Abstract
Objective
To evaluate the potential economic benefits of keeping a meningitis A vaccine at or near ambient temperature for up to 4 days during a mass vaccination campaign.
Methods
During a 10-day mass vaccination campaign against meningitis A in three regions of Chad in 2011, the costs associated with storage and transport of the vaccine in a traditional cold chain system were evaluated. A mathematical model was used to estimate the savings that could have been achieved if the vaccine had been stored at or near ambient temperature – in a “controlled temperature” chain – at the peripheral levels of the supply chain system.
Findings
The cost of the cold chain and associated logistics used in the campaign in Chad was 0.24 United States dollars (US$) per person vaccinated. In the modelled scenario for a controlled temperature chain, however, these costs dropped by 50% and were estimated to be only US$ 0.12 per person vaccinated.
Conclusion
The implementation of a “controlled temperature” chain at the most peripheral levels of the supply chain system – assuming no associated loss of vaccine potency, efficacy or safety – could result in major economic benefits and allow vaccine coverage to be extended in low-resource settings.
Résumé
Objectif
Évaluer les avantages économiques potentiels de la conservation d'un vaccin contre la méningite A à température ambiante ou à une température proche de la température ambiante jusqu'à 4 jours, au cours d'une campagne de vaccination de masse.
Méthodes
Au cours d'une campagne de vaccination de masse de 10 jours contre la méningite A dans trois régions du Tchad en 2011, on a évalué les coûts associés au stockage et au transport du vaccin dans un système de chaîne du froid traditionnel. Un modèle mathématique a été utilisé pour estimer les économies qui auraient pu être réalisées si les vaccins avaient été conservés à température ambiante ou à une température proche de la température ambiante – dans une chaîne de «température contrôlée» – à des niveaux périphériques du système de la chaîne d'approvisionnement.
Résultats
Le coût de la chaîne du froid et de la logistique associée utilisées dans la campagne de vaccination au Tchad était de 0,24$ par personne vaccinée. Toutefois, dans le scénario modélisé d'une chaîne de température contrôlée, ces coûts ont chuté de 50% et ont été estimés à seulement 0,12$ par personne vaccinée.
Conclusion
La mise en œuvre d'une chaîne de «température contrôlée» aux niveaux les plus périphériques du système de chaîne d'approvisionnement – en supposant qu'il n'y a aucune perte d'activité, d'efficacité ou de sécurité du vaccin associé – pourrait entraîner des avantages économiques importants et permettre l'extension de la couverture vaccinale dans des régions à revenu faible.
Resumen
Objetivo
Evaluar los beneficios económicos potenciales de conservar las vacunas contra la meningitis A a (o a aproximadamente) temperatura ambiente durante un máximo de 4 días en una campaña de vacunación masiva.
Métodos
Se evaluaron los costes asociados al almacenaje y transporte de la vacuna en un sistema de cadena de frío tradicional durante una campaña de vacunación masiva contra la meningitis A de 10 días de duración en tres regiones de Chad realizada en 2011. Se empleó un modelo matemático para estimar el ahorro que podría haberse logrado si la vacuna se hubiera almacenado a (o a aproximadamente) temperatura ambiente, en una cadena de «temperatura controlada», en los niveles periféricos del sistema de cadena de suministro.
Resultados
El coste de la cadena de frío y la logística asociada usada en la campaña en Chad fue de 0,24 dólares de los Estados Unidos (US$) por persona vacunada. En el modelo empleado para una cadena de temperatura controlada, sin embargo, estos costes descendieron un 50 % y se estimó que solo representaban US$ 0,12 por persona vacunada.
Conclusión
La aplicación de una cadena de «temperatura controlada» en los niveles más periféricos del sistema de cadena de suministro, suponiendo que no haya pérdida asociada de potencia de la vacuna, la eficacia o la seguridad, podría ofrecer importantes beneficios económicos y permitir que la cobertura de la vacuna se amplíe en entornos de bajos recursos.
ملخص
الغرض
تقييم الفوائد الاقتصادية المحتملة لحفظ لقاح التهاب السحايا أ عند درجة الحرارة المحيطة أو بالقرب منها لمدة 4 أيام أثناء حملة تطعيم واسعة النطاق.
الطريقة
خلال حملة تطعيم واسعة النطاق لمدة 10 أيام ضد التهاب السحايا أ في ثلاث مناطق في تشاد في 2011، تم تقييم التكاليف المرتبطة بتخزين اللقاح ونقله في أحد أنظمة السلسلة الباردة التقليدية. وتم استخدام نموذج رياضي لتقييم الوفورات التي سيتم تحقيقها إذا تم تخزين اللقاح عند درجة الحرارة المحيطة أو بالقرب منها – في سلسلة "درجة حرارة خاضعة للسيطرة" – عند المستويات الطرفية لنظام سلسلة الإمداد.
النتائج
بلغت تكلفة السلسلة الباردة واللوجيستيات ذات الصلة المستخدمة في الحملة في تشاد 0.24 دولاراً أمريكياً لكل شخص حصل على التطعيم. ومع ذلك، انخفضت هذه التكاليف في السيناريو النموذجي لسلسلة درجة حرارة خاضعة للسيطرة، بنسبة 50 % وبلغت وفق التقديرات 0.12 دولاراً أمريكياً فقط لكل شخص حصل على التطعيم.
الاستنتاج
من الممكن أن ينتج عن تنفيذ سلسلة "درجة الحرارة الخاضعة للسيطرة" على المستويات الأكثر طرفية في نظام سلسلة الإمداد – بافتراض عدم الفقدان المرتبط بفعالية اللقاح أو تأثيره أو مأمونيته - فوائد اقتصادية كبرى ويسمح بتوسيع نطاق تغطية اللقاح في المناطق المنخفضة الموارد.
摘要
目的
评估大规模预防接种活动中将A型脑膜炎疫苗存放在环境温度或附近温度最长4 天的潜在经济效益。
方法
在2001年在乍得三个地区开展的为期10 天针对A型脑膜炎的大规模疫苗接种活动中,对传统冷链系统中疫苗的储存和运输的相关成本进行了评估。使用数学模型评估在供应链系统外围层次将疫苗在处于或接近环境温度下(“控制温度”链)存放时可能产生的节省。
结果
乍得接种疫苗人均冷链和相关物流成本是0.24 美元。但是在受控温度链模拟场景中,这些成本降低50%,据估计人均接种疫苗的成本仅为0.12 美元。
结论
在供应链的最外围层次实施“受控温度”链(假设没有疫苗效力、疗效或安全性的相关损失)具有重大的经济效益,可以在资源匮乏的地区推广疫苗覆盖。
Резюме
Цель
Оценить потенциальный экономический эффект от хранения вакцины против менингита А при температуре окружающей среды (или температурах, близких к этому значению) на протяжении 4 дней во время проведения кампании по массовой вакцинации.
Методы
Во время 10-дневной кампании 2011 года по массовой вакцинации против менингита A в трех регионах республики Чад была произведена оценка расходов на хранение и транспортировку вакцины с использованием традиционной системы подвоза с поддержанием низкой температуры груза. Для расчета экономии средств, которой можно было бы достичь в случае хранения вакцины при температуре окружающей среды (или температурах, близких к этому значению) – в системе подвоза с «регулируемой температурой» – на периферийных уровнях системы цепочки поставок, была использована соответствующая математическая модель.
Результаты
Стоимость системы подвоза с поддержанием низкой температуры груза и связанным с ней транспортным обеспечением, использовавшимся во время кампании в республике Чад, составила 0,24 доллара США на одного вакцинированного. В моделируемом сценарии с использованием «регулируемой температуры» расходы снизились на 50% и, в соответствии с расчетами, составили всего 0,12 долларов США на одного вакцинированного.
Вывод
Внедрение системы подвоза с использованием «регулируемой температуры» на большинстве периферийных уровней системы цепочки поставок – при условии, что это не приведет к утрате вакциной ее свойств и не повлияет на ее эффективность или безопасность – может принести значительные экономические выгоды и позволить расширить поставки вакцины с низкими накладными расходами.
Introduction
It is difficult to keep vaccines cool in countries where the mean daytime temperature exceeds 30 °C, electricity is unavailable in rural areas and the cold chain infrastructure is inadequate.1–3 The challenge is all the more acute during mass vaccination campaigns, when a wide-reaching cold chain and logistics (CCL) system is necessary if the target population is to receive a vaccine that has not lost potency because of exposure to excessively high or low temperatures. Unfortunately – irrespective of the actual thermostability of the vaccine – almost all vaccines used today are licensed by their manufacturers for storage at 2–8 °C at all points of the supply chains. One conjugate vaccine produced against meningitis A – MenAfriVac™ (Serum Institute of India, Pune, India) – is undamaged by exposure for a few days to temperatures up to 40 °C.4 In November 2012, this vaccine became the first of the vaccines prequalified by the World Health Organization (WHO) to receive regulatory approval for on-label use during mass vaccination campaigns that allowed vaccine storage at temperatures at or below 40 °C for up to 4 days.4 As there is no associated loss of safety, potency or efficacy, this approval opened the possibility of keeping doses of this vaccine in a relatively simple controlled temperature chain (CTC) for a few days – rather than in a standard cold chain system. In a CTC, the risk of accidental freezing of vaccines is eliminated. Storing vaccines in a CTC could reduce the operational costs of mass vaccination campaigns against meningitis A while extending the reach of such campaigns to the populations in need.5–7
The subject of the present study was a mass campaign against meningitis A that covered three regions of Chad and resulted in the vaccination of 1 807 158 individuals in December 2011.8 Although 12 districts, 668 fixed health posts, 287 outreach posts and 55 mobile teams implemented the campaign, the infrastructure supporting the CCL system used throughout the campaign was weak.8 The campaign therefore provided a good case study for exploring the benefits of CTC. The main aim of the present study was to model the economic benefits that could have been obtained had the vaccine used in this campaign been kept at or near ambient temperatures at the peripheral levels of the supply chain system. The flexibility offered by being able to keep vaccines in a CTC at the periphery could have far-reaching benefits, especially for the 450 million people at risk of meningitis who live in the African “meningitis belt”.9
Methods
Since the label change to MenAfriVac™ to allow for use in a CTC in a mass vaccination campaign had not been approved in 2011 at the time of our study, we concentrated on analysing the full costs of a CCL system in a standard campaign setting. We then modelled the direct savings that could have been expected in the same campaign if the CCL system had been replaced – for up to 4 days before the vaccines were used – by storage, transport and administration at or near ambient temperature in a CTC.
Data collection and sources
During a field visit to Chad from 12 to 16 December 2011, we collected both qualitative information – through interviews and on-site observations – and quantitative data drawn from a variety of primary and secondary sources. Primary data were collected from the national immunization programme, from the central vaccine store, from each of the three regions targeted by the then-current mass vaccination campaign – N’Djamena, Chari Baguirmi and Mayo Kebbi Est – and from the 12 districts within these three regions that were implementing the campaign.8 In addition, primary data were collected from a sample of fixed health posts, outreach posts and mobile teams spread across the 12 implementing districts. A specific survey instrument was developed and adapted locally to account for context-specific elements in Chad. Data on costs were gathered using an “ingredients” approach, in which a general description of the vaccine supply chain was broken down into the components that represented the utilization of resources – namely prices, quantities and time. Important secondary data were collected from pre-campaign planning and budgeting documents, the district-level micro-plans, the national comprehensive multi-year plan for immunization and the national cold chain inventory.8,10–12
Although we collected data on the economic costs of using the resources that were available from Chad’s routine immunization programme before the campaign began, we included in the final analysis only the direct financial costs associated with the CCL for the mass campaign. As a result, we only estimated the direct campaign-specific CCL costs that would have been avoided had a CTC strategy been implemented.
Cost categories
The CCL costs of the campaign were divided into three categories: those associated with the cold chain system, those associated with vaccine transportation and those associated with the human resources involved in the vaccine supply chain during the campaign. All costs were converted from African Financial Community francs into United States dollars (US$), using the exchange rate in use at the end of 2011.
Cold chain system
The costs associated with all of the equipment used to store the vaccines between 2 and 8 °C – at all points of the supply chain until the vaccine was injected – were evaluated. This equipment included walk-in cold and freezer rooms at national level and refrigerators, ice-pack freezers, cold boxes, vaccine carriers and ice packs at lower levels of the supply chain. Substantial amounts of kerosene were needed to run the many absorption refrigerators used at the subnational level10,12 and the costs of this fuel were included.
It is important to note that temporary cold chain equipment had to be deployed specifically for the mass campaign that we studied. In 8 of the 12 districts targeted by the campaign, only 50% of the cold chain equipment available as part of the national immunization programme before the campaign was launched was found to be functioning at that time.10 The remaining equipment was either broken, too run down to store vaccines or unable to produce frozen ice packs for vaccine transportation and storage at the service level. To respond to this constraint, 52 refrigerators or ice pack freezers and 332 cold boxes that had been purchased by the national immunization programme were temporarily deployed specifically for the mass campaign.12 To deploy this additional equipment, a moving company was hired to transport these refrigerators, freezers and cold boxes in advance of the campaign and to collect them at the end of the campaign. These additional costs were included in the analysis.
Transportation system
An elaborate system for the transportation of the vaccine was required for the mass campaign. This included planned delivery circuits to resupply vaccination posts and storage points with vaccine, cold boxes and fresh ice packs two or three times a day. The costs of using the vehicles from the routine immunization programme that were mobilized for use during the campaign, the cost of renting vehicles specifically for the campaign, and the cost of using personal vehicles – particularly at the service delivery levels – for the campaign were included in the analysis. Running costs were primarily based on the distances travelled, fuel consumption and the travel-related per diems provided to health workers.
Human resources
Using information on monthly salaries received from the Ministry of Health, we estimated the salary costs of the staff who – although employed to work on the routine immunization programme – were mobilized to work on CCL-related aspects of the planning and implementation of the mass campaign. In addition, all health workers at regional, district and service level – whether in the paid employment of the national immunization programme or volunteers – were given campaign-specific per diems.
Cost adjustments
Several adjustments to cost items were required. First, all capital costs were annualized using a straight-line depreciation technique and standard published values for useful life–years for each type of cold chain equipment and vehicle.13,14 Second, costs in each of the three cost categories were adjusted to reflect the utilization of resources during the campaign and time on CCL-related activities. For simplicity, it was assumed that, in the areas targeted, the entire cold-chain system was used only for the campaign during the 10 days that the campaign ran. Field observations indicated that this assumption was a reasonable one.12 The large amount of temporary cold chain equipment that was deployed specifically for the campaign was intended to be used only for the campaign.
A similar assumption could not reasonably be made for the other two cost categories. The costs of the transportation system and human resources were therefore adjusted to reflect the proportion of “time in use” during the campaign period that related to campaign-specific CCL activities. For the transportation system, this time allocation was based on interviews with drivers and estimates of the numbers of journeys, distances travelled and time spent on the CCL system during the campaign. It was extremely variable and depended on the type of vehicle used and on where vaccine was being transported in the supply chain. On average, half of the utilization of vehicles for the campaign was attributable to CCL-related activities. Human resource time was separated into two categories: “campaign planning or training” related to CCL and “campaign implementation” time associated with CCL. The time spent in campaign implementation included all the time that the relevant health workers were operating the CCL system for the campaign – to ensure vaccines were kept between 2 and 8 °C throughout the entire distribution system. This included time for freezing, conditioning and distributing ice packs, organizing cold boxes and vaccine carriers, travelling to and from vaccination sites with vaccines, and replacing thawed ice packs and vaccinating. Overall, the attribution of staff time – and hence costs – to these campaign-specific CCL activities was approximately 20 to 25% for vaccinators, 25 to 30% for campaign supervisors, 50 to 55% for drivers and 75 to 100% for cold-chain “officers”.
Modelled scenario
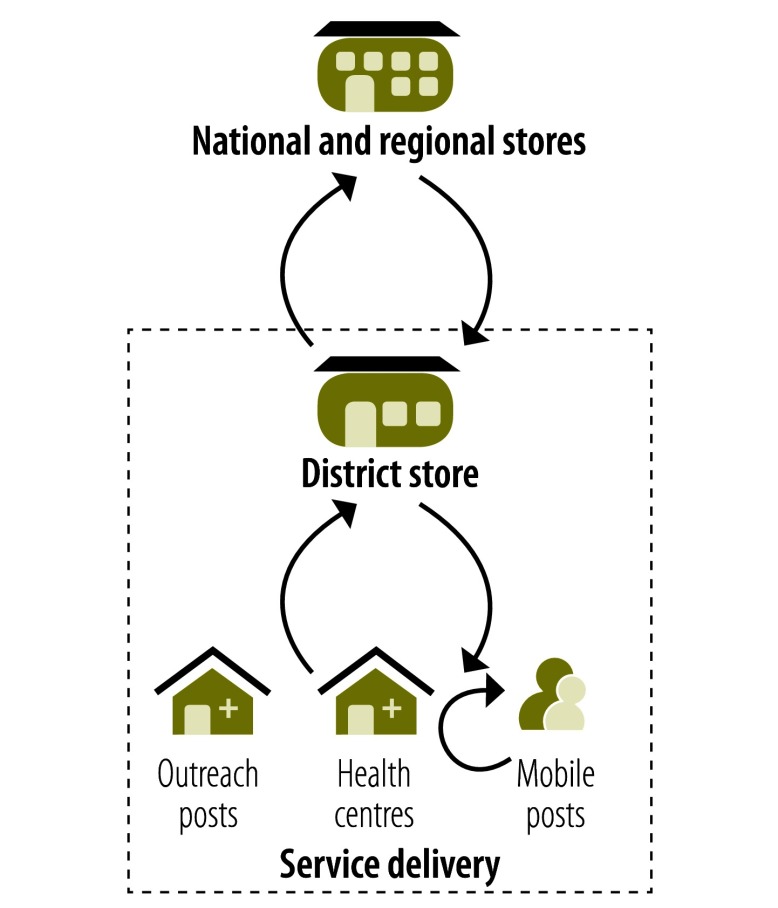
Defining an appropriate scenario to model was primarily conceptual and based on the need to ensure that the vaccine is kept at or near ambient temperatures of no more than 40 °C for up to 4 days. In general, for this to be possible vaccines had to be already edged out to the periphery of the supply chain system before they entered a CTC. In the illustrative scenario that we selected, the CTC therefore started at the district-level storage point for vaccines, which is the last level in the supply chain before service delivery. In this situation, vaccines would be kept at 2 to 8 °C at all times, from the national vaccine store down to – and including – their transport to district storage points. The vaccines would then be stored and transported in a CTC – at or near ambient temperature – up to the point at which they are administered at a vaccination post – which might be fixed, outreach or mobile. Once the scenario was defined, we compared the total CCL costs of the campaign implemented in Chad with the total CCL costs estimated from the modelled scenario in which a CTC strategy was implemented between district-level storage and vaccine administration (Fig. 1).
Fig. 1.
Vaccine distribution in Chad and the modelled scenario
CTC, controlled temperature chain.
Note: The figure illustrates both the actual system used to distribute vaccine doses in the mass campaign in Chad in December 2011 and the modelled scenario. In the model, the cold chain between the initiation of storage at the delivery level and vaccine use was replaced with a CTC – indicated by the part of the system within the dashed box. The circular arrows indicate the supply of ice boxes, vaccine doses and frozen ice packs at the start of each day, the subsequent resupply of doses and frozen ice packs and collection of thawed ice packs – for refreezing – during each day, and the collection of ice boxes, ice packs and unused vaccine doses at the end of each day when not using CTC.
Results
Chad campaign
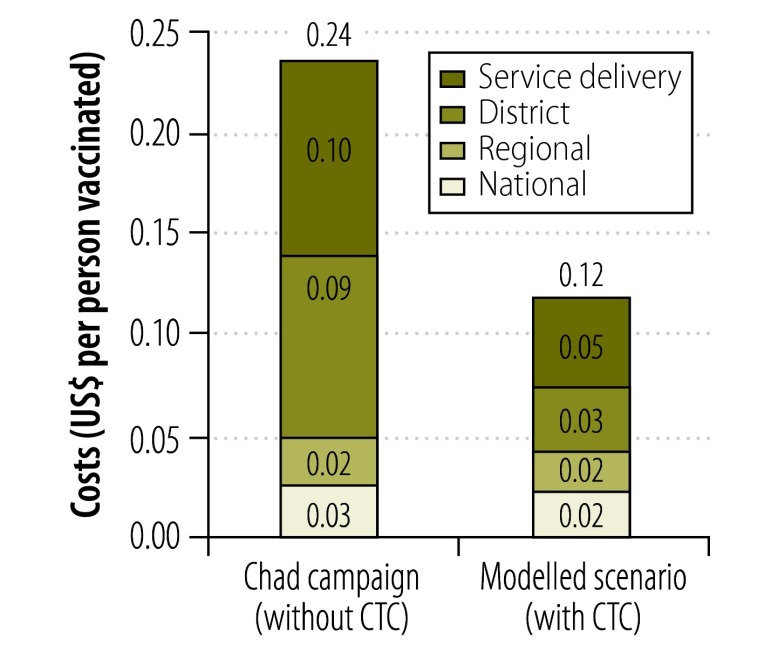
The total cost of operating the CCL system during the campaign that we studied in Chad was US$ 425 181 – equivalent to US$ 0.24 per person vaccinated (Table 1). Among the three regions that implemented the campaign, the cost per person vaccinated varied from US$ 0.20 in the N’Djamena region to US $0.28 in the more challenging Chari Baguirmi region. At the district level, the costs of the CCL system varied from US$ 0.13 to US$ 0.57 per person vaccinated. They were four times lower in the more accessible districts of the capital city of N’Djamena than in the more remote and less densely populated districts of Mayo Kebbi Est.
Table 1. Costs of the cold chain system in the mass vaccination campaign against meningitis A, by region, Chad, 2011.
| Costs | Region |
|||
|---|---|---|---|---|
| Chari Baguirmi | Mayo Kebbi Est | N’Djamena | All three | |
| Total (in US$) | 136 976 | 131 235 | 156 970 | 425 181 |
| Fraction of total attributed to | ||||
| Cold chain system, % | 44 | 46 | 40 | 43 |
| Transport system, % | 25 | 19 | 15 | 20 |
| Human resources, % | 32 | 35 | 45 | 37 |
| Per person vaccinated (in US$), mean, (range)a | 0.28 (0.24–0.31) | 0.24 (0.21–0.57) | 0.20 (0.13–0.35) | 0.24 (0.13–0.57) |
US$, United States dollars.
a Of the mean values recorded at the district level in each region.
Equipment and human resources represented 43% and 37% of the total cost of the CCL system, respectively (Table 1). A substantial amount of time was spent on organizing the vaccination sites in advance of vaccination sessions. This involved not only staff with well defined vaccine management responsibilities – such as cold chain managers, logisticians and drivers – but also vaccinators and supervisors. The organization of vaccination sites included both the supply of each site with vaccine doses, cold boxes and frozen ice packs early on each morning of the campaign and the regular resupply of each site with vaccine doses and frozen ice packs during each vaccination session (Fig. 1).
Modelled scenario
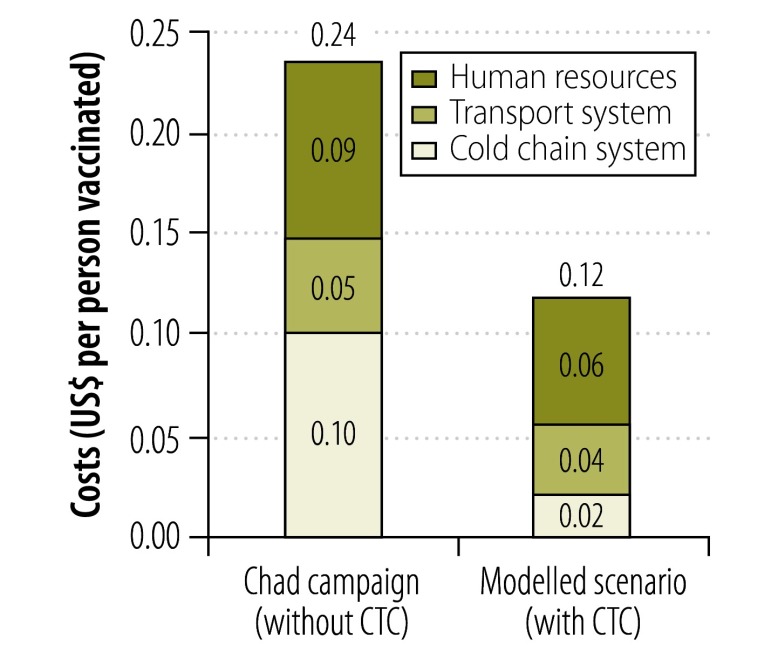
Analysis of the modelled scenario indicated that substantial savings could have been achieved in the Chad campaign if the vaccine could have been kept at or near ambient temperatures in a CTC when it was at the district and service delivery levels (Fig. 2 and Fig. 3). In this situation, the overall CCL costs of the campaign would have dropped to an estimated US$ 213 499. This cost – equivalent to US$ 0.12 per target – represents a saving of US$ 211 682 (Table 2). If we assume that a dose of the vaccine used costs US$ 0.50, the estimated savings from implementing a CTC at the district level represents more than 20% of the cost of the vaccine doses for the campaign.
Fig. 2.
Costs of the cold chain and associated logistics, by cost category, in three regions of Chad, 20011
CTC, controlled temperature chain; US$, United States dollars.
Note: The data shown are the actual costs recorded in the mass vaccination campaign that was run in three regions of Chad in December 2011 and the costs estimated in a modelled scenario in which a CTC was implemented.
Fig. 3.
Costs of the cold chain and associated logistics, by level of the cold chain, in three regions of Chad, 2011
CTC, controlled temperature chain; US$, United States dollars.
Note: The data shown are the actual costs recorded in the mass vaccination campaign that was run in three regions of Chad in December 2011 and the costs estimated in a modelled scenario in which a CTC was implemented.
Table 2. Estimated cost savings with a controlled temperature chain, by region, Chad, 2011.
| Savingsa | Region |
|||
|---|---|---|---|---|
| Chari Baguirmi | Mayo Kebbi Est | N’Djamena | All three | |
| Total (in US$) | 69 119 | 65 744 | 76 819 | 211 682 |
| Fraction of total attributed to | ||||
| Cold chain system, % | 82 | 80 | 89 | 84 |
| Transport system, % | 4 | 4 | 2 | 3 |
| Human resources, % | 14 | 16 | 9 | 13 |
| Per person vaccinated (in US$), mean, (range)b | 0.14 (0.09–0.17) | 0.12 (0.10–0.33) | 0.10 (0.06–0.19) | 0.12 (0.06–0.33) |
US$, United States dollars.
a Estimated from the modelled scenario in which the cold chain between the initiation of storage at delivery level and vaccine use was replaced with a controlled temperature chain (CTC).
b Of the mean values recorded at the district level in each region.
In some districts, the estimated savings from implementing the CTC reached US$ 0.33 per person vaccinated – or more than 65% of the cost of the vaccine (Table 2). At the regional level, the estimated savings per person vaccinated varied from US$ 0.10 in N’Djamena region to US$ 0.14 in Chari Baguirmi region (Table 2). The N’Djamena region is generally urban and already had a relatively strong CCL system before the study campaign began. In this region, no temporary cold chain equipment had to be deployed specifically for the campaign. Chari Baguirmi and Mayo Kebbi Est are generally more rural and less densely populated than the N’Djamena region and had a cold chain infrastructure that was inadequate for the campaign. In both of these regions, cold chain equipment had to be temporarily deployed specifically for the campaign.
In the modelled scenario, shortening of the cold chain was the largest determinant of the cost savings; it represented 84% of the overall savings for the campaign. The cost savings were greater in the human resources category than in the transportation system category (Fig. 2), presumably because the vaccine transportation required was largely unaffected by the type of storage system employed.
Discussion
In this study, we modelled the economic benefits of keeping vaccines at or near ambient temperature in a CTC during a mass vaccination campaign against meningococcal meningitis in Chad. The results indicate the substantial cost savings that could be obtained in similar campaigns if the cold chain system were replaced with a CTC between the initiation of district-level storage and vaccination.
This study has three important limitations that need to be acknowledged. First, the savings modelled in this study are based on a single illustrative scenario. In reality, a CTC may be used very differently from the way in which it was modelled in our scenario. For the future application of such an approach, each country would need to devise a strategy relevant to its own setting and supply chain network – and such specificities are likely to affect the savings that use of a CTC could bring. Second, we could not estimate the costs that might increase as a result of the implementation of such a chain. Such costs include those of replacing vaccine that has been inadvertently damaged by excessive exposure to temperatures above 40 °C – or, at least, that needs to be discarded because exposure to such temperatures has been indicated by vaccine vial monitors or peak threshold indicator cards that have been placed inside the vaccine carriers. Similarly, it was not possible to factor in the additional savings that might be achieved if use of a CTC reduced the incidence of vaccine freezing. Third, the three regions involved in the mass campaign are not representative of the entire country, since they are relatively accessible areas near the capital and with relatively dense populations. Our estimates of the savings – per person vaccinated – that might result from using a CTC are therefore likely to be underestimates of the savings that could be obtained in the other 19 regions of Chad. Linked to this, our results are perhaps also not generalizable across a wider set of “meningitis belt” countries in sub-Saharan Africa because the routine immunization system and cold chain infrastructure that existed in Chad immediately before the mass campaign that we studied were particularly weak. In countries that have stronger systems for routine vaccination and established and efficient cold chain infrastructures, the savings achievable by use of a CTC may be less than those that we estimated for Chad.15,16
Despite these limitations, this analysis indicates the potential economic benefits of allowing a thermostable vaccine to reach ambient temperatures for a few days. The findings are similar to those from a parallel study conducted in Ghana during a vaccination campaign against yellow fever. In the Ghanaian study it was estimated that the use of a CTC would bring savings of US$ 0.10 to 0.16 per person vaccinated – depending on the scenario that was simulated.17
Beyond the potential economic benefits illustrated by our research, it is worth reflecting on the much broader potential benefits – for global vaccination policy and practice – that might be seen if CTCs were to be more widely used. First, the use of such chains could reduce the workloads of health workers who often struggle to maintain a functioning CCL system during a vaccination campaign. Vaccination campaigns are often criticized for diverting scarce human resources from routine health services. Second, CTCs may allow the thermostability of a vaccine to be exploited fully and make it possible for vaccinators to extend their reach to areas and populations that are so remote that they could never be reached by a cold chain system – while avoiding the vaccine damage caused by inadvertent freezing.18–20 If the requirement for a cold chain can be reduced, it may also be possible to use vaccination activities as a better delivery platform for other maternal and child health interventions.21 Third, the use of a CTC for a relatively thermostable vaccine could free up valuable space in cold storage for other vaccines that have poorer thermostability.22
For epidemic meningococcal meningitis, prevention by vaccination – at a vaccine cost of approximately US$ 0.50 per dose – appears to yield a much better return on investment than treatment – at a mean cost for the health sector of about US$ 26 per meningitis case managed.23 With a meningitis A vaccine now licensed for use in a CTC during mass vaccination campaigns, considerable efficiencies and savings could be achieved if the on-label use of the vaccine in such a chain could be adopted and implemented more broadly in Africa’s meningitis belt. To this effect, WHO recently issued guidance for decision-makers and managers involved in immunization programmes on the use of meningitis A vaccine in CTCs.24 Given the public health importance of controlling meningitis outbreaks through mass vaccination – and the difficulties faced by national governments in mobilizing the financial resources needed to conduct the ambitious series of campaigns planned between now and 2016 – such chains may become essential. The potential economic benefits illustrated in this study will hopefully guide decisions and policy around the future adoption and implementation of CTCs in the meningitis belt. They should also help improve the efficiency of mass vaccination campaigns against meningitis in low-resource settings.
Acknowledgements
We thank our colleagues in the World Health Organization and United Nations Children’s Fund for supporting the logistics and operation of the study in Chad and developing the study protocol and data-collection instruments.
Competing interests:
None declared.
References
- 1.Humphreys G. Vaccination: rattling the supply chain. Bull World Health Organ. 2011;89:324–5. doi: 10.2471/BLT.11.030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabot O, Yadav P, Zaffran M. Maximizing every dose and dollar: the imperative of efficiency in vaccine delivery Seattle: National Bureau of Asian Research; 2011. Available from: http://www.nbr.org/downloads/pdfs/CHA/CHA_MazimizingEveryDoseandDollar.pdf [accessed 16 September 2013].
- 3.Zaffran M, Vandelaer J, Kristensen D, Melgaard B, Yadav P, Antwi-Agyei KO, et al. The imperative for stronger vaccine supply and logistics systems. Vaccine. 2013;31(Suppl 2):B73–80. doi: 10.1016/j.vaccine.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization [Internet]. Revolutionary meningitis vaccine breaks another barrier: first to gain approval to travel outside cold chain. Geneva: WHO; 2012. Available from: http://www.who.int/immunization/newsroom/menafrivac_20121114/en/index.html [accessed 16 September 2013].
- 5.Halm A, Yalcouyé I, Kamissoko M, Keïta T, Modjirom N, Zipursky S, et al. Using oral polio vaccine beyond the cold chain: a feasibility study conducted during the national immunization campaign in Mali. Vaccine. 2010;28:3467–72. doi: 10.1016/j.vaccine.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 6.Zipursky S, Boualam L, Cheikh DO, Fournier-Caruana J, Hamid D, Janssen M, et al. Assessing the potency of oral polio vaccine kept outside of the cold chain during a national immunization campaign in Chad. Vaccine. 2011;29:5652–6. doi: 10.1016/j.vaccine.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Zipursky S, Préaud J-M, Djingarey MH. MenAfriVac™ planned for use in a controlled temperature chain Ferney Voltaire: PATH; 2012.
- 8.Meningitis A campaign plan for Chad N’Djamena: Ministry of Health; 2011.
- 9.PATH [Internet]. Map of Africa’s meningitis belt. Ferney Voltaire: PATH; 2012. Available from: http://www.path.org/menafrivac/meningitis-belt.php [accessed 16 September 2013].
- 10.Evaluation report for the EPI cold chain and logistics in Chad. N’Djamena: Ministry of Health; 2010.
- 11.Comprehensive multi year plan (cMYP) for the Expanded Programme on Immunization in Chad (2008–2012). N’Djamena: Ministry of Health; 2007.
- 12.Lydon P, Zipursky S. Economic analysis of using meningitis A in a controlled temperature chain (CTC) – evidence from the MenAfriVac™ campaign in Chad Geneva: World Health Organization; 2012.
- 13.Guidelines for estimating costs of introducing new vaccines into the national immunization system Geneva: World Health Organization; 2002. Available from: http://whqlibdoc.who.int/hq/2002/WHO_V&B_02.11.pdf [accessed 2 December 2013].
- 14.Immunization costing & financing: a tool and user guide for comprehensive multi-year planning (cMYP). Geneva: World Health Organization; 2006 (WHO/IVB/06.15). Available from: http://whqlibdoc.who.int/hq/2006/WHO_IVB_06.15_eng.pdf [accessed 2 December 2013].
- 15.Toboe D. Rapport d’appui technique à l’équipe du bureau de la représentation de l’OMS au Tchad pour l’introduction du nouveau vaccin conjugué contre la méningite A (MenAfriVac™). Brazzaville: World Health Organization Regional Office for Africa; 2012. French.
- 16.Botoko Dolia JP. Rapport de fin de contrat d’appui logistique à la campagne méningite au Tchad du 26 Octobre 2011 au 26 Janvier 2012. Brazzaville: World Health Organization Regional Office for Africa; 2012. French.
- 17.Dillavou C. Cold chain costing: a study of the costs associated with the yellow fever vaccination campaign in Ghana. April 2012 Geneva: World Health Organization; 2012.
- 18.Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Soewarso TI. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ. 1999;77:119–26. [PMC free article] [PubMed] [Google Scholar]
- 19.Otto BF, Suarnawa IM, Stewart T, Nelson C, Ruff TA, Widjaya A, et al. At-birth immunisation against hepatitis B using a novel pre-filled immunisation device stored outside the cold chain. Vaccine. 1999;18:498–502. doi: 10.1016/S0264-410X(99)00242-X. [DOI] [PubMed] [Google Scholar]
- 20.Pegurri E, Fox-Rushby JA, Damian W. The effects and costs of expanding the coverage of immunisation services in developing countries: a systematic literature review. Vaccine. 2005;23:1624–35. doi: 10.1016/j.vaccine.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 21.Johri M, Sharma JK, Jit M, Verguet S. Use of measles supplemental immunization activities (SIAs) as a delivery platform for other maternal and child health interventions: opportunities and challenges. Vaccine. 2013;31:1259–63. doi: 10.1016/j.vaccine.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Lee BY, Cakouros BE, Assi T-M, Connor DL, Welling J, Kone S, et al. The impact of making vaccines thermostable in Niger’s vaccine supply chain. Vaccine. 2012;30:5637–43. doi: 10.1016/j.vaccine.2012.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colombini A, Badolo O, Gessner BD, Jaillard P, Seini E, Da Silva A. Costs and impact of meningitis epidemics for the public health system in Burkina Faso. Vaccine. 2011;29:5474–80. doi: 10.1016/j.vaccine.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 24.Use of MenAfriVac™ (meningitis A vaccine) in a controlled temperature chain (CTC) during campaigns: guidance for immunization programme decision-makers and managers Geneva: World Health Organization; 2013. Available from: http://apps.who.int/iris/bitstream/10665/86018/1/WHO_IVB_13.04_eng.pdf [accessed 16 September 2013].