Abstract
The Wnt family of proteins plays key roles during central nervous system development and has been involved in several neuropathologies during adulthood, including spinal cord injury (SCI). However, Wnts expression knowledge is relatively limited during adult stages. Here, we sought to define the Wnt family expression pattern after SCI in adult mice by using quantitative polymerase chain reaction (qPCR) and immunohistochemistry (IHC). Under physiological conditions, the messenger RNAs (mRNAs) of most Wnt ligands, inhibitors, receptors, and coreceptors are constitutively expressed in healthy adult mice. After dorsal hemisection, we found significant time-dependent variations, with a prominent up-regulation of Wnt inhibitory factor 1 (Wif1). IHC against Frizzled (Fz) 1 and Fz4, as representatives of late and acute up-regulated receptors, showed a differential expression in the uninjured spinal cord of Fz1 by neurons and oligodendrocytes and Fz4 by astrocytes. After injury, both receptors were maintained in the same type of cells. Finally, by using BATgal reporter mice, our results revealed active β-catenin signaling in neurons of the dorsal horn and cells of the central canal of uninjured spinal cords, besides a lack of additional SCI-induced activation. In conclusion, we demonstrate Wnt expression in the adult spinal cord of mice that is modulated by SCI, which differs from that previously described in rats. Further, Fz receptors are differentially expressed by neurons and glial cells, suggestive for cell-specific patterns and thus diverse physiological roles. Further studies will help toward in-depth characterization of the role of all Wnt factors and receptors described and eventually allow for the design of novel therapies.
Key words: : adult, mouse, spinal cord injury, Wnt
Introduction
Spinal cord injury (SCI) is a major cause of disability that currently has no clinically accepted treatment.1 Functional deficits are caused by the initial mechanical damage, secondary neural death, and the limited ability of axons to regenerate.2–6 Therapeutic strategies that seek to limit the extent of secondary damage and promote regeneration have been shown to improve functional outcomes after SCI in animal models.7–11 However, the pathophysiological factors and mechanisms that underlie SCI are still largely unknown. Therefore, uncovering these components has become a major field of research aimed at the development of novel, effective therapies to treat SCI.
Wnts are a well-characterized family of glycoproteins that play prominent roles during neural development, cell proliferation and patterning, cell polarity and motility, axonal guidance, neuronal survival and connectivity, and cell-cell adhesion.12–14 The fact that Wnts can control such a wide variety of biological processes can be explained by their ability to activate a complex network of signaling cascades15,16 through the well-orchestrated spatiotemporal expression of more than 50 proteins, including ligands, secreted inhibitors, receptors, and coreceptors. A growing body of evidence suggests that Wnt signaling may be involved in homeostasis and disease progression in adult tissues,17–26 including the spinal cord.20,27–31
Previously, we reported on the constitutive expression of most Wnt ligands, receptors, and inhibitors as well as active canonical Wnt signaling in the spinal cord of adult rats.27,31 We found that spinal cord contusion induced Wnt expression and activation of the canonical pathway in cells around the wound core,27 which included reactive glia and microglia/macrophage cells. Further, we recently demonstrated that almost all of the 10 known Frizzled (Fz) and receptor related to tyrosine kinase (Ryk) receptors were expressed in specific spatial patterns in the uninjured spinal cords, and their expression patterns were altered during temporal progression of SCI.31 By contrast, the only report describing a role of Wnts in mice SCI claimed that Wnts were not expressed in adulthood and that, after hemisection damage, only a few Wnt ligands (Wnt1, Wnt4, and Wnt5a) and receptors (Ryk and Fz1) were reinduced, playing a key role on corticospinal axonal growth inhibition through noncanonical Wnt5a-Ryk activation.28 In agreement, SCI has been reported to not promote Wnt canonical activation in BATgal transgenic mice.32 However, new recent experimental evidences are providing contradictory data in support of Wnt expression, with key roles in both the healthy and damaged spinal cord of adult mice. In this line of research, a recent wide screening in the spinal cord of adult mice has shown a constitutive expression of most Wnt family members, as well as the expression of several Wnt ligands (Wnt1, Wnt3a, Wnt4, and Wnt5a), receptors (Fz1 and Fz2), β-catenin, and, remarkably, Wnt inhibitory factor 1 (Wif1) by neurons and astrocytes as a result of amyotrophic lateral sclerosis progression.25,33,34 In a different study, Tang and colleagues have reported that Wnt3a, Wnt5a, and the noncanonical receptor, receptor tyrosine kinase-like orphan receptor 2, were constitutively expressed by neurons and glial cells of the adult spinal cord of mice and that, in experimental models of multiple sclerosis and peripheral nerve injury, they might play a role on the process of remyelination and induction of neuropathic pain by, at least, activating the β-catenin canonical pathway.24,29,30 Moreover, a previous thorough in situ hybridization mapping of key factors during development revealed that expression of most Wnt ligands and receptors was prolonged in the adult central nervous system (CNS) of mice, including the spinal cord.35 Therefore, we decided to reassess, by means of quantitative polymerase chain reaction (qPCR) and immunohistochemistry (IHC), a broad analysis of Wnt expression patterns in healthy and post–hemisection injury of the adult spinal cord of mice.
Methods
Surgical procedures and experimental design
Adult female C57BL/6J mice, weighing 20–25 g, were used throughout the study. Animals were obtained from Harlan (Barcelona, Spain) and housed in climate-controlled quarters with a 12-h light/dark cycle. To minimize both suffering and the number of animals used, animal handling was conducted in accord with the laws of the European Union and National Institutes of Health guidelines (2010/63/EU) for animal experimentation, and experimental protocols were approved by the bioethics committee of The National Hospital of Paraplegics (permit nos.: 51/2009 and 45/2008). Throughout the procedures, sterile surgical techniques and methods were performed in a room specifically designated for these experiments. Briefly, animals were anesthetized throughout the surgical procedures with a mixture of oxygen (1.5%) and isoflurane (3%). Laminectomies were performed between the T6 and T10 vertebral levels, followed by four 0.5-mm-deep dorsal incisions at each intervertebral level; finally, overlying muscle and skin layers were sutured. After surgery, mice were allowed to recover on a warmed blanket with access to water and food, during which time they also received subcutaneous injections of saline solution containing enrofloxacin (3 mg/kg) and buprenorphine (0.05 mg/kg). For the qPCR studies (n=4), 24 animals were randomly distributed among each of the following six experimental groups: uninjured control (C); 6 and 24 h postinjury (hpi); and 3, 7, and 14 days postinjury (dpi). For the histological studies (n=3), 9 animals were randomized into three experimental groups (C, 6 hpi, and 14 dpi).
BATgal mice with an FvB background were obtained from the Millar laboratory,36 bred to C57BL/6J mice (Harlan), and maintained as a heterozygous line. Following the surgical procedures described above, a total of 9 BATgal adult mice were used for histological assesment of Wnt canonical activation in uninjured controls (n=3) and after T8 dorsal hemisection injury at 24 hpi (n=3) and 7 dpi (n=3).
RNA isolation and reverse-transcription quantitative polymerase chain reaction analysis
At each of the time points chosen for study, animals were terminally anesthetized with pentobarbital and perfused intracardially (i.c.) with heparinized saline to remove blood from tissue. Total messenger RNA (mRNA) was isolated from a 1-cm-long fragment of spinal cord containing the lesion area using the RNeasy Lipid Mini Kit (Qiagen, Hilden, Germany) and amplified using the MessageAmp II aRNA Amplification Kit (Ambion, Austin, TX), according to the manufacturer's instructions. Complementary DNA (cDNA) synthesis from DNase-treated RNA (6 μg) and relative quantifications were performed as previously described27 using 50 ng of cDNA and specific primers (see Table 1). β-actin (forward 5′-AAGTCCCTCACCCTCCCAAAAG-3′, reverse 5′-AAGCAAT GCTGTCACCTTCCC-3′; GenBank accession no.: NM_007393.3) was quantified in a separate wells as a real-time reporter. Primers not specified in Table 1 were validated elsewhere.37,38
Table 1.
List of qPCR Primers
| Gene name | Forward primer | Reverse primer | Accession no. |
|---|---|---|---|
| Axin2 | 5′-CGTGCGCCGCCCTATAT-3′ | 5′-CATAAACCCCTTTCAAAAACCAA-3′ | NM_015732.4 |
| Dkk1 | 5′-GCCCAAGCAAGTGATTCCA-3′ | 5′-GGCTTCCTTCTGCATAATCTCTTC-3′ | NM_010051.3 |
| Dkk2 | 5′-GTTCCAGCTTCGGACACACA-3′ | 5′-AGCAGACACATGCAGAGCACTAA-3′ | NM_020265.4 |
| Dkk4 | 5′-TGGCCTCAAACGCATGATC-3′ | 5′-GCTCGCCTGTAATCCCAAGA-3′ | NM_145592.2 |
| Fz1 | 5′-GGACTGACGGATGGGTGAGA-3′ | 5′-ACTTCCCCTTCAGCATGCAT-3′ | NM_021457.3 |
| Fz2 | 5′-AGCGGCCTGAGAGATGTTCTAT-3′ | 5′-CCATGCCTGCTGGAAACTG-3′ | NM_020510.2 |
| Fz3 | 5′-AAAGAAGCAAAGCAGGGAGTTG-3′ | 5′-TGTGGAGGCTGCCATGGT-3′ | NM_021458.2 |
| Fz5 | 5′-GCCCTTTCTTGTCGCCAAA-3′ | 5′-CCAAGCCAGGCTGTTTCTTT-3′ | NM_022721.3 |
| Fz6 | 5′-AAATGTGCGAAGGGCAGAGT-3′ | 5′-GCTGCATCTGCTGCACTACAC-3′ | NM_008056.3 |
| Fz7 | 5′-GTCCTCCAATTTTGCAATGTTCT-3′ | 5′-CCCCCTGGCTGGCATTA-3′ | NM_008057.3 |
| Fz8 | 5′-GCCATTGTCCCAGGTCTGA-3′ | 5′-ACTCCTCTCCGCCCCTTCT-3′ | NM_008058.2 |
| GSK-3β | 5′-CTTGGAAACCCCCTGGACTT-3′ | 5′-TGTTTTTCCCCAAAGCAGTGT-3′ | NM_019827.6 |
| Kremen1 | 5′-ATCTGGACCATTTTCTATGAACCCT-3′ | 5′-CATCTTGTTGACTCTGACCCTTGA-3′ | NM_032396.3 |
| Kremen2 | 5′-TCGACCTTGTGCCCCTTTAA-3′ | 5′-TTCTGGTCTTCCCGAGATGATC-3′ | NM_028416.1 |
| LEF1 | 5′-TGACCCCAAGGAACACTGACA-3′ | 5′-TCGGGTCAGCGCTAGCA-3′ | NM_010703.3 |
| LRP5 | 5′-GACCATACAGGCCCTACGTCAT-3′ | 5′-GTGCTGCACGGTGTTGTTG-3′ | NM_008513.3 |
| Ryk | 5′-GGCGGAGCTACGCACAGA-3′ | 5′-CAAAACTAAAACCCCCCAAACC-3′ | NM_013649.3 |
| Wif1 | 5′-CCCCATCTTGAATGCATACAATC-3′ | 5′-CGCCCCATCAAAACTGAACT-3′ | NM_011915.2 |
| Wnt1 | 5′-CTTCGGCAAGATCGTCAACC-3′ | 5′-GCGAAGATGAACGCTGTTTCT-3′ | NM_021279.4 |
| Wnt2b | 5′-GGATTTGCTCCGGTTTCACA-3′ | 5′-GACCACAAGGGCCCTGACTA-3′ | NM_009520.3 |
| Wnt5a | 5′-AATAACCCTGTTCAGATGTCA-3′ | 5′-TACTGCATGTGGTCCTGATA-3′ | NM_009524.2 |
| Wnt7b | 5′-GGCCGGGCACCACAT-3′ | 5′-GCAGGAACACAGCCCATCA-3′ | NM_009528.3 |
| Wnt8a | 5′-CACGTGCTGCCTAGTTGCA-3′ | 5′-TGTCCCCTAGGCCAGAAGTTC-3′ | NM_009290.2 |
| Wnt9a | 5′-CCGGTACAGCAGTGGACTCA-3′ | 5′-GAAATCACAGGCTGCACAAGAC-3′ | NM_139298.2 |
| Wnt10b | 5-TGGGACCTCGGGTGACAAT-3′ | 5′-TCCCTGCCCTCTGTCCTTT-3′ | NM_011718.1 |
| Wnt15 | 5′-TTACCACACCGTGGCTGTCTT-3′ | 5′-GGACGTTCATGGGTGTTGAGA-3′ | NM_011719.4 |
Primers used for qPCR analyses of the genes assessed in this study, including the gene symbol, primer sequence (forward and reverse sequence, respectively), and GenBank accession number.
Immunohistochemistry
As stated above, 3 animals from the C, 6-hpi and 14-dpi groups were i.c. perfused with heparinized saline solution followed by 4% paraformaldehyde (PFA); spinal cords were then postfixed for 4 h in 4% PFA, cryoprotected by immersion in 30% sucrose for 48 h, embedded in Neg-50 frozen medium (Richard-Allan Scientific, Kalamazoo, MI), and stored at −20°C. From each spinal cord, a 1-cm-long fragment containing the area of injury was cut using a cryostat to obtain 30-μm-thick parallel sections.
To characterize the protein expression patterns of Fz1 (mRNA expression pattern with late induction) and Fz4 (mRNA expression pattern with early induction) in uninjured and after hemisection damage, a set of parallel sections of each experimental group were assessed by a single IHC analysis, as previously described.27 In this regard, the following primary antibodies (Abs) produced in rabbit and obtained from Abcam (Cambridge, MA) were used: anti-Fz1 (1:10; ab71342) and anti-Fz4 (1:1000; ab83042). Primary Abs were visualized by sequential incubations with a horseradish peroxidase (HRP)-conjugated anti-rabbit secondary Aby (1:500; Thermo Scientific, Waltham, MA) and “Nova Red Kit” (Vector Laboratories, Burlingham, CA), according to the manufacturer's instructions. Finally, sections were dehydrated in a graded ethanol series, cleared with xylene, and DPX (distrene, plasticizer, xylene) cover slipped (Panreac, Barcelona, Spain). Bright-field images were analyzed and acquired by using a BX61 Motorized Research Microscope (Olympus, Tokyo, Japan).
To evaluate cellular Fz1 and Fz4 expression patterns in both nonlesioned and lesioned spinal cords, double IHC was performed in a set of parallel sections for each experimental group to visualize both receptors in astrocytes (glial fibrillary acidic protein; GFAP), neurons (neuronal nuclei; NeuN), oligodendrocytes (adenomatous polyposis coli; APC), axons (neurofilament 200; NF200), and microglia/macrophages (CD11b). Immediately before use, sections were warmed to room temperature, washed in Tris-buffered saline, and incubated in 5 mg/mL of NaBH4 for 30 min. Then, sections were processed after the same protocol detailed in a previous report.27,31 Both receptors were visualized by using the same primary Ab detailed above for single IHC at dilutions of 1:25 for Fz1 and 1:1000 for Fz4, and the corresponding Dylight594 (ab96897; Abcam) or Dylight488 (ab96899; Abcam) linked anti-rabbit secondary Ab (1:500). Sections were then incubated with the following primary Abs produced in mice, such as anti-GFAP (1:1000; G3893; Sigma-Aldrich, St. Louis, MO), anti-NeuN (1:250; MAB377; Millipore, Billerica, MA), anti-APC (1:25; OP80; Calbiochem, San Diego, CA), and anti-NF200 (1:200; N0142; Sigma-Aldrich), or in rats, such as anti-CD11b (1:200; ab6332; Abcam). Subsequently, the corresponding Dylight488-linked anti-mouse (ab96879; Abcam) or Dylight594-linked anti-rat (SA5-10020; Thermo Scientific) secondary Ab was used (1:500). For double IHC analyses where the primary Abs were produced in mice, we used the specific M.O.M. kit (Vector Laboratories), and the protocol was conducted according to the manufacturer's instructions. Finally, sections were analyzed using both the BX61 Motorized Research Microscope (Olympus) and a Leica TCS SP5 confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany).
For analyses of BATgal mice, whole spinal cords were serially cut in 50-μm-thick sections on a cryostat and assayed by IHC, as described above, using the following Abs: rabbit anti-β-galactosidase (β-gal; 1:5000 for fluorescent detection and 1:10000 for 3, 3′-diaminobenzidine (DAB) detection; ab616-1; Abcam), mouse anti-β-Gal (1:1000; clone 40-1a; Developmental Studies Hybridoma Bank [DSHB), University of Iowa, Iowa City, IA), mouse anti-NeuN (1:1000; MAB377; Millipore), rabbit anti-GFAP (1:20,000; Z0334; Dako Denmark A/S, Glostrup, Denmark), goat anti-SRY (sex determining region Y)-box 9 (Sox9; 1:100; AF3075; R&D Systems, Minneapolis, MN), or mouse anti-radial glial cells (Vimentin; 1:100; clone 3CB2; DSHB). Fluorophore-conjugated secondary Abs (Molecular Probes, Eugene, OR) were used at a 1:1000 dilution. For DAB IHC, samples were incubated with a biotinylated secondary antiserum (1:200; Vector Laboratories) and then visualized using an avidin-biotin-HRP protocol using the ABC kit (Vector Laboratories) with DAB (Sigma-Aldrich) as the chromogen.
All sections were processed at the same time and following the same experimental protocol, and sections were processed without the primary Ab were used as controls (data not shown). For double IHC and to detect putative cross-reactivity between both single IHCs, sections were processed without the second primary Ab and used as controls. No nonspecific staining was observed.
Statistical analysis
All values are expressed as the mean±standard error of the mean. Statistical comparisons were performed using one-way analysis of variance, followed by Tukey's post-hoc test, to determine the individual differences between the means. In all cases, p<0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism (version 4.0; GraphPad Software Inc., La Jolla, CA).
Results
Wnt ligands expression is prolonged in the spinal cord of adult mice and is modulated by injury
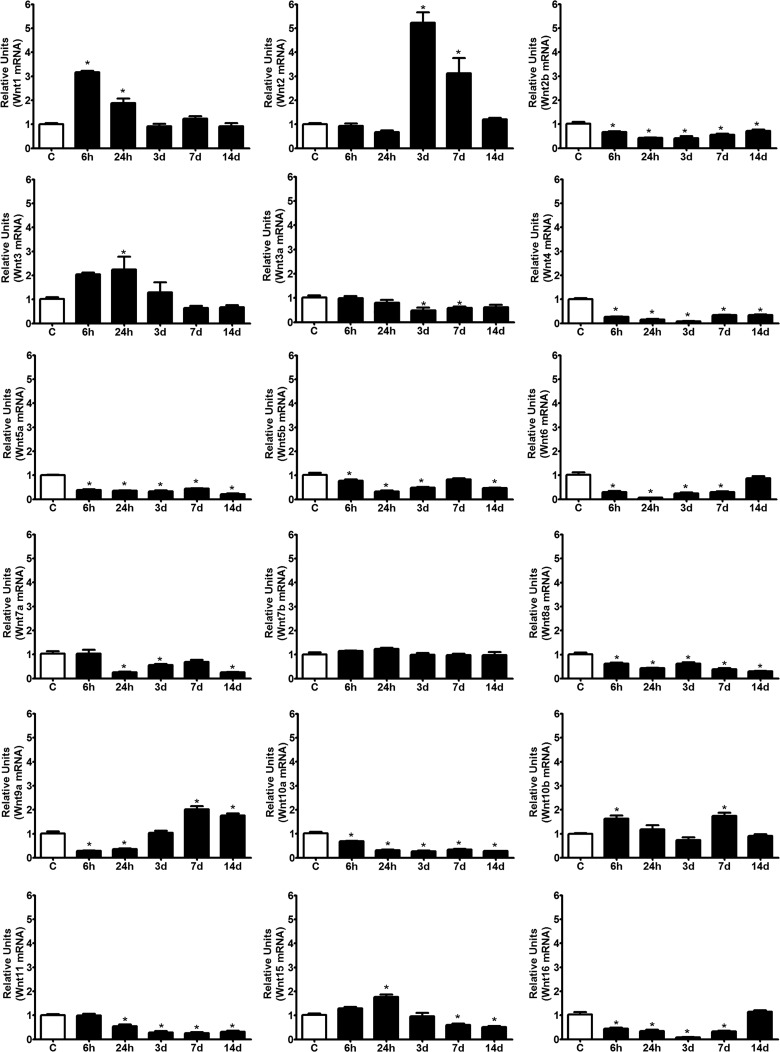
In a recent study, we showed that Wnts are constitutively expressed in the uninjured spinal cord of adult rats and that this expression is modulated after contusion injury.27 However, current experimental evidences regarding the expression of Wnts in the adult spinal cord of mice and their possible involvement in response to injury are controversial.24,25,28–30,33–35,39 Therefore, by using a similar experimental design to our previous study in rats, but with spinal cord dorsal hemisection as an injury paradigm to reproduce the only report describing a role for Wnts in traumatic SCI,28 we decided to assess, by qPCR, the temporal pattern of mRNA expression for all Wnt ligands in both uninjured and injured mice up to 14 days after spinal cord hemisection lesion. Consistent with our previous results in rats, most Wnt ligands were found to be expressed in the uninjured adult spinal cord. Only the Wnt8b mRNA was below the level of detection of the qPCR protocol (threshold cycle, >35). However, unlike in rats, mRNA expression of the majority of the Wnt ligands (Wnt2b, -3a, -4, -5a, -5b, -6, -7a, -8a, -10a, -11, and -16) was significantly down-regulated after injury at all or most of the time points analyzed (Fig. 1). Only four ligands were found to be up-regulated: Wnt1 and Wnt3 were up-regulated early between 6 and 24 hpi; Wnt2 was up-regulated late from 3 to 7 dpi; and Wnt10b followed a biphasic pattern, with peaks of expression at 6 hpi and 7 dpi. Only Wnt7b was unaffected by injury. Other ligands were found to be down-regulated early and up-regulated late (Wnt9a) or, inversely, up-regulated early and down-regulated late (Wnt15). Importantly, none of the Wnt ligands that were absent or undetectable in control spinal cords were expressed after injury (Fig. 1).
FIG. 1.
Wnt ligand expression in the spinal cord of adult mice after spinal cord injury (SCI). All of the Wnt ligands, with the exception of Wnt8b, were constitutively expressed in the uninjured adult spinal cord. After SCI, three main profiles of Wnt ligand expression were observed: 1) no change or a dramatic reduction (Wnt2b, -3a, -4, -5a, -5b, -6, -7a, -7b, -8a, -10a, -11, and -16); 2) early induction (Wnt1, -3, and -15); and 3) late induction (Wnt2 and -9). All analyses were performed using total RNA samples isolated from a 1-cm-long fragment of the spinal cord from uninjured control animals (C) and fragments containing the damaged area of the spinal cord from different times postinjury (6 and 24 hpi as well as 3, 7, and 14 dpi). Gene expression of Wnt ligands was measured using quantitative polymerase chain reaction with specific primers, and the results were normalized to β-actin expression. The values for each experimental group and day are expressed as the mean±standard error of the mean (n=4). Each animal/sample was assayed in triplicate in two separate occasions, yielding two independent technical triplicates and six measurements per sample. *p<0.05, compared to C. mRNA, messenger RNA; d, days.
Canonical and noncanonical Wnt receptors are expressed in both healthy and injured spinal cords of adult mice
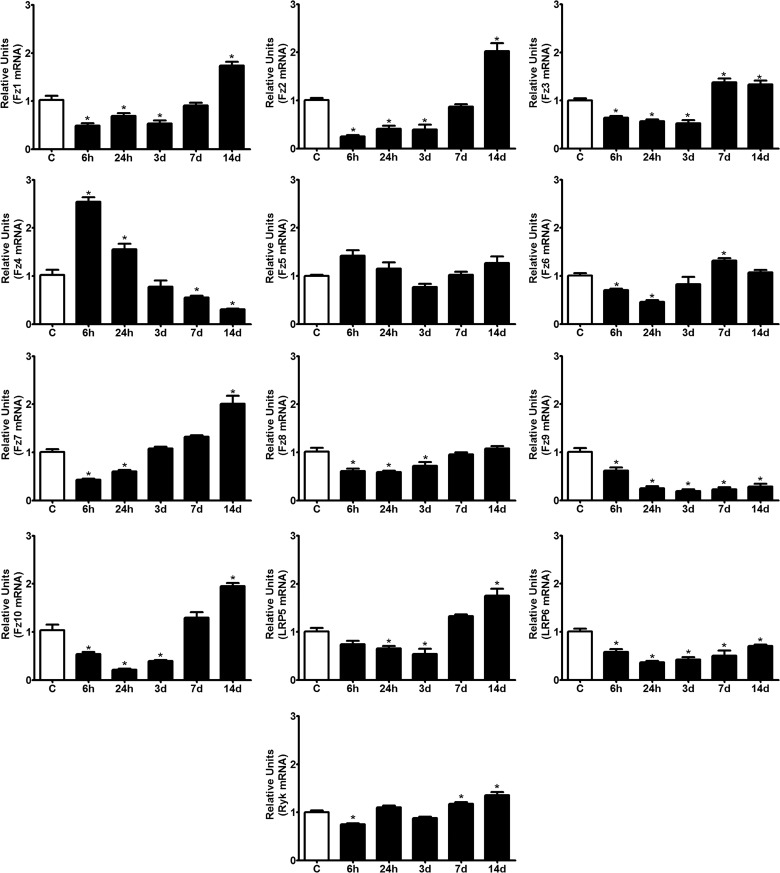
Once we had determined the expression profiles of most Wnt ligands in the spinal cord of adult mice, we next sought to determine mRNA expression profiles of the 10 Fz receptors, the canonical LRP5/6 coreceptors, and the non-canonical Ryk receptor. In this line, recently, we showed that the mRNAs encoding most of the 10 known Fz and Ryk receptors were expressed in both uninjured and injured rats up to 28 days after spinal cord lesion. Further, we showed that the mRNA expression pattern of Fz and Ryk receptors was reflected at the protein level, as detected using single IHC, and interestingly, the Fz and Ryk proteins exhibited distinct spatiotemporal expression patterns.31 Again, consistent with our previous results in rats, we found detectable levels of expression for all of the analyzed receptors in the uninjured spinal cord (Fig. 2). However, after injury, the majority of the Fz receptors showed a dramatic reduction in their expression levels at the early time points analyzed (6–24 hpi), followed by a steady increase in expression from 3–14 or 7–14 dpi, leading to maximal expression at the latest time point (Fz1, -2, -3, -6, -7, -10, and LRP5, and Ryk). Only Fz4 showed a marked up-regulation during 6–24 hpi, after which its mRNA expression levels decreased to below control levels at 7 dpi. The remaining receptors that were analyzed showed either no significant change in their expression profiles after injury (Fz5) or exhibited a dramatic reduction (Fz8 and -9, and LRP6) at almost all of the time points evaluated (Fig. 2).
FIG. 2.
Expression of Fz receptors, LRP5/6 coreceptors, and the noncanonical Ryk receptor in the spinal cord of adult mice after spinal cord injury (SCI). All genes were constitutively expressed in the uninjured adult spinal cord. After SCI, the majority of Fz receptors showed a dramatic reduction in expression during the early time points we analyzed (6–24 hpi), followed by a steady increase in expression beginning at 3 or 7 dpi and lasting until 14 dpi, when maximal expression was observed (Fz1, -2, -3, -6, -7, and -10, LRP5, and Ryk). Only Fz4 showed significant up-regulation during 6–24 hpi, after which mRNA levels decreased to below control levels during 7–14 dpi. Fz5 showed no significant changes in its expression profile, and Fz8, 9, and LRP6 exhibited dramatic reductions at nearly all of the time points analyzed. Expression levels of all genes were measured using quantitative polymerase chain reaction with specific primers, and the results were normalized to the β-actin expression. The values for each experimental group and day are expressed as the mean±standard error of the mean (n=4). Each animal/sample was assayed in triplicate in two separate occasions, yielding two independent technical triplicates and six measurements per sample. *p<0.05, compared to C (control). Fz, Frizzled; mRNA, messenger RNA; d, days; LRP, lipoprotein receptor-related protein; Ryk, receptor related to tyrosine kinase.
Next, using single and double IHC, we investigated the protein expression of Fz1 and Fz4, as representatives of late and early mRNA expression pattern receptors, respectively. Consistent with the results of the mRNA analyses, the receptors were found to be expressed both in the uninjured spinal cords and after injury (peaks of mRNA expression) of adult mice, with Fz-expressing cells located in both the gray and white matter areas (Figs. 3A and 3B and 4A and 4B).
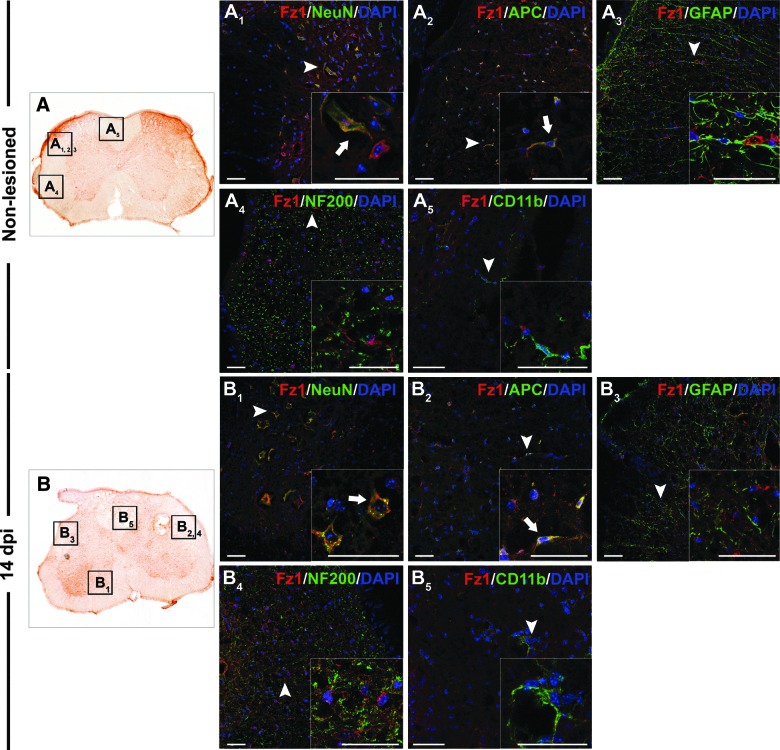
FIG. 3.
Cellular protein expression pattern of Frizzled 1 in nonlesioned spinal cords and at 14 dpi. Representative images obtained from the microscopic evaluation of sections processed by double immunohistochemistry to visualize Frizzled (Fz) 1 in neurons (NeuN), oligodendrocytes (APC), astrocytes (GFAP), axons (NF200), and microglia/macrophages (CD11b) in nonlesioned spinal cords (A, A1, A2, A3, A4, and A5) and at 14 days postinjury (dpi) (B, B1, B2, B3, and B5). Squares in the images showing the entire spinal cord sections (A and B) correspond to the areas of higher magnification. Scale bars=40 μm. dpi, days postinjury; Fz, Frizzled; NeuN, neuronal nuclei; APC, adenomatous polyposis coli; DAPI, 4',6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein. Color image is available online at www.liebertpub.com/neu
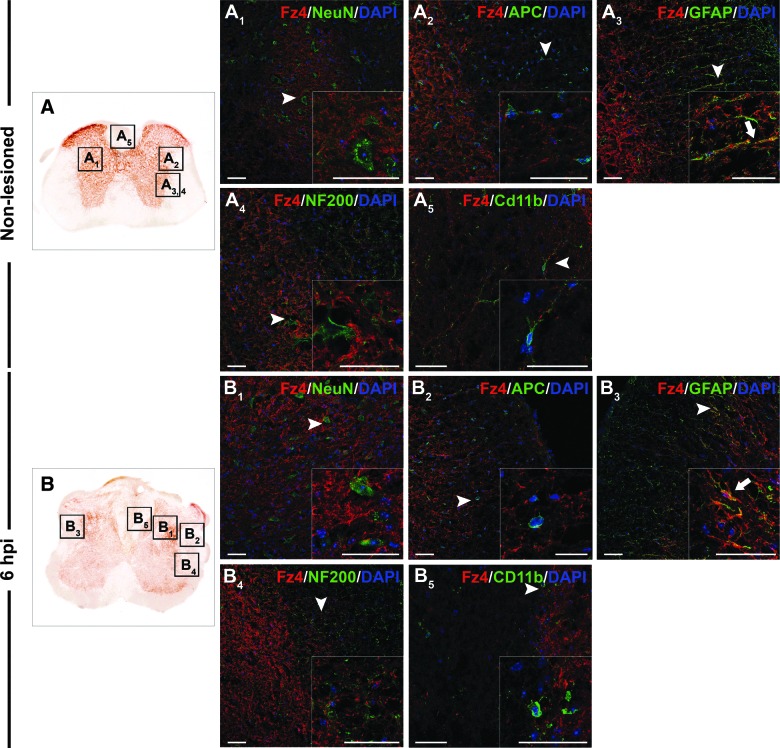
FIG. 4.
Cellular protein expression pattern of Frizzled 4 in nonlesioned spinal cords and at 6 hpi. Representative images obtained from microscopic evaluation of sections processed by double immunohistochemistry to visualize Frizzled (Fz) 4 in neurons (NeuN), oligodendrocytes (APC), astrocytes (GFAP), axons (NF200), and microglia/macrophages (CD11b) in the non-lesioned spinal cords (A, A1, A2, A3, A4 and A5) and at 6 h postinjury (hpi) (B, B1, B2, B3, and B5). Squares in the images showing the entire spinal cord sections (A and B) correspond to the areas of higher magnification. Scale bars=40 μm. hpi, hours postinjury; Fz, Frizzled; NeuN, neuronal nuclei; APC, adenomatous polyposis coli; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein. Color image is available online at www.liebertpub.com/neu
In the uninjured spinal cord, Fz1 expression was detected in neurons located in all spinal cord laminae (Fig. 3A1) analyzed and in oligodendrocytes (Fig. 3A2), but not in astrocytes, quiescent microglial cells, or axonal projections (Fig. 3A3 to 3A5). At 14 dpi (Fig. 3B), Fz1 was still expressed in neurons and oligodendrocytes (Fig. 3B1 and 3B2) in the preserved spinal cord parenchyma that surrounded injured tissue. No Fz1 expression was detected in axons, reactive microglia/macrophages, or astrocytes (Fig. 3B3 to 3B5) at this time postinjury.
Moreover, when we analyzed Fz4, we observed that in the uninjured spinal cord (Fig. 4A), receptor expression was restricted to astrocytes (Fig. 4A3). No Fz4 expression was detected in neurons, oligodendrocytes, axonal projections, or quiescent microglial cells (Fig. 4A1, 4A2, 4A4, and 4A5). At 6 hpi (Fig. 4B), cellular receptor expression was maintained, so that we only observed coexpression in astrocytes (Fig. 4A1 to 4A5).
Wnt inhibitors and modulators are differentially expressed in both healthy and injured spinal cords of adult mice
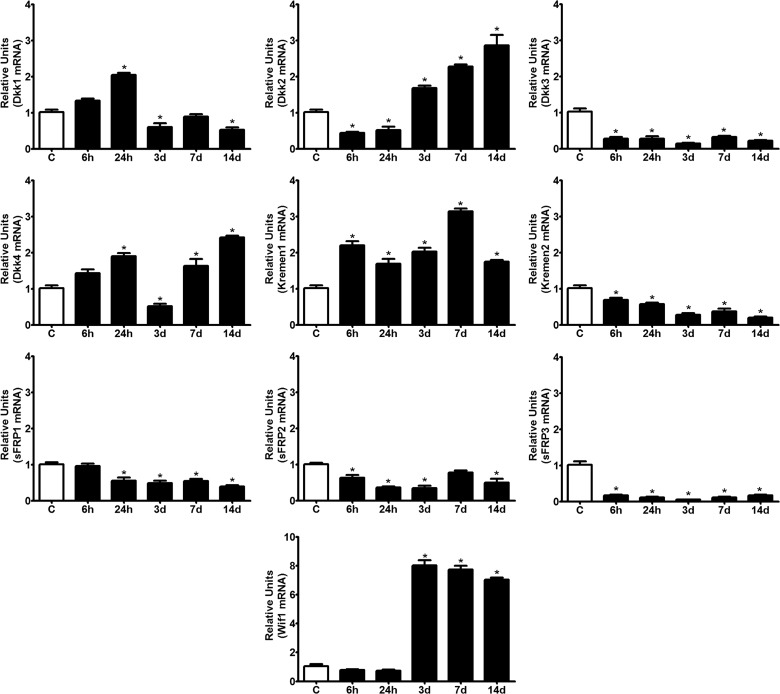
In addition to expression of Wnt ligands and receptors and coreceptors, another important aspect of Wnt signaling is the extracellular secretion of a diverse number of Wnt inhibitors and modulators. Among these molecules, we analyzed the expression of members from several of the most well-known families involved in nervous system function, including the following: Dkk-1/4, which interferes with Wnt activity and antagonizes canonical Wnt signaling by binding to and sequestering LRP5 or LRP6, as well as sFRP-1/5 and Wif1, which can bind to and sequester Wnts, thereby inhibiting both canonical and noncanonical Wnt signaling. As observed with the ligands and receptors, we found that most of the Wnt inhibitors were expressed in the uninjured adult spinal cord, with the exception of sFRP4 and sFRP5 mRNAs (Fig. 5).
FIG. 5.
Wnt inhibitor expression in the spinal cord of adult mice after spinal cord injury (SCI). All of the inhibitors, with the exception of sFRP4 and -5, were detected in the uninjured spinal cord. After SCI, expression of the Dkks was differentially modulated. Dkk1 was induced early, with a maximal peak of expression at 24 hpi, and had a significant decrease in expression from 3 dpi onward. Dkk2 exhibited an early down-regulation during 6–24 hpi, followed by a steady increase in expression from 3 dpi onward. Dkk3 was consistently down-regulated at all time points analyzed, and Dkk4 showed a biphasic up-regulation with peaks at 24 hpi and 14 dpi. Kremen1 was up-regulated during all time points analyzed. sFRP1, -2, and -3 exhibited strong down-regulation during all experimental time points, and Wif1 expression was significantly up-regulated from 3 dpi until the end of the study. Expression levels of inhibitors were measured using quantitative polymerase chain reaction with specific primers, and the results were normalized to β-actin expression. Values for each experimental group and day are expressed as the mean±standard error of the mean (n=4). Each animal/sample was assayed in triplicate in two separate occasions, yielding two independent technical triplicates and six measurements per sample. *p<0.05, compared to C (control). Dkk, dickkopf; mRNA, messenger RNA; d, days; sFRP, secreted Frizzled-related protein; Wif1, Wnt inhibitory factor 1.
Interestingly, we observed differential modulation of all four members of the Dkk family after injury. Dkk1 was induced early with a maximum peak of expression at 24 hpi and that had significantly decreased by 3 dpi. By contrast, Dkk2 exhibited an early down-regulation during 6–24 hpi, followed by a steady up-regulation from 3 dpi onward. Dkk3 was found to be consistently down-regulated at all time points analyzed, and Dkk4 showed a biphasic up-regulation with peaks at 24 hpi and 14 dpi. Consistent with these results, the expression of Kremen-1 and -2, which are coreceptors required for Dkk-mediated inhibition of canonical Wnt signaling, was affected by injury; Kremen-1 was consistently up-regulated, and Kremen-2 was consistently down-regulated at all time points assessed after SCI (Fig. 5). All of the sFRPs that had detectable levels of expression in the uninjured adult spinal cord (sFRP1, -2, and -3) also exhibited strong down-regulation at all of the time points assayed after injury. Finally, Wif1, a broadly acting inhibitor of Wnt signaling, showed no changes in expression for 3 dpi, after which time it became strikingly up-regulated (an 8-fold increase, when compared to controls) and remained significantly elevated until the end of the study (Fig. 5).
Canonical Wnt signaling is constitutively active in the spinal cord of adult mice
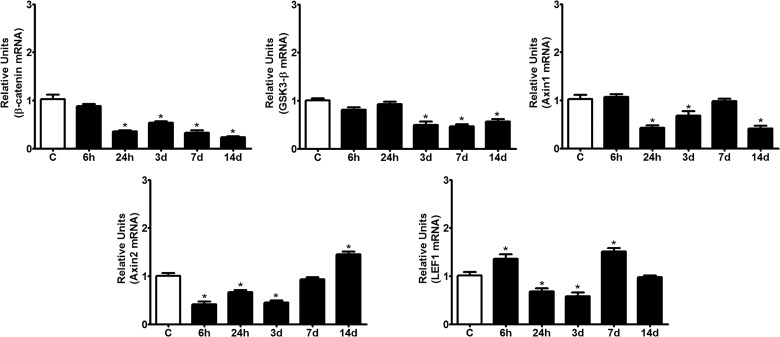
Based on the observed concomitant expression of most Wnt ligands and receptors in the spinal cord of adult mice, we next sought to determine the mRNA expression profiles of several key components of the canonical pathway. As expected, β-catenin, GSK-3β, LEF1, Axin1, and Axin2 were constitutively expressed (Fig. 6). After injury, expression levels of β-catenin, GSK-3β, Axin1, and Axin2 were unaffected or down-regulated during most of the time points analyzed, with the notable exception of Axin2, which was significantly up-regulated at 14 dpi. Finally, the mRNA of the transcription factor, LEF1, was up-regulated in a biphasic manner with two significant peaks at 6 hpi and 7 dpi, whereas it was down-regulated between these peaks during the 24-hpi and 3-dpi time points (Fig. 6).
FIG. 6.
Intracellular Wnt-signaling component expression in the spinal cord of adult mice after spinal cord injury (SCI). The intracellular Wnt-signaling components β-catenin, GSK-3β, Axin, and LEF1 were detected in the uninjured spinal cord. Following SCI, β-catenin and GSK-3β showed significant decreases in their expression levels during most of the time points analyzed. Both Axin1 and Axin2 showed significant decreases in expression during early time points, but only the Axin2 mRNA showed a significant increase at 14 dpi. LEF1 mRNA exhibited a biphasic pattern of expression with two significant peaks at 6 hpi and 7 dpi. Expression levels of modulators were measured using quantitative polymerase chain reaction with specific primers, and the results were normalized to β-actin expression. Values for each experimental group and day are expressed as the mean±standard error of the mean (n=4). Each animal/sample was assayed in triplicate in two separate occasions, yielding two independent technical triplicates and six measurements per sample. *p<0.05, compared to C (control). mRNA, messenger RNA; d, days; GSK-3β, glycogen synthase kinase 3 beta; LEF1, lymphoid enhancer-binding factor-1.
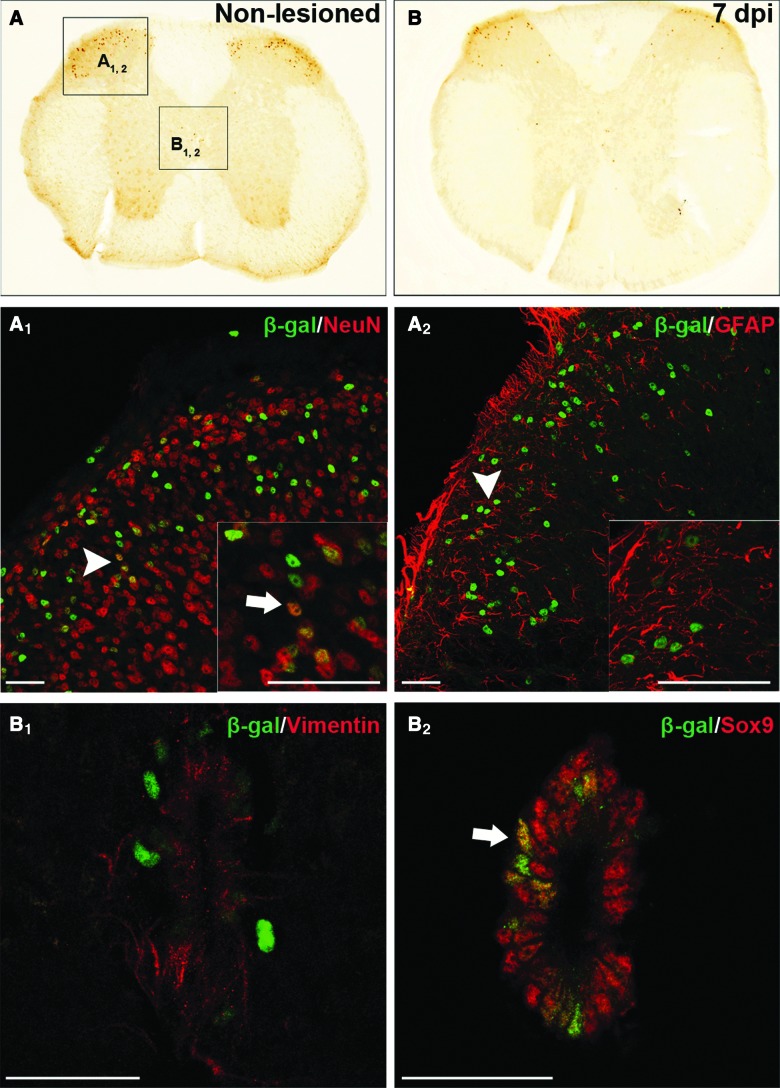
Next, by determining the transcriptional activity induced in BATgal mice, we assessed whether Wnt canonical signaling was active in the adult spinal cord and its modulation by SCI. As previously reported by White and colleagues,32 our results show that canonical Wnt signaling is constitutively active in neurons of the dorsal horns and the central canal of uninjured spinal cords in adult mice (Fig. 7A). After SCI, we did not observe any increase in the number of β-Gal-positive cells at 24 hpi and 7 dpi (Fig. 7B).
FIG. 7.
β-catenin-induced transcription is active in neurons of the dorsal horn and cells around the central canal with a neural stem-like phenotype. BATgal transgene expression in the uninjured spinal cord (A) and 7 days after dorsal hemisection (B). Higher magnification views highlighting Wnt-responsive cells of uninjured spinal cords in the dorsal horn (A1 and A2) and the central canal (B1 and B2). β-galactosidase is expressed in the dorsal horn by cells positive for the mature neural markers of neurons (NeuN; A1), but not astrocytes (GFAP; A2), whereas the central canal appears closely associated with radial glial filaments (Vimentin; B1) and colabels with a marker for adult NSCs (Sox9; B2). Scale bars=40 μm. dpi, days postinjury; β-gal, beta-galactosidase; NeuN, neuronal nuclei; GFAP, glial fibrillary acidic protein; Sox9, SRY (sex determining region Y)-box 9. Color image is available online at www.liebertpub.com/neu
As expected, colabeling of β-Gal-expressing cells of the dorsal horn was observed with NeuN, but not GFAP (Fig. 7A1 and 7A2), whereas cells from the central canal displayed characteristics of NSCs (Fig. 7B1 and B2). First, a close association of β-Gal-positive nuclei was observed with filaments stained with the 3CB2 Ab that labels radial glia40 (Fig. 7B1). This is provocative, given the known association of NSCs with radial glia.41,42 Additionally, some of the β-Gal-labeled cells were positive for Sox9, a transcription factor expressed in neuroepithelial progenitors that has been linked to glial fate choice in development and suggested to be a marker of NSCs in the adult, as well as the main source of precursor cells contributing to glial scarring in mouse SCI43–45 (Fig. 7B2).
Discussion
In the present study, we demonstrate prolonged expression of the Wnt family of glycoproteins as well as receptors (Fz and Ryk), inhibitors (Dkk, sFRP, and Wif1), and key downstream mediators of intracellular Wnt signaling (GSK-3β, Axin, LEF1, and β-catenin) in the spinal cord of adult mice. We also show that damage induces the down-regulation of the mRNAs for many ligands and receptors as well as an up-regulation of the antagonists, Dkks and Wif1, which suggests that Wnt signaling is broadly inhibited after injury (Fig. 8A). Indeed, SCI was ineffective to induce Wnt canonical signaling over the constitutive activation observed in neurons of the dorsal horn and putative NSCs of the central canal of uninjured spinal cords. Moreover, the mRNA expression of Fz receptors was reflected at the protein level, as detected using single IHC. Interestingly, our results showed that Fz1 and Fz4 in the uninjured spinal cords were expressed in different cell types, such as neurons and oligodendrocytes or astrocytes, respectively.
FIG. 8.
Representations of the spinal cord injury (SCI)-induced temporal patterns of expression of Wnt mRNAs in mice and their correlation with previously reported patterns in rat. (A) Summary of the integrated mRNA expression for all measured Wnt Ligands (Wnt1 to 19, except for Wnt8b), Fz receptors (Fz1 to 10), the Wnt canonical inhibitors (Dkk-1/4), sFRP (sFRP-1/3), and Wif1. (B) Comparative representation of the patterns of expression obtained for each category between mice and rats in response to SCI (obtained from Fernández-Martos et al., 201127). The results show inverse patterns of expression, with a rat up-regulation and mice down-regulation for Wnt ligands and receptors and, contrarily, rat down-regulation and mice up-regulation of inhibitors, with as a highlight the prominent increase of the unspecific inhibitor, Wif1, observed in mice. Fz, Frizzled; mRNA, messenger RNA; sFRP, secreted Frizzled-related protein; Wif1, Wnt inhibitory factor 1; d, days. Color image is available online at www.liebertpub.com/neu
Consistent with these findings, we previously reported on the constitutive expression of Wnts in the spinal cord of adult rats and found that they were differentially regulated after contusion injury; namely, Wnt signaling was activated by the Wnt/β-catenin pathway (and possibly others) in cells around the wound core, with a pattern suggestive of a role in glial scarring.27 Interestingly, almost all Fz and Ryk receptors are also physiologically expressed in the uninjured adult rat spinal cord with distinct spatial expression patterns and their mRNA and protein spatiotemporal expression patterns are dramatically altered during the progression of a contusive SCI.31 However, in contrast to mice, contusion SCI induced an up-regulation of the mRNAs for Wnt ligands and a down-regulation of the mRNAs for Wnt inhibitors in rats (Fig. 8B); these differences may be associated with the different pathophysiological responses between species or even between strains of a given species.46–48
Our results are in accord with the increasingly accepted role for Wnts in the modulation of fundamental aspects of the homeostasis and neuronal function of the adult CNS.14,17,18,49–53 In this line of thought, we provide supporting evidences of previous reports describing the expression of most Wnt ligands and receptors by PCR39 and in situ hybridization analysis,35 as well as a constitutive active β-catenin-mediated transcription in the dorsal horns and the central canal of the adult spinal cord of mice.22,32 Interestingly, Wnt3a and Wnt5a have been recently described to be expressed by neurons of the ventral and dorsal horn of adult mice, and that the latter received Wnt3a and Wnt5a synapsis from dorsal root ganglia sensory neurons, with at least involvement of the β-catenin pathway, as evidenced by its enrichment in the pre- and postsynaptic contacts.30,33 Moreover, the induction of central neuropathic pain by either peripheral nerve constriction, capsaicin administration, multiple sclerosis progression, or tumor cell implantation has been associated with dorsal horn up-regulation of the Wnt3a and Wnt5a ligands, the Fz1, Fz8, and Ror2 receptors, and the Wnt-canonical signaling pathway.24,29,30 Significantly, specific Wnt inhibition, by either the Wnt5a antagonist, Box5, the β-catenin inhibitor, indomethacin, or the inhibitor of Wnt production (IWP-2) attenuated the induced mechanical allodynia and neuropathic pain, as well as the expression of proinflammatory factors associated with pain induction, such as tumor necrosis factor alpha and interleukins, providing a compelling functional evidence of a relevant Wnt role on spinal cord physiology.24,29 In this sense, it has been proved that the synthesis and secretion of neuronal Wnt proteins was controlled by synaptic activity54–56 and that the β-catenin signaling pathway played a key role in synaptic assembly, remodeling, and plasticity.57–59 Moreover, our results highlight a differential pattern of expression of Fz receptors by the different cell types residing in the spinal cord, as shown for Fz1 in neurons and oligodendrocytes and Fz4 in astrocytes, suggestive for them still to unravel cell-specific patterns of Wnt receptors that might be key for the neuron-glia interplay and, as a result, the modulation of neural transmission in both physiological and pathological conditions. Taken together, our results invite for a further characterization of the putative role of Wnt signaling in the development of neuropathic pain after SCI, which has a dramatic effect on the patient's quality of life, and, as such, its amelioration is even more relevant than the recovery of locomotor function.60
In fact, the number of reports demonstrating a direct correlation between Wnt-pathway disorders and neurological diseases is rapidly growing.17,18,29,30,33,52,53 Intriguingly, the only study describing a role of Wnts on mouse SCI claimed that, by in situ hybridization, none of the 19 Wnt ligands or 10 Fz receptors were expressed at detectable levels in adulthood28; however, they found that expression of Wnt1, -4, and -5a, as well as the Fz1 and Ryk receptors, was acutely reinduced with similar patterns of expression after hemisection. By contrast, we provide new data supporting that most of the Wnt family soluble factors and receptors are indeed expressed in the healthy spinal cord of mice and that, after hemisection SCI, they are differentially regulated. Further, we showed the specific cellular expression pattern of Fz1 and Fz4, and we were able to localize the receptors at the protein level in spinal cord tissue. We propose that a simple explanation for these discrepancies might be linked to the use of different mouse strains among the two studies. In this sense, the studies describing a physiological expression of the Wnt family of proteins in the adult spinal cord of mice and their involvement in amyotrophic lateral sclerosis (ALS) neurodegeneration or the development of neuropathic pain also used an inbred C57BL/6 or BS6JL, instead of an outbred CD1, mouse strain.28–30,39 In agreement, another interesting point arises from our observation that, after hemisection SCI, the expression of Wnt3a and Wnt5a is down-regulated and β-catenin signaling is not up-regulated in either sensory neurons or proliferating reactive astrocytes, in contraposition to the response described for neurodegeneration or peripheral nerve damage.29,30,33 This could be a result of a different pathophysiological response preceding trauma or specific cell damage, such as the massive death of neurons and oligodendrocytes induced by trauma, and have been claimed to express both ligands.29,30,33 Taken together, these results demonstrate a role for Wnt signaling in the complex network of cellular and molecular responses induced after SCI and, by extension, in its pathophysiological events, as discussed below.28,61–64 It is known that neuroinflammation plays a master role in SCI pathophysiology, where it exerts both beneficial and detrimental effects.2,5,6 In this regard, a growing body of evidence links Wnt signaling with neuroinflammation. For example, in neurodegenerative diseases with associated chronic inflammation, an age-dependent accumulation of β-catenin in reactive microglia61 or an up-regulation of Wnt3a expression and β-catenin-induced proliferation in reactive mature astrocytes33 has been described. Moreover, cultured microglia expresses appropriate combinations of Wnt receptors that, in the presence of Wnt3a, activate β-catenin signaling and increase their proinflammatory response. By contrast, other studies have shown that canonical Wnt activation by lithium, Wnt1, or Wnt3a inhibits several inflammatory events, such as transendothelial migration of monocytes65 and proinflammatory cytokine production by activated macrophages.66,67 Alternatively, it has also been reported that noncanonical signaling (e.g., Wnt5a-Fz5 signaling) is associated with CaMKII activation and downstream nuclear factor kappa B transcription, which is a key mechanism of onset and maintenance of inflammation.68 Intriguingly, our data demonstrate distinct patterns of expression between rats and mice, because canonical activation in cells around the wound core and an up-regulation of Wnt5a mRNA occurs in rats, but not in mice, which may contribute to the different inflammatory responses described between these species. On the other hand, it has been recently shown that a constitutive expression of Wnt5a on the spinal cord dorsal horn afferences and the soma of dorsal root ganglia sensory neurons of mice,29,30 which, in response to SCI, might constitute a peripheral source of Wnt5a regulating neuroinflammation and the development of neuropathic pain. Further studies using overexpressed Wnt ligands and/or inhibitors will help to unravel the precise role of Wnt signaling in innate and adaptive inflammatory responses, as well as the increasingly accepted link between glial reactivity, neuroinflammation, and the development of neuropathic pain.69
A major cause of functional deficits after SCI is the secondary death of neurons and oligodendrocytes resulting from excitotoxic and inflammatory apoptosis.2,6 In this regard, substantial evidence suggests that Wnt signaling might play a critical role in determining the balance between neuronal survival and death in a variety of neurodegenerative states,70–75 brain ischemia,64,76 and excitotoxicity.18,77 For example, GSK-3β inhibition by lithium78 or Wnt3a79 (both of which activate Wnt/β-catenin signaling) have been shown to exert neuroprotective effects during SCI. Indeed, Wnt3a is expressed and up-regulated in mice models of neurodegeneration by neurons29,30,33 and oligodendrocytes29 of the spinal cord, whereas canonical Wnt signaling has been shown to be a key regulator of oligodendrogenesis during development and adult remyelination21,80 and promote neuronal differentiation.14,79 In fact, pharmacological Axin2 stabilization accelerates oligodendrocyte precursor differentiation and remyelination in the spinal cord of adult mice,81 whereas administration of Wnt3a promotes the generation of new neurons from endogenous adult precursors after spinal cord contusion in rats.79 Strikingly, Yu and colleagues have recently shown a strong up-regulation of Wif1 by, predominantly, neurons in the locus of neurodegeneration at the middle stages of ALS progression, which has been claimed as a putative mechanism of motor neuron death and neurogenesis inhibition.39 After SCI, our results demonstrate an early induction of both Wnt1 and its inhibitor, Dkk1, as well as a persistent down-regulation of Wnt3a, followed by prominent up-regulation of Wif1, which coincides with the highest period of neuronal death. Moreover, our results show that Fz1 was expressed in neurons and oligodendroglial cells not only in the uninjured spinal cord, but also after injury in the preserved spinal cord parenchyma that surrounded the injured tissue, which are thought to be affected by the secondary cell death processes associated with the progression of SCI. Therefore, these processes may be susceptible to amelioration by therapies that modulate Wnt signaling to affect neuroprotection, remyelination, and/or neuronal replacement.
In addition to neural death, functional recovery is impeded by glial scarring, which creates a strong inhibitory environment for axonal regeneration.5 With this in mind, recent studies have shed new light on the function of Wnt signaling in axon guidance and formation of neuronal connections during embryogenesis and adulthood.82,83 After SCI, Wnt5a is induced in reactive astrocytes surrounding the damaged area, and corticospinal axonal growth is inhibited by noncanonical activation of the Ryk receptor in both mice28 and rats.20 Consistently, inhibition of noncanonical Ryk activation using Ab20,28 or, alternatively, activation of canonical Wnt signaling using either lithium,84,85 exogenous Wnt3a administration,79 or transplantation of Wnt3a-secreting fibroblasts86 improves locomotor recovery by promoting axonal regeneration after SCI. In our study, we observed detectable levels of Wnt5a at the nonlesioned and all of the times we analyzed postinjury, and we observed an increase in the expression of Ryk during late time points. However, when compared to physiological levels of expression in nonlesioned spinal cords, Wnt5a is down-regulated after injury. As already discussed, this could be linked to a massive loss of Wnt5a-expressing dorsal horn neurons after dorsal hemisection,30 concomitant with an induction inferior to physiological levels of Wnt5a expression in putative reactive astrocytes of the glial scar, as shown by in situ hybridization in mice.28 Taken together, our results invite for further research to revise the expression of Wnt5a and all the other detected Wnt family soluble factors and receptors in the different cell types involved in glial scarring and, eventually, develop more-efficient therapies to promote axonal growth than the already proven inhibition of Ryk function.20,28
Wnts are also crucial physiological regulators of stem cells,14,87–89 which is especially relevant because neural precursors have been identified in adult rodent spinal cord.43,90–92 After SCI, resident ependymal stem cells proliferate (peaking at 1–3 days after lesion) and are recruited to the lesion site, where they mainly differentiate into astrocytes that contribute to the glial scar.43,93–95 Further, β-catenin signaling has been shown to be active in NG2-positive precursors and reactive astrocytes after traumatic brain (but not spinal cord) injury at 3, 7, or 28 dpi in BATgal reporter mice.32 Our results provide supporting evidence for prolonged Wnt expression during adulthood as well as for canonical Wnt-signaling activation in the central canal of the spinal cords of uninjured BATgal mice. This activation is observed specifically in cells from the central canal that expressed the neural stem markers, Sox9 and Vimentin; however, we did not find an up-regulation after SCI, as previously reported.32 Taken together, our results suggest that adult neural stem cells respond to Wnts under physiological conditions (because β-Gal-positive cells express Sox9 and are associated with radial glia), although do not seem to be activated by SCI through at least the Wnt canonical pathway. This would fit with the prominent up-regulation of both the canonical Dkk and unspecific Wif1 inhibitors induced by injury; however, we can not discard other possibilities because of intrinsic limitations to the reporter BATgal mice,96 such as that β-catenin could activate Wnt target genes in a LEF-independent manner,97 that TCFs could modulate gene activation in the absence of Wnt activity,98,99 or that Wnts could stimulate β-catenin-independent gene transcription.100 In this sense, Wnt3a and β-catenin signaling has been recently described in astrocytes of the adult spinal cord of mice as constitutively active and associated with glial proliferation in response to neurodegeneration, becasued reactive astrocytes expressed cyclin D1 and incorporated bromodeoxyuridine.33 Further studies aimed at unraveling Wnt expression patterns and functions in endogenous populations of adult spinal cord neural precursors will likely contribute to the development of novel cell-replacement therapies.
The results of this study provide new, compelling evidences of a broad expression of the Wnt family of glycoproteins, which, together with the growing body of literature, strongly suggest that the Wnt family of proteins may play a role in the physiology of both healthy and damaged adult spinal cords. Nevertheless, further investigation will be necessary to clarify the cellular patterns of expression and molecular mechanisms underlying the function of all the described Wnt soluble factors and receptors, which eventually will lead to the development of therapies able to modulate the multiple pathophysiological events associated with either neurodegenerative or traumatic spinal cord damage.
Acknowledgments
This work was supported by grants obtained from the Fundación para la Investigación Sanitaria de Castilla-La Mancha (FISCAM; grant PI2008-39) and Fondo de Investigaciones Sanitarias (FIS; grant PI08-1475 and PI12-02895 with Fondo Europeo de Desarrollo Regional [FEDER] cofunding). Carlos González-Fernández was supported by FISCAM (MOV- 2009_JI-06). The funding agencies played no role in the study design, data collection and analyses, decision to publish, or preparation of this article.
The authors thank Steven Piccolo for permission to use the BATgal mice and Sarah Millar for providing us with founder BATgal mice. The authors thank Dr. P. González for his generous and significant histological contributions and for his critical review of the manuscript and also thank Virginia Pérez and Sandra Vázquez for their technical support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Knafo S., and Choi D. (2008). Clinical studies in spinal cord injury: moving towards successful trials. Br. J. Neurosurg. 22, 3–12 [DOI] [PubMed] [Google Scholar]
- 2.Profyris C., Cheema S.S., Zang D., Azari M.F., Boyle K., and Petratos S. (2004). Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol. Dis. 15, 415–436 [DOI] [PubMed] [Google Scholar]
- 3.Rowland J.W., Hawryluk G.W., Kwon B., and Fehlings M.G. (2008). Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg. Focus 25, E2. [DOI] [PubMed] [Google Scholar]
- 4.Kwon B.K., Tetzlaff W., Grauer J.N., Beiner J., and Vaccaro A.R. (2004). Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 4, 451–464 [DOI] [PubMed] [Google Scholar]
- 5.Silver J, and Miller J.H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 [DOI] [PubMed] [Google Scholar]
- 6.Hausmann O.N. (2003). Post-traumatic inflammation following spinal cord injury. Spinal Cord 41, 369–378 [DOI] [PubMed] [Google Scholar]
- 7.Cadotte D.W., and Fehlings M.G. (2011). Spinal cord injury: a systematic review of current treatment options. Clin. Orthop. Relat. Res. 469, 732–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon B.K., Okon E., Hillyer J., Mann C., Baptiste D., Weaver L.C., Fehlings M.G., and Tetzlaff W. (2011). A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma 28, 1545–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahni V., and Kessler J.A. (2010). Stem cell therapies for spinal cord injury. Nat. Rev. Neurol. 6, 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tetzlaff W., Okon E.B., Karimi-Abdolrezaee S., Hill C.E., Sparling J.S., Plemel J.R., Plunet W.T., Tsai E.C., Baptiste D., Smithson L.J., Kawaja M.D., Fehlings M.G., and Kwon B.K. (2011). A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 28, 1611–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon B.K., Sekhon L.H., and Fehlings M.G. (2010). Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine (Phila Pa 1976) 35, Suppl. 21, S263–S270 [DOI] [PubMed] [Google Scholar]
- 12.Logan C.Y., and Nusse R. (2004). The wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 13.Freese J.L., Pino D., and Pleasure S.J. (2010). Wnt signaling in development and disease. Neurobiol. Dis. 38, 148–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelidis T.M., and Lie D.C. (2008). Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res. 331, 193–210 [DOI] [PubMed] [Google Scholar]
- 15.van Amerongen R., Mikels A., and Nusse R. (2008). Alternative wnt signaling is initiated by distinct receptors. Sci. Signal 1, re9. [DOI] [PubMed] [Google Scholar]
- 16.Mikels A.J., and Nusse R. (2006). Wnts as ligands: processing, secretion and reception. Oncogene 25, 7461–7468 [DOI] [PubMed] [Google Scholar]
- 17.Inestrosa N.C., and Arenas E. (2010). Emerging roles of wnts in the adult nervous system. Nat. Rev. Neurosci. 11, 77–86 [DOI] [PubMed] [Google Scholar]
- 18.Caraci F., Busceti C., Biagioni F., Aronica E., Mastroiacovo F., Cappuccio I., Battaglia G., Bruno V., Caricasole A., Copani A., and Nicoletti F. (2008). The wnt antagonist, dickkopf-1, as a target for the treatment of neurodegenerative disorders. Neurochem. Res. 33, 2401–2406 [DOI] [PubMed] [Google Scholar]
- 19.De Ferrari G.V., and Moon R.T. (2006). The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene 25, 7545–7553 [DOI] [PubMed] [Google Scholar]
- 20.Miyashita T., Koda M., Kitajo K., Yamazaki M., Takahashi K., Kikuchi A., and Yamashita T. (2009). Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J. Neurotrauma 26, 955–964 [DOI] [PubMed] [Google Scholar]
- 21.Fancy S.P., Baranzini S.E., Zhao C., Yuk D.I., Irvine K.A., Kaing S., Sanai N., Franklin R.J., and Rowitch D.H. (2009). Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garbe D.S., and Ring R.H. (2012). Investigating tonic wnt signaling throughout the adult CNS and in the hippocampal neurogenic niche of BatGal and ins-TopGal mice. Cell Mol. Neurobiol. 32, 1159–1174 [DOI] [PubMed] [Google Scholar]
- 23.Niu L.J., Xu R.X., Zhang P., Du M.X., and Jiang X.D. (2012). Suppression of Frizzled-2-mediated Wnt/Ca(2)(+) signaling significantly attenuates intracellular calcium accumulation in vitro and in a rat model of traumatic brain injury. Neuroscience 213, 19–28 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y.K., Huang Z.J., Liu S., Liu Y.P., Song A.A., and Song X.J. (2013). Wnt signaling underlies the pathogenesis of neuropathic pain in rodents. J. Clin. Invest. 123, 2268–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S., Guan Y., Chen Y., Li X., Zhang C., Yu L., Zhou F., and Wang X. (2013). Role of Wnt1 and Fzd1 in the spinal cord pathogenesis of amyotrophic lateral sclerosis-transgenic mice. Biotechnol. Lett. 35, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 26.Park J.H., Min J., Baek S.R., Kim S.W., Kwon I.K., and Jeon S.R. (2013). Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with wnt3a-secreting fibroblasts. Acta. Neurochir. (Wien) 155, 809–816 [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Martos C.M., Gonzalez-Fernandez C., Gonzalez P., Maqueda A., Arenas E., and Rodriguez F.J. (2011). Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One 6, e27000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Wang X., Lu C.C., Kerman R., Steward O., Xu X.M., and Zou Y. (2008). Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J. Neurosci. 28, 8376–8382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan S., Shi Y., and Tang S.J. (2012). Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. J. Neuroimmune Pharmacol. 7, 904–913 [DOI] [PubMed] [Google Scholar]
- 30.Shi Y., Yuan S., Li B., Wang J., Carlton S.M., Chung K., Chung J.M., and Tang S.J. (2012). Regulation of Wnt signaling by nociceptive input in animal models. Mol. Pain 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez P., Fernandez-Martos C.M., Gonzalez-Fernandez C., Arenas E., and Rodriguez F.J. (2012). Spatio-temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats. PLoS One 7, e50793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White B.D., Nathe R.J., Maris D.O., Nguyen N.K., Goodson J.M., Moon R.T., and Horner P.J. (2010). Beta-catenin signaling increases in proliferating ng2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain. Stem Cells 28, 297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Guan Y, Zhang Z, Liu H, Wang S, Yu L, Wu X, Wang X. (2012). Wnt signaling pathway is involved in the pathogenesis of amyotrophic lateral sclerosis in adult transgenic mice. Neurol Res 34, 390–399 [DOI] [PubMed] [Google Scholar]
- 34.Li X., Guan Y., Chen Y., Zhang C., Shi C., Zhou F., Yu L., Juan J., Wang X. (2013). Expression of wnt5a and its receptor fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. Int. J. Clin. Exp. Pathol. 6, 1245–1260 [PMC free article] [PubMed] [Google Scholar]
- 35.Lein E.S., Hawrylycz M.J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A.F., Boguski M.S., Brockway K.S., Byrnes E.J., Chen L., Chen T.M., Chin M.C., Chong J., Crook B.E., Czaplinska A., Dang C.N., Datta S., Dee N.R., Desaki A.L., Desta T., Diep E., Dolbeare T.A., Donelan M.J., Dong H.W., Dougherty J.G., Duncan B.J., Ebbert A.J., Eichele G., Estin L.K., Faber C., Facer B.A., Fields R., Fischer S.R., Fliss T.P., Frensley C., Gates S.N., Glattfelder K.J., Halverson K.R., Hart M.R., Hohmann J.G., Howell M.P., Jeung D.P., Johnson R.A., Karr P.T., Kawal R., Kidney J.M., Knapik R.H., Kuan C.L., Lake J.H., Laramee A.R., Larsen K.D., Lau C., Lemon T.A., Liang A.J., Liu Y., Luong L.T., Michaels J., Morgan J.J., Morgan R.J., Mortrud M.T., Mosqueda N.F., Ng L.L., Ng R., Orta G.J., Overly C.C., Pak T.H., Parry S.E., Pathak S.D., Pearson O.C., Puchalski R.B., Riley Z.L., Rockett H.R., Rowland S.A., Royall J.J., Ruiz M.J., Sarno N.R., Schaffnit K., Shapovalova N.V., Sivisay T., Slaughterbeck C.R., Smith S.C., Smith K.A., Smith B.I., Sodt A.J., Stewart N.N., Stumpf K.R., Sunkin S.M., Sutram M., Tam, A, Teemer C.D., Thaller C., Thompson C.L., Varnam L.R., Visel A., Whitlock R.M., Wohnoutka P.E., Wolkey C.K., Wong V.Y., Wood M., Yaylaoglu M.B., Young R.C., Youngstrom B.L., Yuan X.F., Zhang B., Zwingman T.A., and Jones A.R. (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 [DOI] [PubMed] [Google Scholar]
- 36.Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A.B., Volpin D., Bressan G.M., and Piccolo S. (2003). Mapping wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. U. S. A. 100, 3299–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawal N., Castelo-Branco G., Sousa K.M., Kele J., Kobayashi K., Okano H. and Arenas E. (2006). Dynamic temporal and cell type-specific expression of wnt signaling components in the developing midbrain. Exp. Cell Res. 312, 1626–1636 [DOI] [PubMed] [Google Scholar]
- 38.Hirsch C., Campano L.M., Wohrle S., and Hecht A. (2007). Canonical wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp. Cell Res. 313, 572–587 [DOI] [PubMed] [Google Scholar]
- 39.Yu L., Guan Y., Wu X., Chen Y., Liu Z., Du H., and Wang X. (2013). Wnt signaling is altered by spinal cord neuronal dysfunction in amyotrophic lateral sclerosis transgenic mice. Neurochem. Res. 38, 1904–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreels M, Vandenabeele F., Deryck L., and Lambrichts I. (2005). Radial glial cells derived from the neonatal rat spinal cord: morphological and immunocytochemical characterization. Arch. Histol. Cytol. 68, 361–369 [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Buylla A., Garcia-Verdugo J.M., and Tramontin A.D. (2001). A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2, 287–293 [DOI] [PubMed] [Google Scholar]
- 42.Merkle F.T., Tramontin A.D., Garcia-Verdugo J.M., and Alvarez-Buylla A. (2004). Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. U. S. A. 101, 17528–17532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meletis K., Barnabe-Heider F., Carlen M., Evergren E., Tomilin N., Shupliakov O., and Frisen J. (2008). Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 6, e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wegner M., and Stolt C.C. (2005). From stem cells to neurons and glia: a soxist's view of neural development. Trends Neurosci. 28, 583–588 [DOI] [PubMed] [Google Scholar]
- 45.Sottile V., Li M., and Scotting P.J. (2006). Stem cell marker expression in the bergmann glia population of the adult mouse brain. Brain Res. 1099, 8–17 [DOI] [PubMed] [Google Scholar]
- 46.Stokes B.T., and Jakeman L.B. (2002). Experimental modelling of human spinal cord injury: a model that crosses the species barrier and mimics the spectrum of human cytopathology. Spinal Cord 40, 101–109 [DOI] [PubMed] [Google Scholar]
- 47.Sroga J.M., Jones T.B., Kigerl K.A., McGaughy V.M., and Popovich P.G. (2003). Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol 462, 223–240 [DOI] [PubMed] [Google Scholar]
- 48.Donnelly D.J., and Popovich P.G. (2008). Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 209, 378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gogolla N., Galimberti I., Deguchi Y., and Caroni P. (2009). Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron 62, 510–525 [DOI] [PubMed] [Google Scholar]
- 50.Lie D.C., Colamarino S.A., Song H.J., Desire L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., and Gage F.H. (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 [DOI] [PubMed] [Google Scholar]
- 51.Rowe W.B., Blalock E.M., Chen K.C., Kadish I., Wang D., Barrett J.E., Thibault O., Porter N.M., Rose G.M., and Landfield P.W. (2007). Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 27, 3098–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boonen R.A., van Tijn P., and Zivkovic D. (2009). Wnt signaling in alzheimer's disease: Up or down, that is the question. Ageing Res. Rev. 8, 71–82 [DOI] [PubMed] [Google Scholar]
- 53.Niu L.J., Xu R.X., Zhang P., Du M.X., and Jiang X.D. (2012). Suppression of Frizzled-2-mediated Wnt/Ca(2+) signaling significantly attenuates intracellular calcium accumulation in vitro and in a rat model of traumatic brain injury. Neuroscience 213, 19–28 [DOI] [PubMed] [Google Scholar]
- 54.Ataman B., Ashley J., Gorczyca M., Ramachandran P., Fouquet W., Sigrist S.J., and Budnik V. (2008). Rapid activity-dependent modifications in synaptic structure and function require bidirectional wnt signaling. Neuron 57, 705–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J., Park C.S., and Tang S.J. (2006). Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J. Biol. Chem. 281, 11910–11916 [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Li B., Wan X., Zhang W., Zhong L., and Tang S.J. (2012). NMDA receptor activation stimulates transcription-independent rapid wnt5a protein synthesis via the MAPK signaling pathway. Mol. Brain 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmeisser M.J., Grabrucker A.M., Bockmann J., and Boeckers T.M. (2009). Synaptic cross-talk between N-methyl-D-aspartate receptors and LAPSER1-beta-catenin at excitatory synapses. J. Biol. Chem. 284, 29146–29157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brigidi G.S., and Bamji S.X. (2011). Cadherin-catenin adhesion complexes at the synapse. Curr. Opin. Neurobiol. 21, 208–214 [DOI] [PubMed] [Google Scholar]
- 59.Arikkath J. (2009). Regulation of dendrite and spine morphogenesis and plasticity by catenins. Mol. Neurobiol. 40, 46–54 [DOI] [PubMed] [Google Scholar]
- 60.Anderson K.D. (2004). Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 [DOI] [PubMed] [Google Scholar]
- 61.Halleskog C., Mulder J., Dahlstrom J., Mackie K., Hortobagyi T., Tanila H., Kumar Puli, L., Farber K., Harkany T., and Schulte G. (2011). Wnt signaling in activated microglia is proinflammatory. Glia 59, 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daneman R., Agalliu D., Zhou L., Kuhnert F., Kuo C.J., and Barres B.A. (2009). Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 106, 641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L., Yang X., Yang S., and Zhang J. (2011). The Wnt/beta-catenin signaling pathway in the adult neurogenesis. Eur. J. Neurosci. 33, 1–8 [DOI] [PubMed] [Google Scholar]
- 64.Chong Z.Z., Shang Y.C., Hou J., and Maiese K. (2010). Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid. Med. Cell Longev. 3, 153–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tickenbrock L., Schwable J., Strey A., Sargin B., Hehn S., Baas M., Choudhary C., Gerke V., Berdel W.E., Muller-Tidow C., and Serve H. (2006). Wnt signaling regulates transendothelial migration of monocytes. J. Leukoc. Biol. 79, 1306–1313 [DOI] [PubMed] [Google Scholar]
- 66.Chong Z.Z., Li F., and Maiese K. (2007). Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell. Signal. 19, 1150–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neumann J., Schaale K., Farhat K., Endermann T., Ulmer A.J., Ehlers S., and Reiling N. (2010). Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming mycobacterium tuberculosis-infected macrophages. FASEB J. 24, 4599–4612 [DOI] [PubMed] [Google Scholar]
- 68.Sen M, and Ghosh G. (2008). Transcriptional outcome of Wnt-Frizzled signal transduction in inflammation: evolving concepts. J. Immunol. 181, 4441–4445 [DOI] [PubMed] [Google Scholar]
- 69.Milligan E.D., and Watkins L.R. (2009). Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 10, 23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.L'Episcopo F., Tirolo C., Testa N., Caniglia S., Morale M.C., Cossetti C., D'Adamo P., Zardini E., Andreoni L., Ihekwaba A.E., Serra P.A., Franciotta D., Martino G., Pluchino S., and Marchetti B. (2011). Reactive astrocytes and wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of parkinson's disease. Neurobiol. Dis. 41, 508–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.L'Episcopo F., Serapide M.F., Tirolo C., Testa N., Caniglia S., Morale M.C., Pluchino S., and Marchetti B. (2011). A Wnt1 regulated Frizzled-1/beta-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol. Neurodegener. 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Ferrari G.V., Chacon M.A., Barria M.I., Garrido J.L., Godoy J.A., Olivares G., Reyes A.E., Alvarez A., Bronfman M., and Inestrosa N.C. (2003). Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol. Psychiatry 8, 195–208 [DOI] [PubMed] [Google Scholar]
- 73.Inestrosa N.C., and Toledo E.M. (2008). The role of Wnt signaling in neuronal dysfunction in Alzheimer's disease. Mol. Neurodegener. 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toledo E.M., Colombres M., and Inestrosa N.C. (2008). Wnt signaling in neuroprotection and stem cell differentiation. Prog. Neurobiol. 86, 281–296 [DOI] [PubMed] [Google Scholar]
- 75.Zhang Z., Hartmann H., Do V.M., Abramowski D., Sturchler-Pierrat C., Staufenbiel M., Sommer B., van de Wetering M., Clevers H., Saftig P., De Strooper B., He X., and Yankner B.A. (1998). Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395, 698–702 [DOI] [PubMed] [Google Scholar]
- 76.Mastroiacovo F., Busceti C.L., Biagioni F., Moyanova S.G., Meisler M.H., Battaglia G., Caricasole A., Bruno V., and Nicoletti F. (2009). Induction of the wnt antagonist, dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J. Cereb. Blood Flow Metab. 29, 264–276 [DOI] [PubMed] [Google Scholar]
- 77.Wei H., Qin Z.H., Senatorov V.V., Wei W., Wang Y., Qian Y., and Chuang D.M. (2001). Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of huntington's disease. Neuroscience 106, 603–612 [DOI] [PubMed] [Google Scholar]
- 78.Cuzzocrea S., Genovese T., Mazzon E., Crisafulli C., Di Paola R., Muia C., Collin M., Esposito E., Bramanti P., and Thiemermann C. (2006). Glycogen synthase kinase-3 beta inhibition reduces secondary damage in experimental spinal cord trauma. J. Pharmacol. Exp. Ther. 318, 79–89 [DOI] [PubMed] [Google Scholar]
- 79.Yin Z.S., Zu B., Chang J., and Zhang H. (2008). Repair effect of Wnt3a protein on the contused adult rat spinal cord. Neurol Res 30, 480–486 [DOI] [PubMed] [Google Scholar]
- 80.Rosenberg S.S., and Chan J.R. (2009). Modulating myelination: knowing when to say Wnt. Genes Dev. 23, 1487–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fancy S.P., Harrington E.P., Yuen T.J., Silbereis J.C., Zhao C., Baranzini S.E., Bruce C.C., Otero J.J., Huang E.J., Nusse R., Franklin R.J., and Rowitch D.H. (2011). Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 14, 1009–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ciani L., and Salinas P.C. (2005). Wnts in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 6, 351–362 [DOI] [PubMed] [Google Scholar]
- 83.Bovolenta P., Rodriguez J., and Esteve P. (2006). Frizzled/Ryk mediated signalling in axon guidance. Development 133, 4399–4408 [DOI] [PubMed] [Google Scholar]
- 84.Dill J., Wang H., Zhou F., and Li S. (2008). Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J. Neurosci. 28, 8914–8928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yick L.W., So K.F., Cheung P.T., and Wu W.T. (2004). Lithium chloride reinforces the regeneration-promoting effect of chondroitinase abc on rubrospinal neurons after spinal cord injury. J. Neurotrauma 21, 932–943 [DOI] [PubMed] [Google Scholar]
- 86.Suh H.I., Min J., Choi K.H., Kim S.W., Kim K.S., and Jeon S.R. (2011). Axonal regeneration effects of wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir. (Wien) 153, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 87.Kléber M., and Sommer L. (2004). Wnt signaling and the regulation of stem cell function. Curr. Opin. Cell Biol. 16, 681–687 [DOI] [PubMed] [Google Scholar]
- 88.Nusse R. (2008). Wnt signaling and stem cell control. Cell Res. 18, 523–527 [DOI] [PubMed] [Google Scholar]
- 89.Lange C., Mix E., Rateitschak K., and Rolfs A. (2006). Wnt signal pathways and neural stem cell differentiation. Neurodegener. Dis. 3, 76–86 [DOI] [PubMed] [Google Scholar]
- 90.Barnabe-Heider F., Goritz C., Sabelstrom H., Takebayashi H., Pfrieger F.W., Meletis K., and Frisen J. (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470–482 [DOI] [PubMed] [Google Scholar]
- 91.Decimo I., Bifari F., Rodriguez F.J., Malpeli G., Dolci S., Lavarini V., Pretto S., Vasquez S., Sciancalepore M., Montalbano A., Berton V., Krampera M., and Fumagalli G. (2011). Nestin- and doublecortin-positive cells reside in adult spinal cord meninges and participate in injury-induced parenchymal reaction. Stem Cells 29, 2062–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goritz C., Dias D.O., Tomilin N., Barbacid M., Shupliakov O., and Frisen J. (2011). A pericyte origin of spinal cord scar tissue. Science 333, 238–242 [DOI] [PubMed] [Google Scholar]
- 93.Horky L.L., Galimi F., Gage F.H., and Horner P.J. (2006). Fate of endogenous stem/progenitor cells following spinal cord injury. J. Comp. Neurol. 498, 525–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamamoto S., Yamamoto N., Kitamura T., Nakamura K., and Nakafuku M. (2001). Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp. Neurol. 172, 115–127 [DOI] [PubMed] [Google Scholar]
- 95.Ronaghi M., Erceg S., Moreno-Manzano V., and Stojkovic M. (2010). Challenges of stem cell therapy for spinal cord injury: human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells 28, 93–99 [DOI] [PubMed] [Google Scholar]
- 96.Barolo S. (2006). Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene 25, 7505–7511 [DOI] [PubMed] [Google Scholar]
- 97.Filali M., Cheng N., Abbott D., Leontiev V., and Engelhardt J.F. (2002). Wnt-3a/beta-catenin signaling induces transcription from the LEF-1 promoter. J. Biol. Chem. 277, 33398–33410 [DOI] [PubMed] [Google Scholar]
- 98.Hsu S.C., Galceran J., and Grosschedl R. (1998). Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with beta-catenin. Mol. Cell Biol. 18, 4807–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Labbe E., Letamendia A., and Attisano L. (2000). Association of smads with lymphoid enhancer binding factor 1/t cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc. Natl. Acad. Sci. U. S. A. 97, 8358–8363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ziemer L.T., Pennica D., and Levine A.J. (2001). Identification of a mouse homolog of the human BTEB2 transcription factor as a beta-catenin-independent Wnt-1-responsive gene. Mol. Cell. Biol. 21, 562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]