likely to recover after SCI. Last, we showed that an endogenous angiogenic response does occur in the spinal cord after clip-compression injury, and, consistent with other reports, we observed maximal angiogenesis between 3 and 7 days postinjury.3 To date, few studies have examined the spatial and temporal profiles of the clip-compression injury, and, to our knowledge, this is the first study to investigate endogenous angiogenic response after clip-compression injury. Taken together, the results from this research highlight potential targets and time points that may be most suitable for the delivery of vascular therapies post-SCI.
Our study shows that the BSCB is disrupted as early as 1 h postinjury and remains open until 5 days postinjury, with maximum permeability observed at 24 h post-SCI (Fig. 1). The time course of BSCB dysfunction in our results from a clip-compression model of SCI coincide with previous reports by Noble and Wrathall and Popovich and colleagues, which used weight-drop models of SCI.29,34 In our study, we used EB as a marker for spinal cord vascular permeability, which has benefits and disadvantages for assessing BSCB disruption.35–37 EB is a convenient, simple dye to administer and quantify; however, it binds to serum albumin, making it a 70-kDa protein, which is considered a large molecule, and may ultimately make the use of EB a “crude” estimate of vascular permeability.31,32 Previous studies have used horseradish peroxidase, which is approximately 45 kDa, or α-aminoisobutyric acid, which is only 0.1 kDa. In comparison, EB assays may be less sensitive to detecting subtle disruptions to the BSCB, because it may be too large to leak out. Therefore, it may be beneficial to repeat this study using a smaller vascular tracer to improve detection of vascular permeability. In general, in vivo tracers do not selectively identify the dysfunction of endothelial transporters or loss of specific tight junctions and adherens junctions; therefore, future studies would be required to identify the mechanism by which BSCB permeability is being affected. An additional caveat of using in vivo tracers to detect BSCB disruption is the inherent issue of reduced or obstructed blood flow after injury—vascular tracers rely on optimal hemodynamics. By using a circulating vascular marker, our results may be under-represented because the tracer may be physically restricted from reaching certain vascular networks (by blood clots and/or broken or severed vessels). In future studies, histological examination of BSCB proteins and structure may be a complimentary approach in assessing vascular permeability.
BSCB disruption after injury is a double-edge sword. On one hand, an increase in vascular permeability creates an ideal opportunity for the influx of inflammatory mediators and proteins not usually permitted in the CNS. Conversely, a compromised BSCB provides a unique opportunity for therapeutic intervention, because getting drugs and molecules into the highly regulated CNS normally presents a substantial challenge. In our model of clip-compression SCI, we observed that the BSCB remains significantly disrupted for up to 5 days after injury, which is a clinically relevant therapeutic window for administering treatments.
GM vessels are typically smaller vessels or capillaries; therefore, it is not surprising that they (being the most distal structures) would be more affected. However, diminished GM perfusion results in hypoxia ischemia to neuronal cell bodies, and if a decreased blood flow persists, neurons will undergo cell death. From a therapeutic and regenerative medicine prospective, it is therefore critical to revasularize the GM of the cord after trauma. More important, it appears that the extent of the vascular damage is restricted to a relatively small area (spanning a total of 2 mm rostral and caudal), and endogenous angiogenesis also maximally occurs in this region (at 1000 μm rostral and 1000 μm caudal); therefore, effective vascular therapies that are administered locally are likely to provide the most effective repair.
In the present study, we investigated the perfusion of the vasculature using an in vivo FITC-LEA dye. We believed that distinguishing functional vessels from other vessels was an important area of research, especially because it has not been specifically addressed in previous characterizations of vascular damage post-SCI. In comparing FITC-LEA and RECA-1 counts, we noted a decreased number of FITC-LEA vessels, suggesting a reduced number of vessels connected to the bloodstream. Although this may not be a surprising result, it is nevertheless an important finding. In many previous studies, vessels and vascular regeneration are often quantified by histological analysis. Histological assessment (i.e., RECA-1) quantifies all vascular structure without distinguishing whether the vessel had an active perfusion state. FITC-LEA marks the luminal surface of ECs, which have a hemodynamic state. With this, it is important to note that FITC-LEA may misrepresent the number of functional vessels, because some newly formed vessels (depending on lumen size) may be plasma perfused without supporting cellular perfusion and therefore are not truly acting as functional vessels. Whereas this technique provides some useful information, based on the results of this study, we recommend that future research be cautious when interpreting the results because the values may be overestimated.
Considering the extent of research dedicated to promoting vascular regeneration over the past decade, it was interesting to note that very few studies had focused on characterizing the vasculature of the injury itself, and no studies have been completed using the clip-compression model. In order to investigate the effectiveness of vascular therapies, it seems logical that we first understand the endogenous events, which could be used as a baseline for subsequent outcomes. With that, we believe that this research significantly contributes to the field of vascular regeneration after SCI and will aid in designing and administering more-effective vascular therapies for neurotrauma.
Conclusions
As observed by EB and FITC-LEA, the BSCB exhibits increased permeability, and the overall vascular function (perfusion) is decreased, respectively. These changes are observed as early as 24 hours postinjury and the disturbances extend past 10 days. Similarly, RECA-1 immunohistochemistry showed a significant decrease in the number of vessels observed at 2 and 4 mm from the lesion epicenter at 24 h postinjury, compared to uninjured animals, with endogenous revascularization showing a slight increase in vessel counts by 10 days postinjury. Separation of the WM and GM quantification showed that GM vessels are more susceptible to SCI, compared to WM vasculature, suggesting that vascular therapies should strive to target smaller, deeper vascular repair within the spinal cord. Finally, we observed endogenous angiogenesis after SCI. The angiogenic response spanned between 3 and 7 days postinjury, although maximal EC proliferation was observed at day 5. These data, consistent with other research in the field, suggests that angiogenic therapies should be administered locally (in the injury penumbra) and before 5 days postinjury to complement and enhance endogenous revascularization.
Acknowledgments
The authors express their gratitude to Eunice Cho and Sofia Khan for their help with tissue extraction, tissue processing, immunohistochemistry, and cell counting. The authors also thank Jared Wilcox, Behzad Azad, Yang Liu, and other members of the Fehlings' lab who assisted with postoperative animal care. This study was supported by the Gerald and Tootsie Halbert Chair in Neural Repair and Regeneration (held by Dr. Michael G. Fehlings) and by a grant received from the Krembil Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Furlan J.C., and Fehlings M.G. (2008). Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg. Focus 25, E15. [DOI] [PubMed] [Google Scholar]
- 2.Holtz A., Nystrom B., and Gerdin B. (1990). Relation between spinal cord blood flow and functional recovery after blocking weight-induced spinal cord injury in rats. Neurosurgery 26, 952–957 [DOI] [PubMed] [Google Scholar]
- 3.Loy D.N., Crawford C.H., Darnall J.B., Burke D.A., Onifer S.M., and Whittemore S.R. (2002). Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J. Comp. Neurol. 445, 308–324 [DOI] [PubMed] [Google Scholar]
- 4.Tator C.H., and Koyanagi I. (1997). Vascular mechanisms in the pathophysiology of human spinal cord injury. J. Neurosurg. 86, 483–492 [DOI] [PubMed] [Google Scholar]
- 5.Benton R.L., Maddie M.A., Minnillo D.R., Hagg T., and Whittemore S.R. (2008). Griffonia simplicifolia isolectin B4 identifies a specific subpopulation of angiogenic blood vessels following contusive spinal cord injury in the adult mouse. J. Comp. Neurol. 507, 1031–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelhardt B., and Coisne C. (2011). Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling X., and Liu D. (2007). Temporal and spatial profiles of cell loss after spinal cord injury: reduction by a metalloporphyrin. J. Neurosci. Res. 85, 2175–2185 [DOI] [PubMed] [Google Scholar]
- 8.Liu X.Z., Xu X.M., Hu R., Du C., Zhang S.X., McDonald J.W., Dong H.X., Wu Y.J., Fan G.S., Jacquin M.F., Hsu C.Y., and Choi D.W. (1997). Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 17, 5395–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lou J., Lenke L.G., Ludwig F.J., and O'Brien M.F. (1998). Apoptosis as a mechanism of neuronal cell death following acute experimental spinal cord injury. Spinal Cord 36, 683–690 [DOI] [PubMed] [Google Scholar]
- 10.Ribatti D., Vacca A., Nico B., Roncali L., and Dammacco F. (2001). Postnatal vasculogenesis. Mech. Dev. 100, 157–163 [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P. (2000). Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6, 389–395 [DOI] [PubMed] [Google Scholar]
- 12.Glaser J., Gonzalez R., Sadr E., and Keirstead H.S. (2006). Neutralization of the chemokine CXCL10 reduces apoptosis and increases axon sprouting after spinal cord injury. J. Neurosci. Res. 84, 724–734 [DOI] [PubMed] [Google Scholar]
- 13.Kaneko S., Iwanami A., Nakamura M., Kishino A., Kikuchi K., Shibata S., Okano H.J., Ikegami T., Moriya A., Konishi O., Nakayama C., Kumagai K., Kimura T., Sato Y., Goshima Y., Taniguchi M., Ito M., He Z., Toyama Y., and Okano H. (2006). A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 12, 1380–1389 [DOI] [PubMed] [Google Scholar]
- 14.Yoshihara T., Ohta M., Itokazu Y., Matsumoto N., Dezawa M., Suzuki Y., Taguchi A., Watanabe Y., Adachi Y., Ikehara S., Sugimoto H., and Ide C. (2007). Neuroprotective effect of bone marrow–derived mononuclear cells promoting functional recovery from spinal cord injury. J. Neurotrauma 24, 1026–1036 [DOI] [PubMed] [Google Scholar]
- 15.Ohab J.J., Fleming S., Blesch A., and Carmichael S.T. (2006). A neurovascular niche for neurogenesis after stroke. J. Neurosci. 26, 13007–13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters K.G., De Vries C., and Williams L.T. (1993). Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc. Natl. Acad. Sci. U. S. A. 90, 8915–8919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raab S., and Plate K. (2007). Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 113, 607–626 [DOI] [PubMed] [Google Scholar]
- 18.Bearden S.E., and Segal S.S. (2004). Microvessels promote motor nerve survival and regeneration through local VEGF release following ectopic reattachment. Microcirculation 11, 633–644 [DOI] [PubMed] [Google Scholar]
- 19.Casella G.T.B., Marcillo A., Bunge M.B., and Wood P.M. (2002). New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp. Neurol. 173, 63–76 [DOI] [PubMed] [Google Scholar]
- 20.Schumacher P.A., Eubanks J.H., and Fehlings M.G. (1999). Increased calpain I-mediated proteolysis, and preferential loss of dephosphorylated NF200, following traumatic spinal cord injury. Neuroscience 91, 733–744 [DOI] [PubMed] [Google Scholar]
- 21.von Euler M., Seiger Å., and Sundström E. (1997). Clip compression injury in the spinal cord: a correlative study of neurological and morphological alterations. Exp. Neurol. 145, 502–510 [DOI] [PubMed] [Google Scholar]
- 22.Grossman S.D., Rosenberg L.J., and Wrathall J.R. (2001). Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp. Neurol. 168, 273–282 [DOI] [PubMed] [Google Scholar]
- 23.Tator C.H., and Fehlings M.G. (1991). Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 75, 15–26 [DOI] [PubMed] [Google Scholar]
- 24.Noble L.J., and Wrathall J.R. (1989). Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 482, 57–66 [DOI] [PubMed] [Google Scholar]
- 25.Noble L.J., and Wrathall J.R. (1988). Blood-spinal cord barrier disruption proximal to a spinal cord transection in the rat: time course and pathways associated with protein leakage. Exp. Neurol. 99, 567–578 [DOI] [PubMed] [Google Scholar]
- 26.Noble L.J., and Wrathall J.R. (1987). The blood-spinal cord barrier after injury: pattern of vascular events proximal and distal to a transection in the rat. Brain Res. 424, 177–188 [DOI] [PubMed] [Google Scholar]
- 27.Benton R.L., and Whittemore S.R. (2003). VEGF165 therapy exacerbates secondary damage following spinal cord injury. Neurochem. Res. 28, 1693–1703 [DOI] [PubMed] [Google Scholar]
- 28.Gruner J.A. (1992). A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 9, 123–126 [DOI] [PubMed] [Google Scholar]
- 29.Popovich P.G., Horner P.J., Mullin B.B., and Stokes B.T. (1996). A quantitative spatial analysis of the blood-spinal cord barrier I. Permeability changes after experimental spinal contusion injury. Exp. Neurol. 142, 258–275 [DOI] [PubMed] [Google Scholar]
- 30.Fehlings M.G., and Tator C.H. (1995). The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 132, 220–228 [DOI] [PubMed] [Google Scholar]
- 31.Ikeda Y., Wang M., and Nakazawa S. (1994). Simple quantitative evaluation of blood-brain barrier disruption in vasogenic brain edema. Acta Neurochir. Suppl. (Wien) 60, 119–120 [DOI] [PubMed] [Google Scholar]
- 32.Saria A., and Lundberg J.M. (1983). Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J. Neurosci. Methods 8, 41–49 [DOI] [PubMed] [Google Scholar]
- 33.Hagg T., and Oudega M. (2006). Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma 23, 264–280 [DOI] [PubMed] [Google Scholar]
- 34.Noble L.J., and Wrathall J.R. (1989). Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 482, 57–66 [DOI] [PubMed] [Google Scholar]
- 35.Aoki T., Sumii T., Mori T., Wang X., and Lo E.H. (2002). Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke 33, 2711–2717 [DOI] [PubMed] [Google Scholar]
- 36.Saunders N., Habgood M., and Dziegielewska K.M. (1999). Barrier mechanisms in the brain, I. Adult brain. Clin. Exp. Pharmacol. Physiol. 26, 11–19 [DOI] [PubMed] [Google Scholar]
- 37.Kaya M., and Ahishali B. (2011). Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol. Biol. 763, 369–382 [DOI] [PubMed] [Google Scholar]